FIGURE 4.
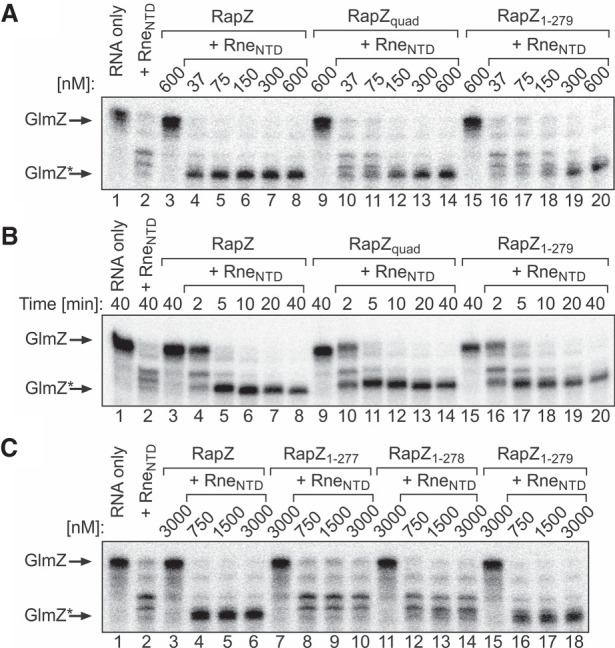
RapZ variants lacking RNA-binding activity but retaining interaction with RNase E promote cleavage of GlmZ by RNase E in vitro. (A) In vitro RNase E cleavage end point assays addressing the activities of the RapZquad and RapZ1–279 variants. Radiolabeled GlmZ was coincubated with 50 nM RneNTD and incremental concentrations of RapZ, RapZquad, or RapZ1–279 for 40 min. To provide controls, GlmZ was incubated alone (lane 1) or with each of the proteins individually (lanes 2,3,9,15). Reactions were separated on denaturing PAA gels and analyzed by phospho-imaging. (B) Time course of GlmZ cleavage by RneNTD (50 nM) in presence of 300 nM RapZ, RapZquad or RapZ1–279. Aliquots were removed and reactions were stopped at indicated times. (C) In vitro RNase E cleavage end point assays comparing the activities of the RapZ1–277, RapZ1–278, and RapZ1–279 variants. Radiolabeled GlmZ and indicated proteins were coincubated for 40 min.
