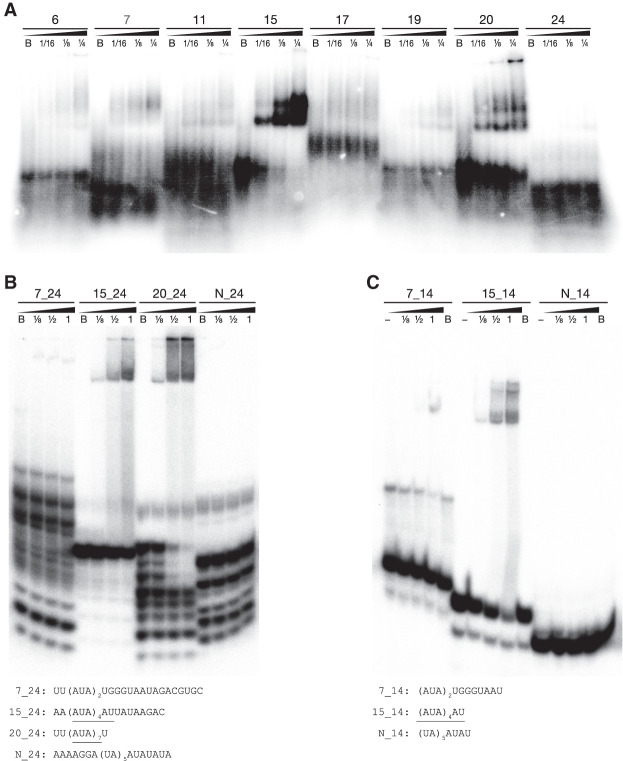
FIGURE 2.
Gel shift (EMSA) assays show specific binding of Dmr1p to RNA fragments containing the trinucleotide AUA repeats. (A) Radiolabeled 80 nt RNA fragments corresponding to probes #15 and #20 clearly show a mobility shift indicative of binding by increasing concentrations of Dmr1p. Fragment #7 and other fragments bind Dmr1p weakly or not at all. (B) Radiolabeled 24 nt fragments containing the 14- or 21-nt stretch of AUA repeats (fragments 15_24 and 20_24) show mobility shift indicative of binding by Dmr1p, whereas the fragment containing fewer repeats (7_24) and a control fragment containing dinucleotide UA/AU repeats (N_24) do not interact with the protein. (C) Radiolabeled 14 nt fragment comprising the minimal recognized motif (AUA)4AU (15_14) shows clear mobility shift by increasing amounts of Dmr1p, whereas the fragment containing fewer repeats (7_14) exhibits only minimal shift at the maximum Dmr1p concentration, and the control fragment containing dinucleotide UA/AU repeats (N_14) shows no interaction. Dmr1p concentrations increase from left to right for each fragment, and are labeled by fractions of the maximum (1), which corresponded to 0.8 µg per reaction. Total protein amount in each reaction was kept constant at 0.8 µg by addition of BSA. B is negative control (only BSA), “-” denotes negative control with no protein. Sequences of fragments used in B and C are shown below respective gels, with the AUA trinucleotide repeats underlined. The autoradiograms (originally recorded as high bit depth TIFF files) were linearly transformed to visualize weaker bands, resulting in an overexposure of the stronger bands.