Abstract
A vaccine, when available, will likely become our best tool to control the current COVID-19 pandemic. Even in the most optimistic scenarios, vaccine shortages will likely occur. Using an age-stratified mathematical model, we determined optimal vaccine allocation for four different metrics (deaths, symptomatic infections, and maximum non-ICU and ICU hospitalizations) under a wide variety of assumptions. We find that a vaccine with effectiveness ≥50% would be enough to substantially mitigate the ongoing pandemic provided that a high percentage of the population is optimally vaccinated. When minimizing deaths, we find that for low vaccine effectiveness, it is optimal to allocate vaccine to high-risk (older) age-groups first. In contrast, for higher vaccine effectiveness, there is a switch to allocate vaccine to high-transmission (younger) age-groups first for high vaccination coverage. While there are other societal and ethical considerations, this work can provide an evidence-based rationale for vaccine prioritization.
Introduction
As of 13 August 2020, over 750 thousand people have died due to the ongoing SARS-CoV-2 pandemic (1). Different countries have enacted different containment and mitigation strategies, but the world awaits impatiently for the arrival of a vaccine as the ultimate tool to fight this disease and to allow us to resume our normal activities. There are over 100 vaccines under development (2,3), with some currently undergoing phase 3 clinical trials (3). However, there are many unknowns surrounding a potential vaccine, including how efficacious it would be, how long it would be protective, how effective it would be in older individuals, how many doses would be immediately available and how long scaling up the vaccine production would take. Furthermore, should early vaccines have low effectiveness, what are the potential trade-offs between using a low-effectiveness vaccine and waiting for a vaccine with a more desirable vaccine effectiveness? With the hope of producing a vaccine in the near future comes the difficult task of deciding who to vaccinate first as vaccine shortages are inevitable (4–6). Here we utilized a mathematical model paired with optimization algorithms to determine the optimal use of vaccine for 100 combinations of vaccine effectiveness (VE) and number of doses available under a wide variety of scenarios.
Results
Briefly, we developed a deterministic age-structured mathematical model of SARS-CoV-2 transmission with a population stratified into 16 age-groups (Fig. S1, SM). Because, historically, vaccine is distributed to each state in the United States proportional to its population, and the allocation strategy is then determined at the state level (7), we chose a state level model with a population similar to Washington State in size and demographics; however, our results are generalizable to other populations. We assumed that children were less susceptible to infection than middle-aged adults (20 to 65 years old), while older adults (older than 65) were relatively more susceptible (8). We assumed that both natural and vaccine-induced immunity last at least one year (our time horizon). At the beginning of our simulations, 20% of the population have already been infected and are immune (additional results for 10, 30 and 40% of the population can be found in the SM) and all social distancing interventions have been lifted. Here, we consider that front-line health care workers, who should obviously be prioritized, have already been vaccinated.
For the vaccine optimization, we collated the 16 age-groups into five vaccination groups: children (aged 0–19), adults between 20 and 49 years old, adults between 50 and 64 years old, adults between 65 and 74 years old, and those 75 and older. This stratification reflects our current knowledge of disease severity and mortality based on age (9, 10). We developed an optimization routine that combined a coarse global search algorithm with a fast optimizer to explore the entire space of possible combinations of vaccine allocation. We compared the optimal allocation strategy given by the optimizer to a pro-rata allocation, where the vaccination coverage to each vaccination group is distributed proportionally to population size in each group. We considered VE ranging from 10% to 100% and vaccination coverage ranging from 10% to 100% of the total population. We evaluated four objective functions reflecting different metrics of disease burden that could be considered by decision makers: minimization of the total number of symptomatic infections, total number of deaths, number of cases requiring hospitalization (non-ICU) at the epidemic peak, and number of cases requiring ICU hospitalization at the epidemic peak. The last two objective functions were chosen because hospital bed (non-ICU and ICU) occupancy is a key metric currently used to determine county/state/country readiness to move between different interventions strategies. Here, we utilized the total number of licensed ICU beds in WA state and its current goal of staying below 10% of hospital beds occupied by COVID-19 cases (11,12) as references when interpreting our results.
Epidemic mitigation and containment:
Our model suggests that herd immunity will be achieved once 60% of the population is infected (equivalently 40% vaccinated with a perfect vaccine assuming 20% of the population has already immunity) (Fig. 1J, Fig. S2 and Fig. 2A).
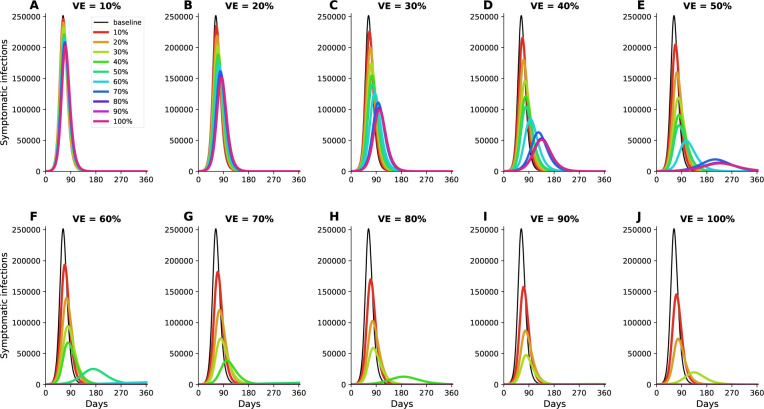
Figure 1:
Simulated prevalence of symptomatic COVID-19 infections for VE ranging from 10% (A) to 100% (J) in 10% increments under optimal distribution of vaccine. Colors represent different vaccination coverage, ranging from 0 (black, “baseline”) to 100% (magenta). For clarity, we present here epidemic curves for the main set of parameters only and show a complete figure with uncertainty bounds in Fig. S2.
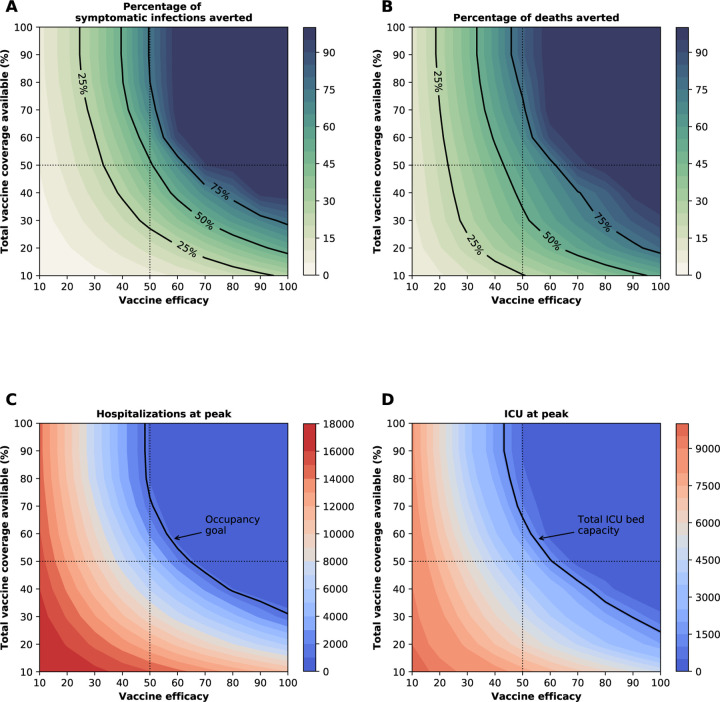
Figure 2:
Four key metrics of COVID-19 burden under optimal distribution of vaccine. Proportion of symptomatic infections (A) and deaths (B) averted, number of maximum non-ICU (C) and ICU (D) hospitalizations as a function of VE and vaccination coverage (total vaccine available as a percentage of the population). The dotted lines correspond to VE = 50% and vaccine available to cover 50% of the population. The isocline in (C) indicate the current goal for WA state of having 10% of licensed general (non-ICU) hospital beds occupied by COVID-19 patients and the isocline in (D) indicates the total ICU licensed hospital beds in WA state respectively.
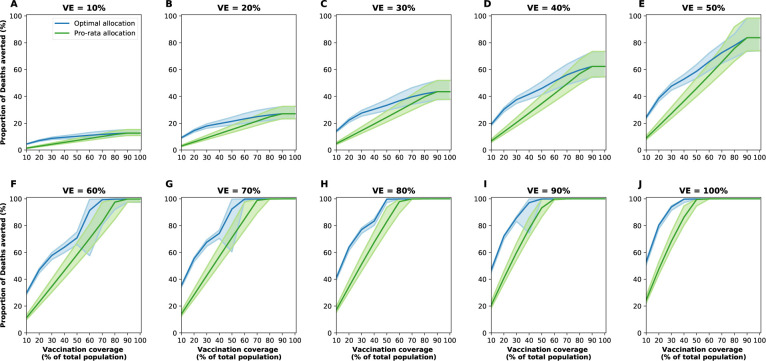
The epidemic can be substantially slowed with any vaccine with a VE ≥ 50% as long as a majority of the population is vaccinated (Fig. 1E, Fig. 2A), and over 50% of deaths could be averted with as little as 35% of the population vaccinated (Fig. 2A, B). If VE = 60%, the epidemic is completely contained if we optimally vaccinate 70% of the population, but we would only need to vaccinate 50% of the population if VE = 70% (Fig. 2A and Fig. 1F, I). Only vaccines with VE ≥ 50% can maintain the number of non-ICU hospitalizations below the established goal (≤ 10% hospital-bed occupancy by COVID-19 patients) and can prevent an overflow of the ICUs. With VE = 60%, 54% of the population would have to be optimally vaccinated to satisfy both conditions (Figs. 2C, D, S3F and S4F). In contrast, with the same VE, over 67% of the population would have to be vaccinated with the pro-rata allocation in order to maintain hospitalizations (both non-ICU and ICU) below the desired goals (Figs. S6, S7, S8, S9). Utilizing the optimal allocation strategy matters most when less vaccine is available, with a maximum difference of 32% deaths averted (for VE = 100% and with enough vaccine to cover 20% of the population) and 32% symptomatic infections averted (for VE = 60% and vaccination coverage of 60%) when compared with a pro-rata allocation strategy (Fig. 3, S5, S10). As VE increases, both strategies tend to perform similarly as vaccination coverage increases (Fig. 3, S5 S10).
Figure 3:
Proportion of deaths averted for the optimal allocation strategy (blue) and the pro-rata strategy (green) for VE ranging from 10% (A) to 100% (J) in 10% increments and vaccination coverage ranging from 10% to 100% of the total population. The shaded areas represent results of 1,000 simulations with the top and bottom 2.5% simulations removed.
Optimal vaccine allocation changes with VE and vaccination coverage:
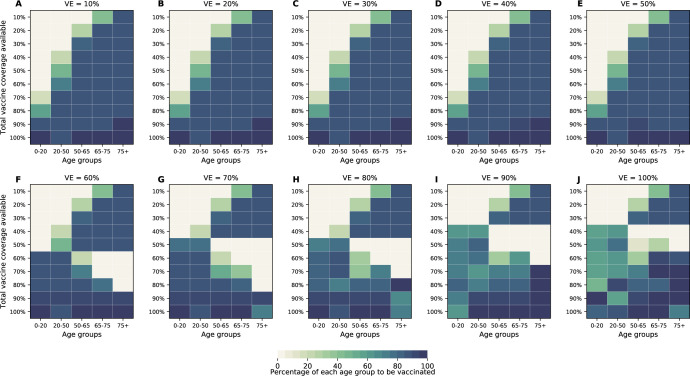
The optimal allocation strategy to minimize total deaths is identical for vaccine efficacies between 10% and 50%: with low vaccination coverage, it is optimal to allocate vaccine first to the highest risk group (people over 75 years old) and then to the younger vaccination groups as more vaccine becomes available (Fig. 4A–E). However, there is a threshold phenomenon observed for VE ≥ 60%: for low coverage, the optimal allocation is still to vaccinate the high-risk groups first, but when there is enough vaccine to cover roughly half of the population (60% for VE = 60%, 50% for VE = 70% and 40% for VE ≥ 80%), there is a switch to allocate vaccine to the high-transmission groups (those aged 20–50 and children) first. This is because directly vaccinating those who are driving the epidemic results in a much slower epidemic curve and hence in fewer deaths (Fig. 1F–H). As more vaccine becomes available the optimizer allocates it to high-risk groups again (Fig. 4F–J).
Figure 4:
Optimal allocation strategies for minimizing deaths for VE ranging from 10% (A) to 100% (J) in 10% increments (additional figures for minimizing symptomatic infections, number of non-ICU hospitalizations at peak and number of ICU hospitalizations at peak are given in SM). For each plot, each row represents the total vaccination coverage available (percentage of the total population to be vaccinated) and each column represents a different vaccination group. Colors represent the percentage of the population in a given vaccination group to be vaccinated.
Optimal vaccine allocation differs for different objective functions:
Next, we investigated how the optimal allocation strategy changed for different objective functions and present results for VE = 60%. The optimal vaccine allocation for the four objectives were most different when fewer vaccines are available (enough vaccine to cover less than 30% of the total population). When minimizing symptomatic infections and peak non-ICU hospitalizations, priority was given to the younger vaccination groups, as they have the most contacts in our model and hence drive transmission (Fig. S11A,B). As we move toward more severe outcomes (ICU hospitalizations at peak and deaths), for which older individuals are most at risk, the optimal allocation strategy shifts toward those vaccination groups (Fig. S11C,D). Once more vaccine becomes available, the optimal allocation strategies are very similar for all objective functions. In fact, they are nearly identical for all the objective functions when there is enough vaccine to cover 60% and 70% of the population. For high coverage, the optimal allocation strategies for all objective functions shifted towards the high-transmission groups. To note, we did not impose the optimizer to use all the available vaccine. As a result, the optimizer found allocation strategies utilizing less than the total vaccine available while performing equally well. This was very prominent when VE and vaccination coverage were very high. For example, when minimizing peak ICU hospitalizations and VE = 90%, the optimizer utilized vaccine to cover 75% of the population even though there was vaccine available to cover the entire population. This is expected, complete containment is attained once a high proportion of the population is vaccinated and any vaccine used above that threshold will result in the same mathematical outcome.
Optimal vaccine allocation as a function of pre-existing immunity:
As the COVID-19 pandemic dynamics have been dramatically different in different parts of the world, we expect to see a range of population-level naturally-acquired immunity when vaccination campaigns start. Hence, we investigated the optimal use of vaccine with 10%, 30% and 40% of the population is already immune at the beginning of the simulations. For all of these, the same pattern is observed when minimizing deaths: for low coverage, it is optimal to allocate all of the vaccine to the high-risk groups, for higher coverage, the optimal vaccination strategy switches to allocate more vaccine to the high-transmission groups. This threshold however varies with the degrees of pre-existing immunity in the population. When only 10% of the population is immune, the switch occurs when 80% of the population is vaccinated, but this threshold is observed when 40% of the population is vaccinated if 40% of the population has infection-acquired immunity prior to vaccination (Fig. S12). In addition, under low pre-existing immunity, the optimal strategy favors more the older vaccination groups (Fig. S12A), while under higher pre-existing immunity, the optimal allocation strategy tends to distribute vaccine more evenly across vaccination groups (Fig. S12D).
Robustness of optimal allocation strategies around major parameters:
We explored the robustness of the optimal allocation strategies around key parameters of the transmission and natural history of SARS-CoV-2. Because susceptibility to SARS-CoV-2 infection remains unclear, we compared the optimal allocation strategy under the assumption of differential susceptibility, as suggested in (8, 13) (presented throughout the text), to one assuming equal susceptibility across age-groups (Figs. S13, S14, S15 and S16), as suggested in (14, 15). The optimal allocation strategies under both equal and differential susceptibility were remarkably consistent for minimizing deaths, with more vaccine allocated to children as VE increases (VE≥60) and more vaccine becomes available (coverage to vaccinate 70% or higher) (Figs. 4 and S13). When minimizing symptomatic infections, optimal allocation strategies under equal susceptibility tended to allocate more vaccine to children than did the strategies under differential susceptibility (Figs. S17 and S14). The major differences were observed when minimizing peak hospitalizations (both non-ICU and ICU). Assuming equal susceptibility resulted in optimal allocation strategies that favored the high-transmission groups (children and adults aged 20 to 49 years old) over the high-risk groups (Figs. S18, S15, S19 and S16). In addition, we selected four parameters for which there is the most uncertainty and re-ran the optimization routine for several combinations (full details in SM). The optimal allocation strategies were very robust under this analysis (Supplemental Files. SF1–SF4). Finally, we compared the optimal allocation strategies when the simulations were started with a higher number of infected individuals (10,000 current infections). This would reflect a situation where the epidemic is in full exponential growth when vaccination becomes available. The optimal allocation strategy was surprisingly robust under this scenario, with nearly identical allocation strategies for all objective functions (Figs. S20, S21, S22, S23).
Discussion
The COVID-19 pandemic has devastated families and societies around the world. A vaccine, when available, would most likely become our best tool to control the spread of SARS-CoV-2. However, in the short term, even in the most optimistic scenarios, vaccine production would likely be insufficient. In this work, we paired a mathematical model of SARS-CoV-2 transmission with optimization algorithms to determine optimal vaccine allocation strategies. Given the current uncertainties surrounding such a vaccine (we do not know yet if and when this vaccine would be available, how efficacious it will be and the number of doses immediately available) we explored 100 combinations of VE and vaccination coverage under a wide variety of scenarios minimizing four metrics of disease burden.
Our results suggest that any vaccine with medium to high effectiveness (VE ≥ 50%) would be able to considerably slow the epidemic while keeping the burden on healthcare systems manageable, as long as a high proportion of the population is optimally vaccinated. Moreover, once VE = 70%, full containment of the epidemic would be possible. This is in agreement with vaccine modeling studies (16,17). Further, we showed that much can be achieved even with low vaccination coverage; indeed, with medium VE, over half of deaths can be averted by optimally vaccinating only 35% of the population. When minimizing deaths, for low VE and a low supply of vaccine, our results suggest that vaccines should be given to the high-risk groups first. For high VE and high vaccination coverage, the optimal allocation strategy switched to vaccinating the high-transmission groups (younger adults and children). This remained true under equal or reduced susceptibility to infection for children, pointing to the importance of children as key players in disease transmission. This finding is consistent with others (18, 19) that have found for other respiratory viruses that protecting the high-transmission groups can be the optimal use of resources.
Here, we utilized mathematical optimization to determine the optimal vaccine allocation and by design, did not impose any restrictions in the allocation strategies. However in practice, implementation of optimal strategies must also account for other factors (ethical, political and societal). When large quantities of vaccine are available, a feasible solution could involve first vaccinating the high-risk groups and then allocating the remaining vaccine to the high-transmission groups.
This study has several limitations. Our model assumes that both naturally- and vaccine-acquired immunity will last for at least one year. We do not yet know how long immunity against SARS-CoV-2 will last and there is some evidence that neutralizing antibodies become undetectable after just a few weeks following infection (20), though it is unclear how this correlates with immunity. If immunity were short-lived, then these results would only be applicable for that duration. Further, we assumed that asymptomatic and symptomatic infections would confer equal immunity. However, some studies have suggested that asymptomatic infections might result in a weaker immune response (21). We utilized mortality and hospitalization rates that were based on the epidemic in Wuhan, but these rates may vary vastly in different regions. Further, we compared modeled peak hospitalizations to current state goals for hospital bed occupancy, but deterministic models tend to overestimate the transmission dynamics, so it is possible that a lower vaccine effectiveness or a lower vaccination coverage could achieve the same goals. To keep the optimization from being unreasonably long, our model does not capture geographical differences or other heterogeneities. We assumed a vaccine that would only reduce susceptibility to infection, but other effects, e.g., a reduction in disease severity, might occur. We have identified optimal allocation strategies and once more information about a vaccine characteristics is known, validating our allocation strategies with more complex models is welcome. To avoid confounding effects from different interventions, we optimized vaccine allocation assuming no social distancing interventions in place. In reality, vaccination, at least at the beginning, would take place while some social distancing interventions remain in effect. Under those circumstances, we would need less vaccine to control the epidemic. In that sense, our results are conservative. We computed the optimal allocation strategies utilizing age as the sole risk factor. However, several studies (22) have shown that, as a result of health systems with systemic health and social inequalities, people from racial and ethnic minority groups are at increased risk for getting sick and dying from COVID-19 in certain countries. This is a crucial consideration that will be included in further studies and can point towards who, within a given age-group, should get the vaccine first.
We believe that these results can provide a quantification of the effectiveness of different allocation scenarios under four metrics of disease burden and can be used as an evidence-based guidance to vaccine prioritization.
Supplementary Material
Acknowledgments
LM acknowledges Mia Moore for helpful discussions regarding the model structure and Michael Gutteridge for help with cluster computing.
Funding: LM, TL and ERB acknowledge support from NIAID, grant 1 UM1 AI148684-01.
Footnotes
Competing interests: LM and TL have received funding from Wellcome Trust for research unrelated to this manuscript. All other authors declare no competing interests.
Data and materials availability: Data are available in the main text or supplementary materials. Code available at https://github.com/lulelita/vaccine_optimization.
References
- 1.Johns Hopkins University and Medicine, Coronavirus COVID-19 global cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) (2020). [Google Scholar]
- 2.Mullard A., The Lancet 395, 1751(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corum J., Grady D., Wee S.-L., Zimmer C., Coronavirus Vaccine Tracker (2020). [Google Scholar]
- 4.Cohen R., et al. , medRxiv (2020). [Google Scholar]
- 5.Usher A. D., The Lancet 395, 1822(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Twohey M., Who Gets a Vaccine First? U.S. Considers Race in Coronavirus Plans (2020). [Google Scholar]
- 7.Centers for Disease Control and Prevention, 2009 H1N1 Flu (2009). [Google Scholar]
- 8.Zhang J., et al. , Science 8001, 1(2020). [Google Scholar]
- 9.CDC, COVID-19 Pandemic Planning Scenarios. [Google Scholar]
- 10.Ferguson N. M., et al. (2020).
- 11.Washington State Coronavirus Response, COVID-19 risk assessment dashboard (2020). [Google Scholar]
- 12.State of California, COVID-19 data and tools (2020). [Google Scholar]
- 13.Jing Q.-L., et al. (2020).
- 14.Bi Q., et al. , Lancet Infectious Diseases (2020). [Google Scholar]
- 15.Havers F. P., et al. , JAMA Internal Medicine (2020). [Google Scholar]
- 16.Bartsch S. M., et al. , medRxiv (2020). [Google Scholar]
- 17.Makhoul M., et al. , medRxiv (2020). [Google Scholar]
- 18.Medlock J., Galvani A. P., Science 325, 1705(2009). [DOI] [PubMed] [Google Scholar]
- 19.Matrajt L., Longini I. M., PLoS ONE 5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibarrondo F. J., et al. , New England Journal of Medicine (2020). [Google Scholar]
- 21.Long Q.-X., et al. , Nature Medicine (2020). [Google Scholar]
- 22.Hooper M. W., Nápoles A. M., Pérez-Stable E. J., JAMA 323, 2466(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.United States Census Bureau, Washington (2020). [Google Scholar]
- 24.Prem K., Cook A. R., Jit M., PLoS Computational Biology 13, e1005697(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du Z., et al. , Emerging Infectious Diseases 26, 1341(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishiura H., Linton N. M., Akhmetzhanov A. R., International Journal of Infectious Diseases 93, 284(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halloran M. E., Haber M., Longini I. M., American Journal of Epidemiology 136, 328(1992). [DOI] [PubMed] [Google Scholar]
- 28.Gao F., Han L., Computational Optimization and Applications 51, 259(2012). [Google Scholar]
- 29.Audet C., Hare W., Derivative-Free and Blackbox Optimization (Springer, Cham, Switzerland, 2017). [Google Scholar]
- 30.The Sage Developers, SageMath, the Sage Mathematics Software System (Version 9.1) (2020). [Google Scholar]
- 31.Kotz S., Balakrishnan N., Johnson N. L., Continuous Multivariate Distributions. Volume 1: Models and Applications (Wiley, 2000). [Google Scholar]
- 32.Virtanen P., et al. , Nature Methods 17, 261(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy J., Eberhart R., Proceedings of IEEE International Conference on Neural Networks 4, 1942(1995). [Google Scholar]
- 34.Miranda L. J. V., The Journal of Open Source Software 3, 433(2018). [Google Scholar]
- 35.Lauer S. A., et al. , Annals of Internal Medicine (2020). [Google Scholar]
- 36.Wei W. E., et al. , Morbidity and Mortality Weekly Report 69, 411(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y.-H., Hong C. M., Kim D. H., Lee T. H., Lee J., Emerging Infectious Diseases Oct (2020). [Google Scholar]
- 38.Mizumoto K., Kagaya K., Zarebski A., Chowell G., Euro Surveillance 25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He X., et al. , Nature Medicine 26 (2020). [Google Scholar]
- 40.Wu J. T., Leung K., Leung G. M., Lancet 395, 689(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao S., et al. , International Journal of Infectious Diseases 92, 214(2020).32007643 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.