Abstract
Stimulants like methylphenidate are increasingly used for cognitive enhancement, but precise mechanisms are unknown. We found that methylphenidate boosts willingness to expend cognitive effort by altering the benefit-to-cost ratio of cognitive work. Willingness to expend effort was greater for participants with higher striatal dopamine synthesis capacity, while methylphenidate and sulpiride – a selective D2 receptor antagonist – increased cognitive motivation more for participants with lower synthesis capacity. A sequential sampling model informed by momentary gaze revealed that decisions to expend effort are related to amplification of benefit-versus-cost information attended early in the decision process, while the effect of benefits is strengthened with higher synthesis capacity and by methylphenidate. These findings demonstrate that methylphenidate boosts the perceived benefits-versus-costs of cognitive effort by modulating striatal dopamine signaling.
One Sentence Summary:
Striatal dopamine increases cognitive effort by respectively amplifying and attenuating the subjective benefits and costs of cognitive control.
Cognitive control is effortful, causing people to avoid demanding tasks (1) and to discount goals (2, 3). Striatal dopamine invigorates physical action by mediating cost-benefit tradeoffs (4). In cortico-striatal loops, dopamine has opponent effects on D1 and D2-expressing medium spiny neurons, which modulate sensitivity to the benefits versus the costs of actions (5). Given that similar mechanisms may govern cognitive action selection (6–8), we hypothesized that striatal dopamine could promote willingness to exert cognitive effort, enhancing attention, planning, and decision-making (8–11).
Converging evidence on cognitive motivation in Parkinson’s disease (12–15) provides an initial basis for this conjecture. Moreover, catecholamine-enhancing psychostimulants alter cognitive effort in rodents (10) and humans (16). This raises the question of whether so called smart drugs act by enhancing the willingness rather than the ability to exert cognitive control. Indeed, the dominant interpretation is that stimulants improve cognitive processing, via direct cortical effects, noradrenaline transmission (17, 18) and/or concomitant working memory improvements (19). We instead hypothesized that methylphenidate (a dopamine and noradrenaline reuptake blocker) boosts cognitive control by increasing striatal dopamine and, accordingly, sensitivity to the benefits-versus-costs of cognitive effort.
50 healthy, young adults (ages 18—43, 25 men) completed a cognitive effort discounting paradigm (2) quantifying the subjective effort costs as the amount of money required to make participants equally willing to perform a hard (N = 2, 3, 4) versus easier (N = 1, 2) level of the N-back working memory task. We defined the subjective value of an offer to complete a harder task (N = 2—4) as the amount offered for the task, minus subjective costs.
Subjective values decreased with N-back load, indicating rising subjective costs (Fig. 1A). Critically, greater willingness to expend cognitive effort corelated with higher dopamine synthesis capacity (measured using [18F]DOPA PET) in the caudate nucleus (independently defined (20); Fig. 1A—C, Fig. S1). A mixed-effects model confirmed that on placebo, subjective values increased with larger offer amounts (€4 versus €2 offers; , ), smaller relative load (, ), and higher dopamine synthesis capacity (, ). These individual difference effects were selective to the caudate nucleus (Fig. S1—S2), consistent with human imaging studies on cognitive motivation (7, 21, 22). Although N-back performance decreased with load, dopamine effects on discounting could not be attributed to performance changes (Supplemental Results). Moreover, there were no drug effects on performance because drugs were administered after N-back.
Fig. 1.
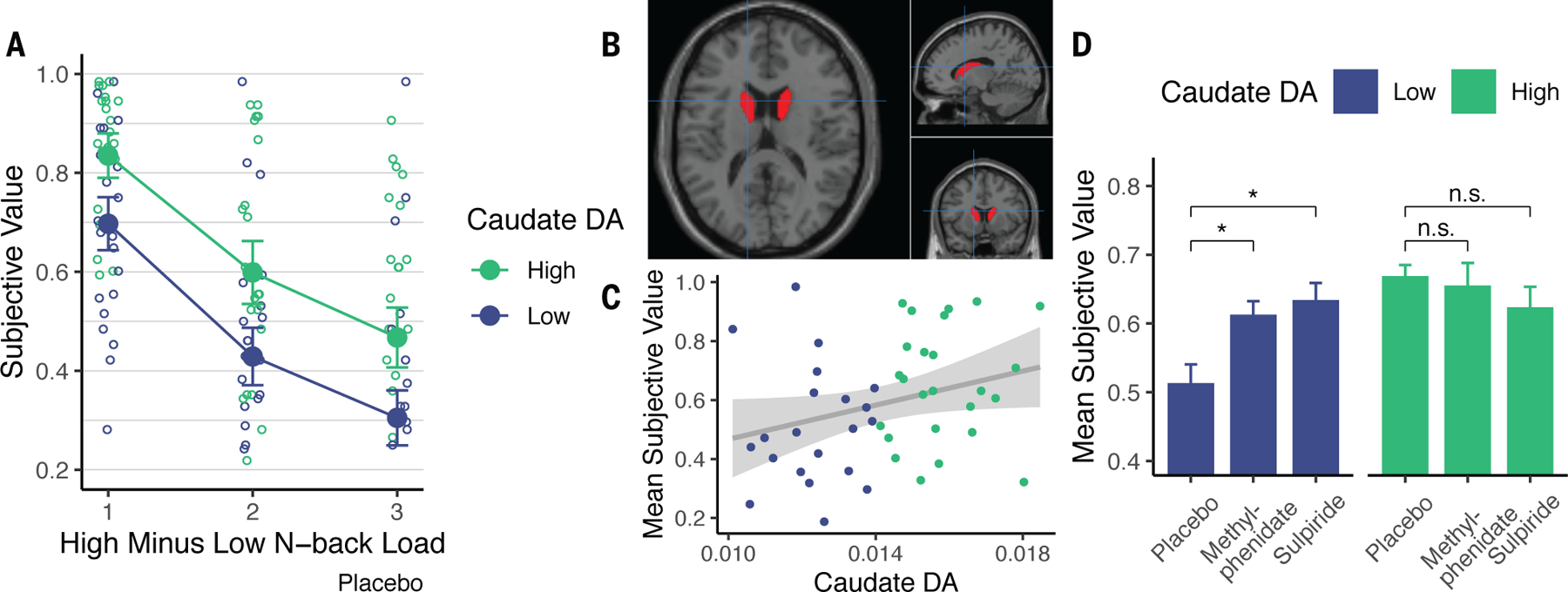
Participants discounted offers as a function of cognitive load, dopamine synthesis capacity (DA), and drug. A. Offers were discounted more for high- versus low-load levels, and more by participants with below- versus above-median dopamine synthesis capacity. Circles show individual’s indifference points. Filled circles show group mean +/− SEM. B. Caudate nucleus mask. Crosshairs at MNI [−14, 10, 16]. C. Participant-averaged subjective values correlated with synthesis capacity on placebo (Spearman , ). D. Methylphenidate (, ) and sulpiride (, ) increased subjective values for participants with low, but not high synthesis capacity ( for both). Error bars are within-subject SEM.
If dopamine mediates cognitive effort, it should be possible to increase motivation pharmacologically. Indeed, both methylphenidate and sulpiride increased subjective values for participants with low, but not high dopamine synthesis capacity (Fig. 1D; Fig. S2B—C). A mixed-effects model revealed that both methylphenidate (, ) and sulpiride (, ) interacted with dopamine synthesis capacity to increase subjective values. Neither drug showed main effects (both ).
The converging effects of synthesis capacity and two separate drugs strongly implicate striatal dopamine. Methylphenidate blocks reuptake, increasing extracellular striatal dopamine tone (23) and can amplify transient dopamine signals (24). Sulpiride is a D2 receptor antagonist which, at low doses can increase striatal dopamine release by binding to pre-synaptic auto-receptors, enhancing striatal reward signals, and learning (6, 25). While sulpiride can block postsynaptic D2 receptors at higher doses (26), both drugs increased behavioral vigor (reaction times and saccade velocities; cf. (6, 26)), especially in low dopamine synthesis capacity participants, corroborating that both drugs increased dopamine release (Supplemental Results).
To assess whether dopamine amplified subjective benefits versus costs, we made a series of offers, in a second task, centered around participants’ indifference points (Fig. 2A). To generate specific predictions, we simulated psychometric choice functions with a computational model of striatal dopamine effects on decision making (5). With higher dopamine, the model predicts enhanced sensitivity to benefits and reduced sensitivity to costs. This manifests as a steeper choice function to the right of indifference, where the ratio of benefits to costs (of the high- versus low-effort option) is larger, but shallower functions to the left, where the benefits-to-costs ratio is smaller (Fig. 2B).
Fig 2.
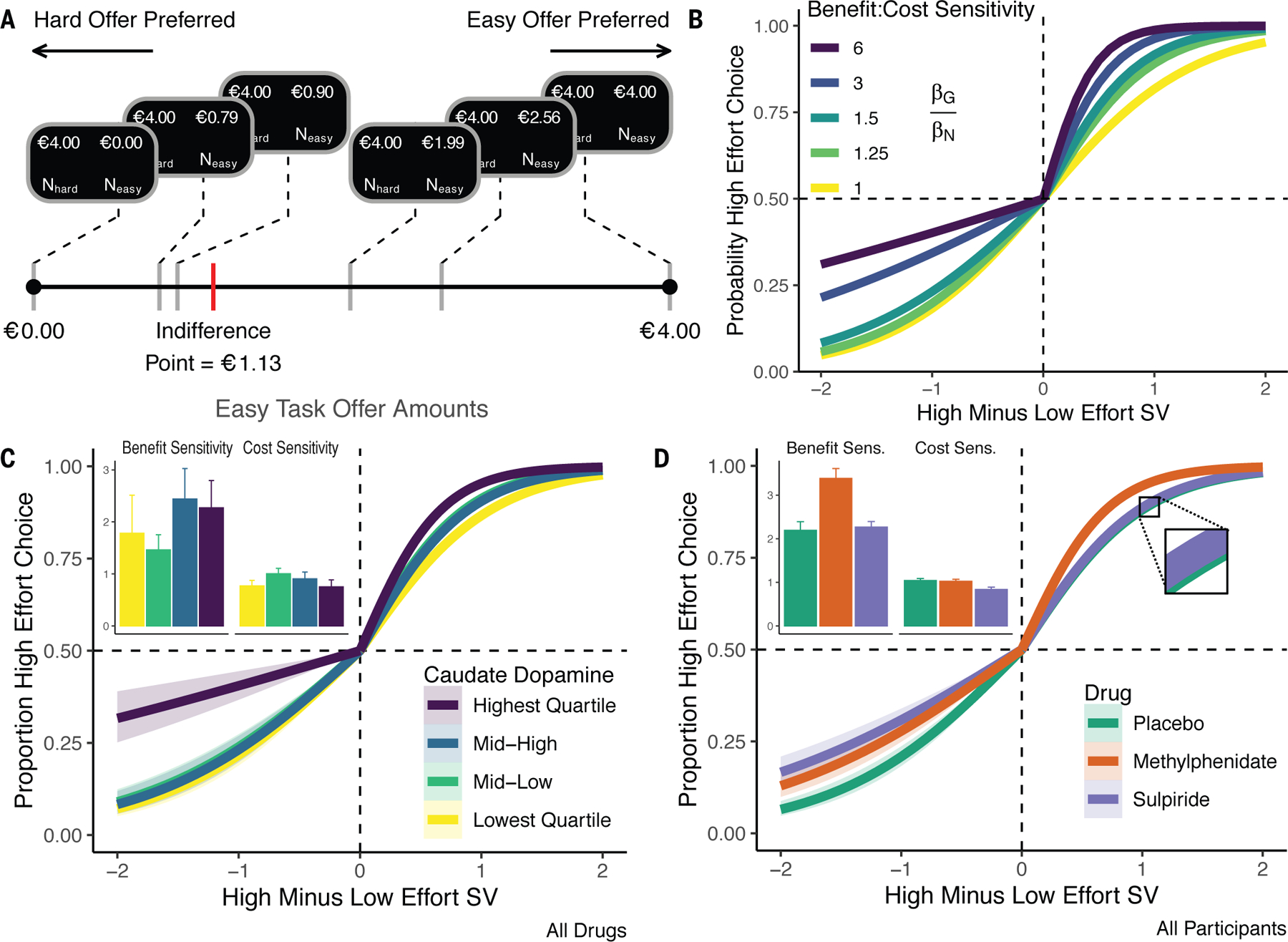
Dopamine alters valuation by re-weighting benefits-versus-costs of cognitive work. A. Low-effort (Neasy) offers were paired with a €4 offer for a high-effort N-back task (Nhard). B. Simulated effects of dopamine on benefit-versus-cost sensitivity are mirrored by empirical effects of C. dopamine synthesis capacity and D. pharmacological agents. Mixed-effects logistic regression curves and 95% CI fit across all drugs for each synthesis capacity quartile (C.) or all participants for each drug (D.). Insets show estimated effect of benefits and costs on choice across participants in (C.) each quartile +/− SEM and on (D.) each drug +/− within-subject SEM.
Choice behavior supported model predictions. Simulated effects were mirrored by effects of variability in dopamine synthesis capacity (Fig. 2C), and of methylphenidate and sulpiride versus placebo (Fig. 2D). Formally, high effort selection was sensitive to both benefits (offer amount differences; , ) and costs (load differences; , ). Critically, the effect of benefits increased with synthesis capacity (, ) and on methylphenidate (, ), while the effect of costs was attenuated on sulpiride (, ). Participants also selected high-effort choices more often with higher dopamine synthesis capacity (, ), and on methylphenidate (, ) versus placebo, but not reliably so for sulpiride (, ). No other interactions or main effects were significant (all ).
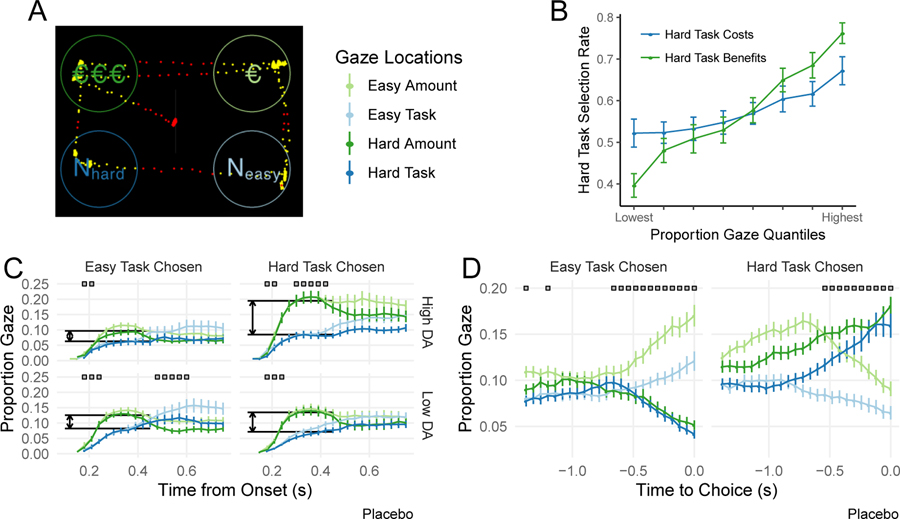
These results clearly implicate dopamine in choice but they do not uncover how decision- making is altered. Dopamine could increase attention to benefits versus costs. Alternatively, it could alter the impact of these attributes on choice without impacting attention itself. We thus tracked eye gaze to quantify attention to attributes and how it interacted with dopamine. Proportion gaze at an offer (either costs or benefits) strongly predicted offer selection, (Fig. 3B; , ; cf. (27, 28)). However, gaze at benefits predicted steeper increases in hard-task selection than gaze at costs (gaze by dimension interaction:, ).
Fig. 3.
Effect of gaze, value, and dopamine synthesis capacity on effort selection. A. Participants decided between offers with costs (N-back load) and benefits (Euros) separated in space. Dots indicate gaze at (yellow) and away from (red) offers. B. Gaze predicted high-effort selection, and more so with gaze at benefits-versus-costs. C—D. Proportional (cross-trial) gaze at the four information quadrants following offer onset and leading up to response. C. Early gaze (250—450ms following offer onset) indicated by grey shading. Boxes indicate time points at which participants gazed reliably more at either benefits or cost information (paired t-tests P < 0.05). D. Boxes indicate time points at which participants gazed reliably more at the selected offer (one-tailed paired t-tests, P < 0.05). All error bars reflect +/− SEM.
Gaze patterns implicated dopamine in enhancing the impact of attention to benefits-versus-costs on the decision to engage in cognitive effort. Early in a trial, participants fixated benefits (of either offer) more than costs and this asymmetry was larger on trials in which they chose the high-effort option (choice effect: , ; Fig. 3C). Moreover, this effect was stronger in participants with higher dopamine synthesis capacity (choice by synthesis capacity interaction: , ; top versus bottom row, Fig. 3C). For those with lower synthesis capacity, methylphenidate strengthened this relationship (interaction between drug, synthesis capacity, and choice: , ; though sulpiride did not: , ). Drugs and synthesis capacity did not impact gaze patterns themselves ( for main effects), indicating that dopamine did not alter attention to benefits, but rather strengthened the impact of attention to benefits-versus-costs on choice.
Gaze may correlate with choice because attention amplifies the perceived value of attended offers, causally biasing choice (27). Alternatively, reversing this causality, participants may simply look more at offers they have already implicitly chosen (28). We found evidence for both: Early in a trial, attention influenced choice while, later, choice influenced attention. To address this, we fit drift diffusion models (29) in which cost and benefit information accumulate in a decision variable rising to a threshold. This variable is the instantaneous difference in the perceived value of the high- versus the low-valued offer. We considered ‘attention-biasing-choice’ models with multiplicative effects (i.e., gaze multiplies the effects of value information), and ‘choice-biasing-attention’ models in which gaze has a simple, additive effect (gaze correlates with choice but does not amplify value) (28). The best fitting model (Fig. 4A—C; Eqn. 1, 30) included both additive and multiplicative effects (Supplemental Results).
Fig. 4.
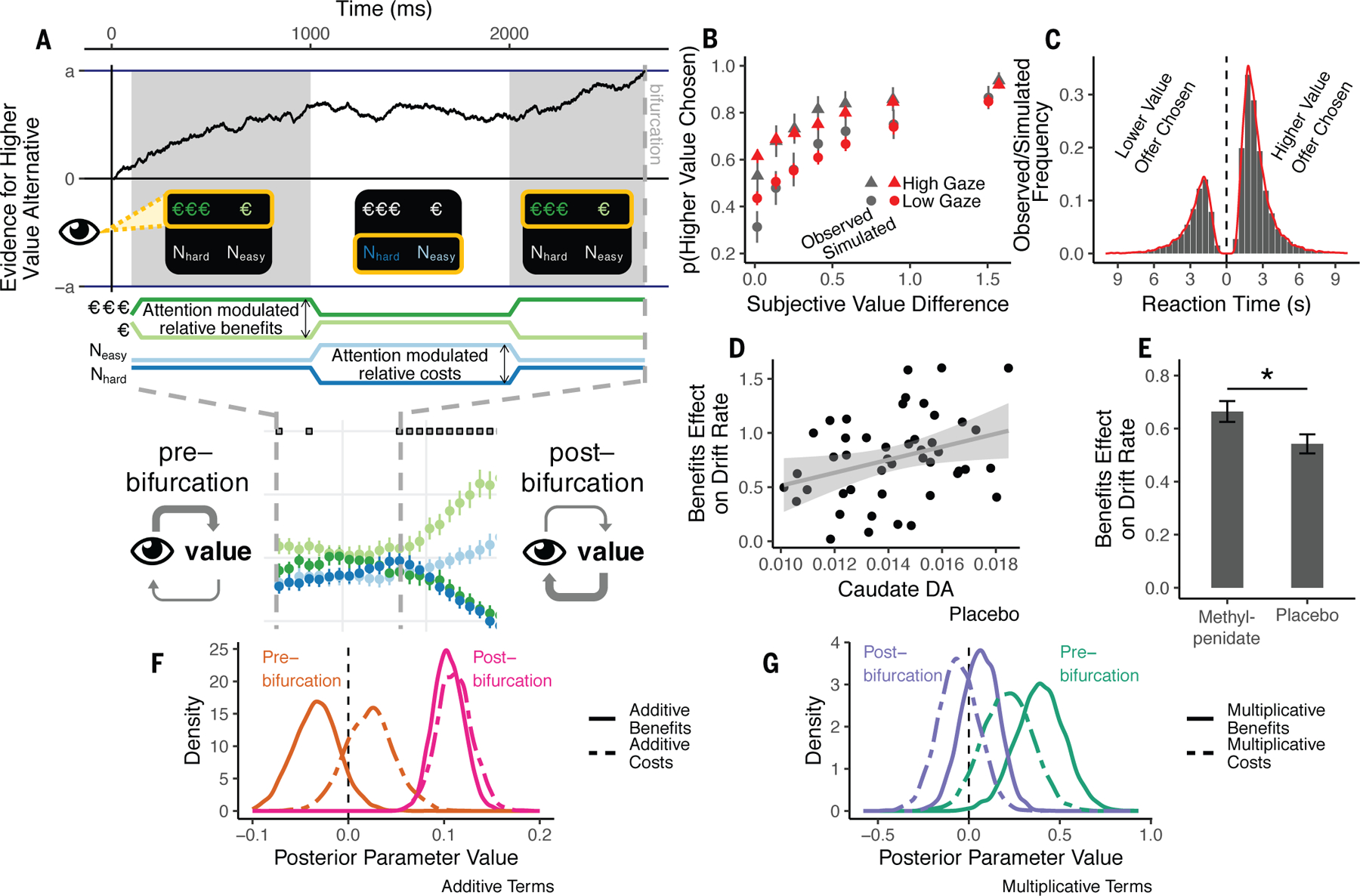
A. Gaze-attribute model: early gaze amplified the effect of attended-versus-unattended attributes on choice. Late gaze reflected the to-be-selected response. B. Model simulations (red) predicted choice (grey), and reaction time distributions (C). D. Benefits effect on drift rate correlate with dopamine synthesis capacity (95% CI shown). E. Methylphenidate enhances benefit effect. F—G. Posterior parameter densities from models fit alternately with pre- or post-bifurcation gaze on placebo. F. Additive benefit (; ) and cost (; ) gaze terms were approximately zero pre-bifurcation, and reliably positive post-bifurcation (; and ; ). G. Multiplicative interaction terms reveal that the effects of benefits (; ) and costs (at trend-level; = 0.12; ) were larger when fixating the respective attribute pre-bifurcation, while neither term was different from zero, post-bifurcation (= 0.07; P = 0.27 and ; ). All error bars are +/− SEM.
We next considered the possibility that the gaze-value interactions changed dynamically across the trial. Indeed, ~775 ms prior to responding participants began committing their gaze towards the to-be-chosen offer (Fig. 3D). Thus, while early gaze appeared to influence choice formation (Fig. 3C), later gaze appears reflect to latent choices, once formed. Based on this, we asked whether early attention causally amplifies attended attributes (a multiplicative combination), while late gaze simply correlates with choice (additive; Fig. 4A). To test our hypothesis, we split trials according to when participants began committing their gaze to the to-be-chosen offer (the “bifurcation”) for each participant and session and refit our model to gaze data from before or after this time point. The result supported our hypothesis. Multiplicative terms were reliably positive pre-bifurcation but near zero post-bifurcation, with the opposite pattern for additive terms (Fig. 4F—G). These results support that while early attention appeared to amplify the effect of benefits-versus-costs, later gaze simply reflected a latent choice.
Finally, we tested whether the effects of dopamine on choice could be attributed to these dynamic decision processes. Indeed, both higher dopamine synthesis capacity (on placebo; Eqn. 1: ; Pearson , ; Fig. 4D) and methylphenidate (, ; Fig. 4E) increased the effect of benefits on evidence accumulation. The corresponding effect of sulpiride on cost was not significant (Eqn. 1: ; ; ; Supplemental Discussion). We further found that methylphenidate amplified the effects of benefits on drift rate even when only modeling pre-bifurcation gaze (; ) – prior to the latent choice. Collectively, our results support that striatal dopamine enhances motivation for cognitive effort by amplifying the effects of benefits versus costs attended early in a decision.
| Eqn. 1 |
Supplementary Material
Acknowledgments:
We wish to thank the individuals who participated in this study, and James Wilmott for eye-tracking code and consultation.
Funding: NWO VICI Grant, 453-14-005 (2015/01379/VI) to R.C.; NIH Grant F32MH115600-01A1 to A.W., and NIH Grant R01MH080066 to M.J.F.
Footnotes
Competing interests: Authors declare no competing interests.
Data and materials availability: Data and analysis scripts will be publicly available at the conclusion of the parent study via http://hdl.handle.net/11633/aac2qvfx.
References and Notes:
- 1.Kool W, McGuire JT, Rosen ZB, Botvinick MM, Decision making and the avoidance of cognitive demand. Journal of Experimental Psychology-General. 139, 665–682 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westbrook A, Kester D, Braver TS, What is the subjective cost of cognitive effort? Load, trait, and aging effects revealed by economic preference. PLoS One. 8, e68210 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apps M, Grima LL, Manohar S, Husain M, The role of cognitive effort in subjective reward devaluation and risky decision-making. Scientific Reports (2015), doi: 10.1038/srep16880. [DOI] [PMC free article] [PubMed]
- 4.Salamone JD et al. , The pharmacology of effort-related choice behavior: Dopamine, depression, and individual differences. Behavioural Processes. 127, 3–17 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Collins AGE, Frank MJ, Opponent actor learning (OpAL): Modeling interactive effects of striatal dopamine on reinforcement learning and choice incentive. Psychological Review. 121, 337–366 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Frank MJ, O’Reilly RC, A mechanistic account of striatal dopamine function in human cognition: Psychopharmacological studies with cabergoline and haloperidol. Behavioral Neuroscience. 120, 497–517 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Aarts E, van Holstein M, Cools R, Striatal dopamine and the interface between motivation and cognition. Frontiers in Psychology. 2 (2011), doi: 10.3389/fpsyg.2011.00163/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westbrook A, Braver TS, Dopamine does double duty in motivating cognitive effort. Neuron. 89, 695–710 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volkow ND et al. , Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Molecular Psychiatry. 16, 1147–1154 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cocker PJ, Hosking JG, Benoit J, Winstanley CA, Sensitivity to cognitive effort mediates psychostimulant effects on a novel rodent cost/benefit decision-making task. Neuropsychopharmacology. 37, 1825–1837 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cools R, The cost of dopamine for dynamic cognitive control. Current Opinion in Behavioral Sciences, 1–8 (2015).
- 12.Aarts E et al. , Greater striatal responses to medication in Parkinson׳s disease are associated with better task-switching but worse reward performance. Neuropsychologia. 62, 390–397 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Manohar SG et al. , Reward pays the cost of noise reduction in motor and cognitive control. Current Biology. 25, 1707–1716 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timmer MHM, Aarts E, Esselink RAJ, Cools R, Enhanced motivation of cognitive control in Parkinson’s disease. European Journal of Neuroscience. 48, 2374–2384 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuigan S et al. , Dopamine restores cognitive motivation in Parkinson’s disease. Brain. 5, 16880 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Froböse MI et al. , Catecholaminergic modulation of the avoidance of cognitive control. Journal of Experimental Psychology-General. 147, 1763–1781 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Hosking JG, Floresco SB, Winstanley CA, Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: A comparison of two rodent cost/benefit decision-making tasks. Neuropsychopharmacology. 40, 1005–1015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spencer RC, Devilbiss DM, Berridge CW, The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex. Biological Psychiatry. 77, 940–950 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cools R, D’Esposito M, Inverted-u-shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry. 69, e113–e125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piray P, den Ouden HEM, van der Schaaf ME, Toni I, Cools R, Dopaminergic modulation of the functional ventrodorsal architecture of the human striatum. Cerebral Cortex. 2010, bhv243 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Schmidt L, Lebreton M, Cléry-Melin M-L, Daunizeau J, Pessiglione M, Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biology. 10, e1001266 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pauli WM, O’Reilly RC, Yarkoni T, Wager TD, Regional specialization within the human striatum for diverse psychological functions. Proceedings of the National Academy of Sciences. 113, 1907–1912 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volkow ND et al. , Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. American Journal of Psychiatry. 155, 1325–1331 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Volkow ND et al. , Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. Journal of Neuroscience. 21, 1–5 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jocham G, Klein TA, Ullsperger M, Dopamine-mediated reinforcement learning signals in the striatum and ventromedial prefrontal cortex underlie value-based choices. Journal of Neuroscience. 31, 1606–1613 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenegger C et al. , Role of dopamine D2 receptors in human reinforcement learning. Neuropsychopharmacology. 39, 2366–2375 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krajbich I, Armel C, Rangel A, Visual fixations and the computation and comparison of value in simple choice. Nature Neuroscience. 13, 1292–1298 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Cavanagh JF, Wiecki TV, Kochar A, Frank MJ, Eye tracking and pupillometry are indicators of dissociable latent decision processes. Journal of Experimental Psychology: General. 143, 1476–1488 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiecki TV, Sofer I, Frank MJ, HDDM: Hierarchical Bayesian estimation of the Drift-Diffusion Model in Python. Frontiers in Neuroinformatics. 7, 1–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Equation 1 - The rate at which participants accumulate evidence in favor of offer A versus B (ν) on trial (i) is given by proportion gaze at benefits () and its interaction with the benefits of A versus B (), proportion gaze at costs () and its interaction with costs (), as well as additive contributions of gaze at offer A for both benefits (), and costs ().
- 31.Swanson JM, Volkow ND, Serum and brain concentrations of methylphenidate: implications for use and abuse. Neuroscience and Biobehavioral Reviews. 27, 615–621 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Wiesel F-A, Alfredsson G, Ehrnebo M, Sedvall G, Prolactin response following intravenous and oral sulpiride in healthy human subjects in relation to sulpiride concentrations. Psychopharmacology. 76, 44–47 (1982). [DOI] [PubMed] [Google Scholar]
- 33.Patlak CS, Blasberg RG, Fenstermacher JD, Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Journal of Cerebral Blood Flow & Metabolism. 3, 1–7 (1983). [DOI] [PubMed] [Google Scholar]
- 34.Muhammed K, Dalmaijer E, Manohar S, Husain M, Voluntary modulation of saccadic peak velocity associated with individual differences in motivation. Cortex, 1–15 (2019). [DOI] [PMC free article] [PubMed]
- 35.Moghaddam B, Bunney BS, Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: An in vivo microdialysis study. Journal of Neurochemistry. 54, 1755–1760 (1990). [DOI] [PubMed] [Google Scholar]
- 36.Wu Q et al. , Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: An in vivo voltammetric study. The Journal of Neuroscience. 14, 6272–6281 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Schaaf ME et al. , Establishing the dopamine dependency of human striatal signals during reward and punishment reversal learning. Cerebral Cortex. 24, 633–642 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.