Abstract
Entry of SARS-CoV-2 is facilitated by endogenous and exogenous proteases. These proteases proteolytically activate the SARS-CoV-2 spike glycoprotein and are key modulators of virus tropism. We show that SARS-CoV-2 naïve serum exhibits significant inhibition of SARS-CoV-2 entry. We identify alpha-1-antitrypsin (AAT) as the major serum protease inhibitor that potently restrict protease-mediated entry of SARS-CoV-2. AAT inhibition of protease-mediated SARS-CoV-2 entry in vitro occurs at concentrations far below what is present in serum and bronchoalveolar tissues, suggesting that AAT effects are physiologically relevant. Moreover, AAT deficiency affects up to 20% of the population and its symptomatic manifestations coincides with many risk factors associated with severe COVID-19 disease. In addition to the effects that AAT may have on viral entry itself, we argue that the anti-inflammatory and coagulation regulatory activity of AAT have implications for coronavirus disease 2019 (COVID-19) pathogenicity, SARS-CoV-2 tissue restriction, convalescent plasma therapies, and even potentially AAT therapy.
Keywords: COVID-19, SARS-CoV-2, Alpha-1-antitrypsin, SERPINA1, alpha-2-macroglobulin, TMPRSS2, protease, convalescent plasma
The COVID-19 pandemic that began in December 2019 has resulted in tens of millions of cases with hundreds of thousands of deaths. The central conundrum of this pandemic is the heterogeneity of disease severity in SARS-CoV-2 infected individuals. The widespread disparities in outcomes has spurred global efforts to better understand SARS-CoV-2 pathogenesis, investigate the factors contributing to the clinical course of COVID-19, and develop viable therapeutics. One of the most promising therapeutic targets is the Spike (S) glycoprotein of SARS-CoV-2, which bears the fusion machinery necessary to mediate viral entry. Vaccines, monoclonal antibodies and convalescent plasma therapy are all premised upon neutralizing SARS-CoV-2 spike (CoV2-S) mediated entry. All three modalities are being developed at unprecedented speed. Unfortunately, the lack of standardized virus neutralization assays (VNAs) or reporting metrics have made it difficult to compare the efficacy across the proliferating number of vaccine platforms and CoV2-S targeted treatment modalities.
Standardizing a SARS-CoV-2 Viral Neutralization Assay
In an accompanying study, we worked to develop a scalable, standardized VNA that reflects the complex interplay between CoV2-S receptor interaction and proteolytic activation. To that end we generated and validated VSVΔG pseudotyped particles bearing SARS-CoV-2 spike (CoV2pp).1 We initially optimized infection conditions in serum free media. Due to the role of proteolytic activation in SARS-CoV-2 entry, we utilized exogenous trypsin as well as soybean trypsin inhibitor to maximize entry of the CoV2pp while limiting cytotoxicity. These conditions were designed noting that proteolytic activation of CoV2-S is required for the receptor-induced conformational changes that result in virus-cell membrane fusion and viral entry. Several endosomal, cell surface, and exogenous proteases, including furin, select cathepsins and TMPRSSs, and trypsin-like proteases have been implicated in mediating these cleavage events for SARS-CoV-2 and enhancing cellular entry (Fig. 1A).2–4 A similar body of evidence also indicates that these proteases and others, such as elastase, play critical roles in the productive processing of the S protein from SARS-CoV-1, MERS-CoV, and other betacoronaviruses.5–7 In the case of MERS-CoV, proteolytic processing of spike is capable of dictating cell tropism and correlates well with virulence.8 For SARS-CoV-2, the role of cell surface protease-mediated entry is of sufficient importance that TMPRSS2 overexpression can render some cell lines refractory to chloroquine mediated inhibition of virus entry.9
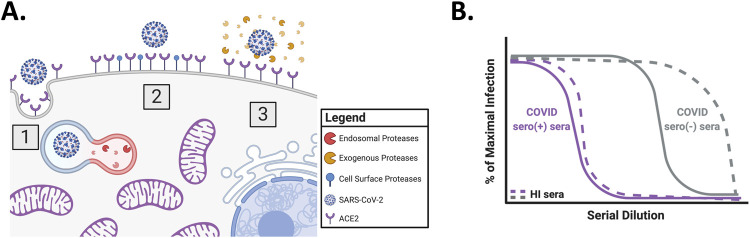
Figure 1. Overview of SARS-CoV-2 entry and inhibition of trypsin treated CoV2pp entry by COVID seronegative sera.
(A) Overview of SARS-CoV-2 entry. Three modes of entry are displayed: (1) Entry mediated by endosomal proteases, such as Cathepsin B, (2) entry mediated by cell surface proteases, such as TMPRSS2, and (3) entry mediated by exogenous proteases, such as trypsin. This model was created in Biorender. (B) Inhibition of trypsin treated CoV2pp entry by COVID seronegative sera. Presented is a schematized version of the results presented in supplemental Figure 3A of our previous publication. For this experiment, sera samples were incubated with trypsin treated CoV2pp for 30mins prior to addition to Vero-CCL81 cells. Both the CoV2pp and the sera samples were diluted in serum free media. The grey lines represent COVID seronegative sera, they purple lines are COVID seropositive sera, and the dashed lines are samples that were heat inactivated (HI) for 1hr at 56°C prior to use for CoV2pp neutralizations.
To standardize our VNA, we initially utilized trypsin-treated CoV2pp for human serum neutralization experiments. However, we observed that under these conditions, sera from patients not exposed to SARS-CoV-2 was capable of neutralizing these pseudoviruses, though, as expected, to a lesser extent than sera from patients that tested positive for SARS-CoV-2 antibodies (Fig. 1B)1.
Uncovering AAT: a heat-labile, CoV2pp neutralizing factor in SARS-CoV-2 naïve serum
To pre-empt the variable neutralizing effect of seronegative serum, we diluted our trypsin-treated CoV2pp in DMEM containing 10% fetal bovine serum (FBS). However, while this provided an easy solution in standardizing our assay for “out-of-the-box” use when sent to multiple labs, it left the question of “why” unanswered. This neutralizing effect of SARS-CoV-2 naïve human sera was ameliorated through heat-inactivation by incubation at 56°C for 1hr (Fig. 1B), and also appeared to be specific to CoV2-S mediated entry as VSV-G pseudotyped particles (VSV-Gpp) were unaffected. Thus, the serum neutralizing factor(s) was unlikely to be cross-reactive antibodies to seasonal coronaviruses or complement per se. We therefore suspected a heat-labile serum factor(s) capable of inhibiting trypsin.10
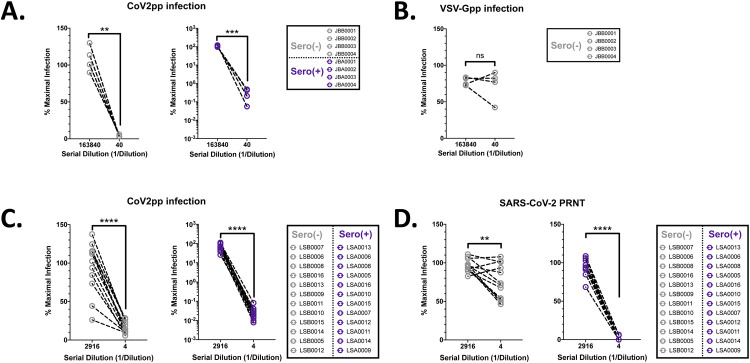
To validate these observations, we tested a panel of non-heat-inactivated human sera for neutralization activity against CoV2pp and VSV-Gpp. SARS-CoV-2 seropositive and seronegative sera were diluted in SFM (Supplemental Fig. 1A). Here, we observed neutralization of trypsin-treated CoV2pp by both seronegative and seropositive sera (Fig. 2A and Supplemental Fig. 1B). Importantly, VSV-Gpp was not inhibited by the samples tested (Fig. 2B and Supplemental Fig. 1C), once again showing that this effect was CoV2pp-specific. At the same time, an external group at Louisiana State University Health Shreveport (LSUHS) independently observed neutralization by seronegative sera under similar experimental conditions using our CoV2pp (Fig. 2C, Supplemental Fig. 1D and 1E). Remarkably, in both groups, SARS-CoV-2 naïve sera inhibited CoV2pp entry by 90–97% (Fig. 2A and 2C, left panels). Nonetheless, seropositive patient sera showed inhibition orders of magnitude beyond this threshold, suggesting antibody mediated inhibition of CoV2pp entry (Fig. 2A and C, right panels). Additionally, using the identical serum samples in Fig. 2C, collaborators at the University of Texas Medical Branch at Galveston (UTMB) observed modest, but significant, neutralization of live virus by seronegative sera as assayed by a plaque reduction neutralization assay (Fig. 2D and Supplemental Fig. 1F).
Figure 2. Negative patient sera inhibit exogenous protease mediated enhancement of CoV2pp.
(A) SARS-CoV-2 seronegative sera inhibit trypsin treated CoV2pp. Seronegative or seropositive samples were first identified based on IgG antibodies against Spike (Supplemental Fig. 1). The indicated sera diluted in serum-free DMEM were incubated with a pre-titered amount of CoV2pp prior to spinoculation on Vero-CCL81 cells as described.1 Sera were not heat inactivated before use in our neutralization assays. Normalized infection data at the highest and lowest dilutions tested are shown as % maximal infection (media only) with results from seronegative (left) and seropositive (right) sera plotted on linear and log scale, respectively. Data points represent the mean of neutralizations performed in triplicate. Dilutions shown were compared using a paired t test (ns, not significant; **, p < 0.01; ***, p < 0.005, and ****, p < 0.0001). Full neutralization curves are shown in Supplemental Fig. 1. (B) SARS-CoV-2 seronegative sera do not inhibit VSV-Gpp. Experiment performed and presented as in Fig. 2A. (C) Inhibition of trypsin treated CoV2pp entry by SARS-CoV-2 seronegative sera independently observed. Collaborators in a different state independently performed the identical experiment described in Fig. 1A with their own cohort of seropositive and seronegative samples. Data shown are means from technical quadruplicates/sample/dilution and presented exactly as for Fig. 2A. (D) Live SARS-CoV-2 is modestly inhibited by seronegative sera. Sera samples presented in Fig. 2C were utilized for plaque reduction neutralization experiments (PRNT) with live virus (left panel) as described in the materials and methods. Presented here are the mean of one experiment done in technical duplicates and error bars show SEM. Data presented as in Fig. 2A.
Upon making these repeated observations, we searched the literature for highly abundant and heat labile products in serum and identified alpha-1-antitrypsin (AAT) and alpha-2-macroglobulin (A2M) as potential candidates.10 These blood products are typically present in human serum at high concentrations (1.1–2.2 mg/mL for AAT and 2–4 mg/mL for A2M) and have been described to inhibit both exogenous and endogenous proteases.11–13 Despite the careful characterizations of the role endogenous and exogenous proteases play in SARS-CoV-2 entry, there have been limited characterizations of the role in vivo protease inhibitors play in modulating SARS-CoV-2 entry. A2M and AAT alone are responsible for approximately 10% and 90% of serum antiprotease capacity, respectively.14
A2M functions to inhibit a broad range of proteases, such as serine and cysteine proteases. In addition to protease inhibitory functions, A2M also inhibits thrombin to prevent coagulation and binds to growth factors and cytokines. No clinical conditions have yet been associated with low plasma levels of A2M.11 On the other hand, AAT is a protease inhibitor that irreversibly binds serine proteases and plays additional roles in the regulation of inflammation and coagulation.15 Notably, decreased plasma concentrations of or function of AAT have been associated with liver and lung disease, particularly pulmonary emphysema due to unregulated neutrophil elastase activity.12 Mutations leading to these conditions are highly prevalent as nearly 20% of individuals have non-wildtype AAT alleles.13
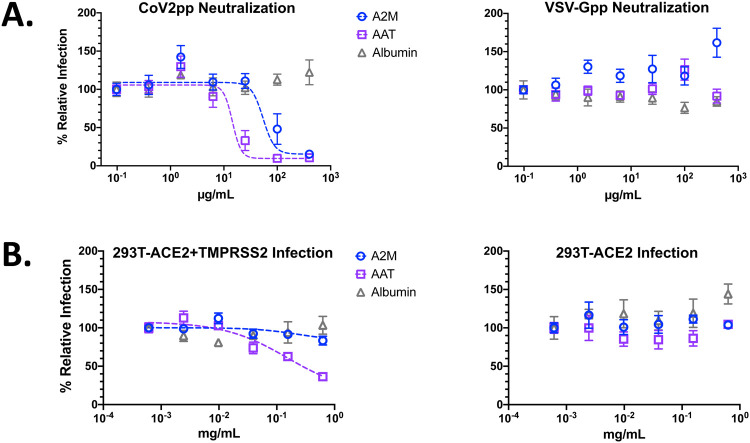
To assess whether AAT and/or A2M alone could inhibit trypsin-treated CoV2pp entry, we added each at the time of infection and observed potent entry inhibition by AAT and modest inhibition by A2M, with IC50s of 14.47μg/mL and 54.20μg/mL, respectively (Fig. 3A, left panel). Importantly, neither protein inhibited VSV-Gpp (Fig. 3A, right panel). Albumin, the most abundant protein in blood, showed no significant reduction of entry of either CoV2pp or VSV-Gpp (Fig. 3A), which underscores that the inhibitory effects of AAT and A2M on CoV2-S mediated entry was specific.
Figure 3. Alpha-1-antitrypsin (AAT) and alpha-2-macroglobulin (A2M) inhibit protease mediated enhancement of CoV2pp entry.
(A) AAT and A2M inhibit trypsin-mediated enhancement of CoV2pp entry. Trypsin treated CoV2pp (left panel) and standard VSV-Gpp (right) were diluted in serum free media, then used to infect Vero-CCL81 cells in the presence of the indicated concentrations of albumin, AAT, or A2M. Data are from two independent experiments and are presented as percent relative infection where each concentration was normalized to the lowest concentration of the test reagent used. Data fit as described in Fig. 1A. (B) AAT inhibits TMPRSS2-mediated enhancement of CoV2pp entry. CoV2pp not treated with trypsin were diluted in DMEM+10% FBS and utilized to infect 293T-ACE2+TMPRSS2 clone F8–2 (left panel) or 293T-ACE2 clone (5–7) in the presence of the indicated concentrations of A2M, AAT, or Albumin. Data points are means +/− SEM a representative experiment performed in triplicates, but otherwise presented as described as in Fig. 3A.
While these findings suggest that AAT, and to a lesser extent A2M, can inhibit exogenous trypsin-like proteases known to enhance SARS-CoV-2 entry, tissue restriction of SARS-CoV-2 infection is also mediated by proteases at the cell surface.2,3 Therefore, we sought to investigate whether either protein could inhibit TMPRSS2, an endogenous serine protease implicated in SARS-CoV-2 pathogenicity. We previously engineered two ultra-permissive 293T clones stably expressing ACE2 (clone 5–7) or ACE2+TMPRSS2 (clone F8–2). Each of these lines was capable of highly efficient CoV2pp entry in the absence of trypsin pre-treatment. To assess entry inhibition by AAT and A2M, we performed VNAs in both clones using CoV2pp without any trypsin pre-treatment but diluted in standard media (DMEM+10% FBS). Here, we observed that AAT inhibited CoV2pp entry into TMPRSS2 expressing F8–2 clones, but not the 5–7 clones (Fig. 3B). For both cell lines, A2M and albumin both displayed no entry inhibition at the concentrations tested. As additional controls, we observe potent inhibition of CoV2pp by sRBD in both cell lines and, as expected, Nafamostat mesylate, a serine protease inhibitor, inhibited entry in only the TMPRSS2-expressing F8–2 clones (Supplemental Fig. 2). Notably, AAT and Nafamostat inhibition of CoV2pp entry into TMPRSS2-expressing F8–2 cells approached a maximal inhibition of ~80%, suggesting that SARS-CoV-2 can enter via other pathways noted in Fig. 1A.
Together, these observations show that not only can SARS-CoV-2 naïve sera potently inhibit protease-mediated entry of SARS-CoV-2, but AAT and A2M appear to be potential regulators of protease-mediated entry by SARS-CoV-2. In concert with the large body of literature about AAT and the proteolytic processing of coronavirus spike proteins, we are led to three distinct, but important hypotheses. The first is that AAT and A2M may play a biologically relevant role in tissue restriction in SARS-CoV-2 infection. The second is that, if the first hypothesis is true, variant AAT genotypes could influence the relative severity of COVID-19. And thirdly, treatment with the already FDA-approved AAT, could play a role in simultaneously controlling viral burden as well as aberrant immune responses.
The suspected roles of protease inhibitors as uncharacterized players in COVID-19 pathogenesis and therapy.
In an accompanying paper, we reported that a factor, or factors, in SARS-CoV-2 seronegative sera is capable of inhibiting trypsin-mediated entry of CoV2pp. Here, we pinpoint AAT and A2M as highly abundant serum factors that inhibit the effect of exogenous proteases on CoV2pp entry. Moreover, at the concentrations tested, we show that AAT, but not A2M, can inhibit TMPRSS2-mediated entry of CoV2pp at subphysiologic concentrations. Two recent pre-peer review reports support these findings. Azouz et al utilized a fluorescent cleavage assay in a screen that identified four small molecules and AAT as inhibitors of TMPRSS2 enzymatic activity.16 Wettstein et al report neutralization of SARS-CoV-2 by polypeptides extracted from pooled bronchoalveolar lavage (BAL) fluids and, after fractionation and mass spectrometry, identify AAT as a component driving this activity.17 Although previously reported, the identification of AAT in the BAL confirms that it can diffuse into lung tissues and suggests that it can be present at the site of SAR-CoV-2 infection and replication. They also show that while the fraction containing AAT inhibits >99% of infection, there is another set of fractions that inhibit just under 90% of infection. We speculate that these fractions may contain A2M which we show to be a less potent inhibitor of trypsin-mediated CoV2pp entry, perhaps due to the requirement of a tetramer to trap two proteases (Fig. 3A).11 Interestingly, this lack of potency may be offset by its broader protease inhibition potential, particularly for cysteine proteases such as Cathepsin B and L, which have been reported to play a role in endosomal mediated SARS-CoV-2 entry. Additionally, elastase—a serine protease released by neutrophils—has been previously reported to play a role in enhancing SARS-CoV-1 and MERS entry.7 Although the role of elastase in SARS-CoV-2 entry has not been elucidated, elevation of neutrophil counts in BAL and serum have been consistently associated with severe COVID-19 cases.18–20 In spite of its name, AAT has a stronger binding affinity to elastase than trypsin, and this is borne out by the clinically significant sequalae associated with AAT deficiency.15
Though the findings presented here focus on protease inhibitors’ ability to inhibit protease-mediated SARS-CoV-2 entry, the inhibitors play significant additional roles. While others have speculated that neutrophil elastase should be considered as a target for potential COVID-19 prophylactics, AAT was not noted specifically.21 AAT, as an acute phase protein, has been characterized to play roles in modulating inflammation by inhibiting elastase among other factors. Elastase is critical for the formation of neutrophil extracellular traps (NETs) in acute pneumonia, which can amplify inflammatory responses if not resolved by AAT. Runaway pulmonary inflammation and NETosis is an emerging theme in COVID-19 pathogenesis.22 AAT is also known to modulate activities that result in downstream IL-6 inhibition, which is heavily implicated in COVID-19 pathogenicity.12,23 Seeking to capitalize on these anti-inflammatory roles and the already well established use of recombinant AAT to treat AAT deficiency, McElvaney et al recently reported the initiation of a clinical trial for AAT treatment of COVID-19 based on their published work.24 Moreover, AAT has regulatory roles in the coagulation cascade25 and, via elastase inhibition, could inhibit NET-triggered immunothromboses.26 Notably, inflammatory dysregulation and coagulopathies have been reported to play a role in the disparate COVID-19 severities between patients.27
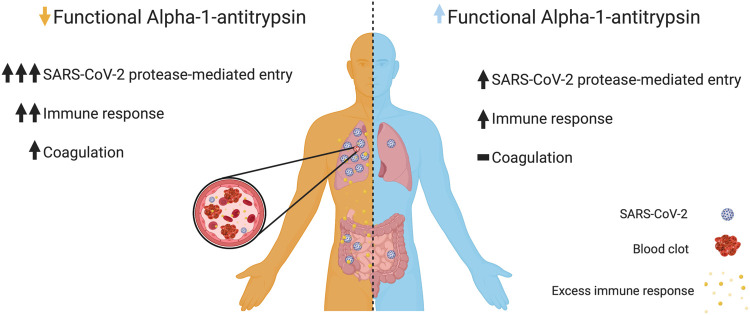
The potent neutralization of protease-mediated cellular entry by SARS-CoV-2 along with the wide range and prevalence of functionally different AAT genotypes implies a potential role for AAT in variable COVID-19 severity. Of particular note, nearly 20% of individuals are either heterozygous or homozygous for non-wildtype alleles, many of which have been described to result in reduced levels of AAT in the blood or reduced AAT function.13 Although many mutations have been characterized, new mutations that impair abundance or function are still being identified. For example, AAT variants such as G373R or the R223C (F allele) have been identified and reported to have wildtype levels in the blood, but impaired inhibitory activity against neutrophil elastase.28,29 We speculate that this CoV2pp VNA assay may be capable of serving as a scalable means by which one could screen for deficient AAT functionality and not simply abundance. Considering that widespread screening of AAT is rarely performed in the absence of emphysema, it is reasonable to expect that there may be more unidentified mutations that impair abundance or function of AAT, which may subsequently result in aberrant response to SARS-CoV-2 infection. This undesired response by individuals with functional AAT deficits may enable effective viral entry, dysregulated inflammation, and/or coagulopathies (Fig. 4). It also raises the possibility that AAT may represent a novel therapeutic approach in the fight against SARS-CoV-2.25
Figure 4. Putative relationship between alpha-1-antitrypsin function and SARS-CoV-2.
Differential alpha-1-antitrypsin (AAT) abundance and/or function may result in a differential response to infection by SARS-CoV-2. Due to our observations that AAT can inhibit CoV2pp protease-mediated entry, we expect that in the presence of functional AAT (light blue, right) there is only modest amounts of SARS-CoV-2 protease-mediated entry relative to those with AAT functional deficiencies (orange, left). In addition to its entry effects, we speculate that normal AAT abundance and/or function may reign in an otherwise dysregulated immune response. Moreover, AAT’s role in regulation of the coagulation cascade may further prevent the development of coagulopathies. For the latter, a normal immune response to infection is indicated by a single up arrow and the absence of coagulopathy is indicated by a dash. Figure generated in Biorender.
However, in the case of convalescent plasma therapies, the presence of AAT in blood plasma from donors may play a beneficial role as non-immunoglobulin products are currently not excluded from transfused plasma. In line with this, a recent meta-analysis from Joyner et al suggests that convalescent plasma therapy is beneficial to its recipients.30 While the purported benefits are attributed to the presence of neutralizing antibodies, the authors also acknowledge that “other biological mechanisms” may contribute to these observations. Additionally, a recent bioRxiv report shows modest benefits from the transfer of standard IVIG, suggesting that neutralizing antibodies are not the only serum components that may play a role in alleviating the burden of COVID-19 in patients.31 In its myriad roles, the transfer of AAT may provide additional benefits to convalescent plasma recipients by inhibiting SARS-CoV-2 entry, restraining inflammation and/or moderating coagulation.
In sum, these findings highlight the importance of protease inhibitors in restricting exogenous and/or endogenous protease-mediated enhancement of SARS-CoV-2 entry. We also speculate that the diversity of AAT genotypes, the complex regulation of its activity, and its myriad roles in inflammation and coagulation, implicates functional AAT levels in COVID-19 pathogenicity. There is an urgent need to address:
The biologically relevant roles AAT, A2M, or other proteases play in tissue restriction in SARS-CoV-2 infection.
The impact of variant AAT genotypes on the relative severity of COVID-19.
Whether treatment with, the already FDA-approved, AAT, may be able to play a role in simultaneously controlling viral burden as well as aberrant immune responses and if simultaneous treatment with A2M should be considered given their ability to form inhibitor complexes.32
METHODS
Maintenance and generation of isogenic cell lines:
Vero-CCL81, parental 293T, and isogenic 293T cells were cultured in DMEM with 10% heat inactivated FBS at 37°C in the presence of 5% CO2. Isogenic 293T cell clones 5–7 and F8–2 were generated by lentivirus transduction to stably express ACE2 only or ACE2 and TMPRSS2, respectively. ACE2 expression was under puromycin selection and TMPRSS2 was under blasticidin selection as previously described.
Production of VSVΔG pseudotyped particles and neutralization studies:
Detailed protocols for the production and use of standardized VSVpp (CoV2pp and VSV-Gpp) are given in Oguntuyo and Stevens et al.1 Briefly, 293T producer cells were transfected to express the viral surface glycoprotein of interest, infected with VSVΔG-rLuc-G* reporter virus, then virus supernatant collected and clarified 2 days post infection prior to use. Trypsin treated CoV2pp were treated as previously described.1 All pseudotyped viruses (PsV) were aliquoted prior to storage at −80°C and tittered prior to usage for neutralization experiments. Neutralization experiments were performed by diluting the appropriate PsV with a 4-fold serial dilution of Albumin (Sigma-Aldrich, A8763), alpha-1-antitrypsin (Sigma-Aldrich, SRP6312), alpha-2-Macroglobulin (Sigma-Aldrich, SRP6314) or Nafamostat mesylate (Selleckchem, S1386). SARS-CoV-2 soluble RBD (sRBD) with human IgG-Fc was produced by the Lee Lab using a recombinant Sendai virus expression platform (manuscript in preparation). De-identified patient sera were obtained via institutional biobanks that allowed use for research purposes. All infections were processed for detection of Renilla luciferase activity at 20hrs post-infection, and luminescence was read on the Cytation3 (BioTek).
Plaque reduction neutralization titration (PRNT) by sera of SARS-CoV-2:
Neutralization experiments with live virus were performed by incubating sera with 50–100 PFU of SARS-CoV-2 for one hour at 37°C. All sera were diluted in serum free DMEM. Serial dilutions started at a four-fold dilution and went through seven three-fold serial dilutions. The virus-serum mixture was then used to inoculate Vero E6 cells for one hour at 37°C and 5% CO2. Cells were overlaid with EMEM medium (no FBS) and 1.25% Avicel, incubated for 3 days, and plaques were counted after staining with 1% crystal violet in formalin.
Supplementary Material
Supplemental Figure 1. Spike ELISA data and full neutralization curves. (A) Spike ectodomain ELISAs for JBA and JBB samples. Our seronegative and seropositive samples were utilized. ELISAs performed as previously described(ref) and shown are the OD490 values from the 1:100 sera dilution. Shown are the median and interquartile range. (B) Full neutralization curves from trypsin treated CoV2pp. Data points are mean +/− SEM from experiment done in triplicates. (C) Full neutralization curves from VSV-Gpp. Presented are the means of an experiment in technical triplicate with error bars showing SEM. (D) Spike ectodomain ELISAs from LSA and LSB samples. Twelve seropositive and twelve seronegative samples were utilized. Shown are the OD450 values from the 1:100 sera dilution. (E) Full neutralization curves from LSU CoV2pp neutralization are shown here. (F) Live virus full neutralization curves. Live virus neutralizations performed as described in the Methods and the same samples as in Supplemental Fig. 1E were used. Presented here are the means of one experiment done in technical duplicate and error bars show SEM and data were fit using variable slope, 4-parameter logistics regression curve (robust fitting method).
Supplemental Figure 2. Nafamostat mesylate inhibits CoV2pp entry into TMPRSS2 expressing cells. CoV2pp were mixed with a serial dilution of either Nafamostat or sRBD prior to infection of isogenic cells stably expressing ACE2+TMPRSS2 (clone F8, left panel) or ACE2 (clone 5–7, right panel). Presented here are the results of an experiment done in technical triplicates. Error bars show SEM and data were fit using variable slope, 4-parameter logistics regression curve (robust fitting method).
ACKNOWLEDGMENTS
The authors acknowledge the following funding: KYO and CS were supported by Viral-Host Pathogenesis Training Grant T32 AI07647; KYO was additionally supported by F31 AI154739. BL acknowledges flexible funding support from NIH grants R01 AI123449, R21 AI1498033, and the Department of Microbiology and the Ward-Coleman estate for endowing the Ward-Coleman Chairs at the ISMMS. JPK and SSI acknowledge funding from a LSUHS COVID-19 intramural grant. JPK and SSI acknowledge additional funding from NIH grants AI116851 and AI143839, respectively. BL wishes to dedicate this paper to Ernest L Robles-Levroney, the first graduate student BL had the privilege to train. Ernie Robles-Levroney was dedicated teacher, role model and trailblazer who passed away unexpectedly during the course of writing this manuscript.
REFERENCES
- 1.Oguntuyo K. Y. and Stevens C. et al. Quantifying absolute neutralization titers against SARS-CoV-2 by a standardized virus neutralization assay allows for cross-cohort comparisons of COVID-19 sera. medRxiv (2020). [DOI] [PMC free article] [PubMed]
- 2.Hoffmann M. et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181, 271–280.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu F. C. et al. Proteolytic activation of the SARS-coronavirus spike protein: Cutting enzymes at the cutting edge of antiviral research. J. Virol. 395, 379–397 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang J. et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. 117, 11727–11734 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shulla A. et al. A Transmembrane Serine Protease Is Linked to the Severe Acute Respiratory Syndrome Coronavirus Receptor and Activates Virus Entry. J. Virol. 85, 873–882 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menachery V. D. et al. Trypsin Treatment Unlocks Barrier for Zoonotic Bat Coronavirus Infection. J. Virol. 94, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belouzard S., Madu I. & Whittaker G. R. Elastase-mediated Activation of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein at Discrete Sites within the S2 Domain. J. Biol. Chem. 285, 22758–22763 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J. E. et al. Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism. Proc. Natl. Acad. Sci. 113, 12262–12267 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann M. et al. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature (2020). doi: 10.1038/s41586-020-2575-3 [DOI] [PubMed] [Google Scholar]
- 10.Sriram Vemuri C. Yu Tony, and N. R. Formulation and Stability of Recombinant Alpha-1-Antitrypsin in Stability and Characterization of Protein and Peptide Drugs (eds. Wang Y. J. & Pearlman R.) 5, 263–285 (Springer US, 1993). [DOI] [PubMed] [Google Scholar]
- 11.Rehman A. A., Ahsan H. & Khan F. H. Alpha-2-macroglobulin: A physiological guardian. J. Cell. Physiol. 228, 1665–1675 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Blanco I. Alpha-1 Antitrypsin Biology. Blanco’s Overview of Alpha-1 Antitrypsin Deficiency 1, (Elsevier Inc., 2017). [Google Scholar]
- 13.Bornhorst J. A., Greene D. N., Ashwood E. R. & Grenache D. G. α1-Antitrypsin phenotypes and associated serum protein concentrations in a large clinical population. Chest 143, 1000–1008 (2013). [DOI] [PubMed] [Google Scholar]
- 14.de Serres F. & Blanco I. Role of alpha-1 antitrypsin in human health and disease. J. Intern. Med. 276, 311–335 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Strnad P., McElvaney N. G. & Lomas D. A. Alpha 1 -Antitrypsin Deficiency. N. Engl. J. Med. 382, 1443–1455 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Azouz N. P., Klingler A. M. & Rothenberg M. E. Alpha 1 Antitrypsin is an Inhibitor of the SARS-CoV2–Priming Protease TMPRSS2. bioRxiv (2020). doi: 10.1101/2020.05.04.077826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wettstein L. et al. Alpha-1 antitrypsin inhibits SARS-CoV-2 infection. bioRxiv (2020). doi: 10.1101/2020.07.02.183764 [DOI] [Google Scholar]
- 18.Zhou Z. et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe 27, 883–890.e2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas C. et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature (2020). doi: 10.1038/s41586-020-2588-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao M. et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 26, 842–844 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Mohamed M. M. A., El-Shimy I. A. & Hadi M. A. Neutrophil Elastase Inhibitors: A potential prophylactic treatment option for SARS-CoV-2-induced respiratory complications? Crit. Care 24, 311 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes B. J. et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 217, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grifoni E. et al. Interleukin-6 as prognosticator in patients with COVID-19. J. Infect. (2020). doi: 10.1016/j.jinf.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McElvaney O. J. et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. (2020). doi: 10.1164/rccm.202005-1583OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janciauskiene S. & Welte T. Well-Known and Less Well-Known Functions of Alpha-1 Antitrypsin. Its Role in Chronic Obstructive Pulmonary Disease and Other Disease Developments. Ann. Am. Thorac. Soc. 13, S280–S288 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Middleton E. A. et al. Neutrophil Extracellular Traps (NETs) Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood (2020). doi: 10.1182/blood.2020007008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jose R. J. & Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir. Med. 8, e46–e47 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cook L., Burdon J. G. W., Brenton S., Knight K. R. & Janus E. D. Kinetic characterisation of alpha-1-antitrypsin F as an inhibitor of human neutrophil elastase. Pathology 28, 242–247 (1996). [DOI] [PubMed] [Google Scholar]
- 29.Laffranchi M. et al. Characterisation of a type II functionally-deficient variant of alpha-1-antitrypsin discovered in the general population. PLoS One 14, e0206955 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joyner M. J. et al. Early Safety Indicators of COVID-19 Convalescent Plasma in 5,000 Patients. medRxiv (2020). doi: 10.1101/2020.05.12.20099879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakoulas G. et al. Intravenous Immunoglobulin (IVIG) Significantly Reduces Respiratory Morbidity in COVID-19 Pneumonia: A Prospective Randomized Trial. medRxiv (2020). doi: 10.1101/2020.07.20.20157891 [DOI] [Google Scholar]
- 32.Dejgaard S., Ortapamuk O. & Özer I. The Trypsin-Inhibitory Efficiency of Human α 2 -Macroglobulin in the Presence of α 1 -Proteinase Inhibitor: Evidence for the Formation of an α 2 -Macroglobulin-α 1 -Proteinase Inhibitor Complex. J. Enzyme Inhib. 14, 391–405 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Spike ELISA data and full neutralization curves. (A) Spike ectodomain ELISAs for JBA and JBB samples. Our seronegative and seropositive samples were utilized. ELISAs performed as previously described(ref) and shown are the OD490 values from the 1:100 sera dilution. Shown are the median and interquartile range. (B) Full neutralization curves from trypsin treated CoV2pp. Data points are mean +/− SEM from experiment done in triplicates. (C) Full neutralization curves from VSV-Gpp. Presented are the means of an experiment in technical triplicate with error bars showing SEM. (D) Spike ectodomain ELISAs from LSA and LSB samples. Twelve seropositive and twelve seronegative samples were utilized. Shown are the OD450 values from the 1:100 sera dilution. (E) Full neutralization curves from LSU CoV2pp neutralization are shown here. (F) Live virus full neutralization curves. Live virus neutralizations performed as described in the Methods and the same samples as in Supplemental Fig. 1E were used. Presented here are the means of one experiment done in technical duplicate and error bars show SEM and data were fit using variable slope, 4-parameter logistics regression curve (robust fitting method).
Supplemental Figure 2. Nafamostat mesylate inhibits CoV2pp entry into TMPRSS2 expressing cells. CoV2pp were mixed with a serial dilution of either Nafamostat or sRBD prior to infection of isogenic cells stably expressing ACE2+TMPRSS2 (clone F8, left panel) or ACE2 (clone 5–7, right panel). Presented here are the results of an experiment done in technical triplicates. Error bars show SEM and data were fit using variable slope, 4-parameter logistics regression curve (robust fitting method).