Fig. 5 |. Prophylactic and therapeutic application of mAb CV07–209 in a COVID-19 hamster model.
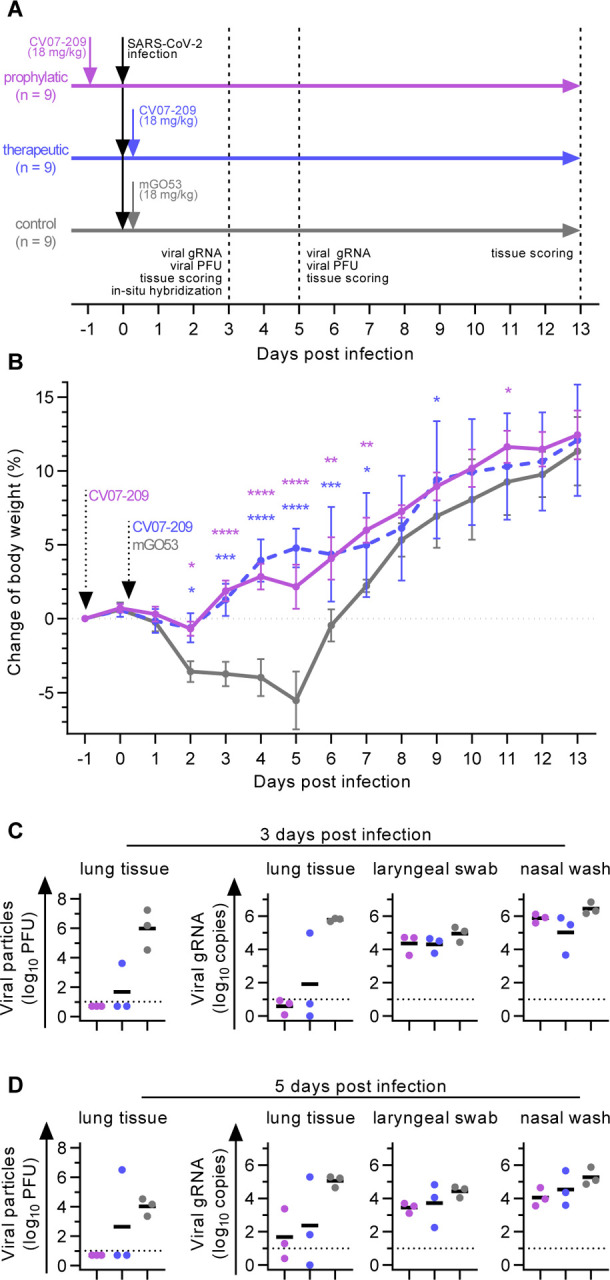
(A) Schematic overview of the animal experiment. (B) Body weight of hamsters after virus challenge and prophylactic (pink) or therapeutic (blue) application of SARS-CoV-2 neutralizing mAb CV07–209 or control antibody (mean±SEM from n=9 animals per group from day −1 to 3, n=6 from days 4 to 5; n=3 from days 6 to 13; mixed-effects model with posthoc Dunnett’s multiple tests in comparison to control group; significance levels shown as * (p<0.05), ** (p<0.01), *** (p<0.001), **** (p<0.0001), or not shown when not significant). (C-D) Quantification of plaque forming units (PFU) from lung homogenates and quantification of SARS-CoV-2 RNA copies per 105 cellular transcripts from samples and timepoints as indicated. PFU were set to 5 when not detected, RNA copies below 1 were set to 1. Bars indicate mean. Dotted lines represent detection threshold.
