Abstract
Background
Research documents bidirectional pathways linking peripheral inflammation and neural circuitries subserving emotion processing and regulation. To extend this work, this paper presents results from two independent studies examining the relationship between inflammation and resting state functional connectivity (rsFC), as measured by fMRI.
Methods
Study 1 involved 90 rural African-American young adults, age 25 years (52% female), and Study 2 involved 82 urban African-American youth, ages 13–14 (66% female). Both studies measured circulating inflammatory biomarkers (CRP, IL6, IL10, TNFα), which were averaged to form a composite. Study 2 also enumerated classical monocytes, a key leukocyte sub-population involved in immune-to-brain signaling. All participants completed a resting state fMRI scan.
Results
Consistent with prediction, higher scores on the inflammatory composite were associated with lower rsFC within an emotion regulation network in Study 1, controlling for sex. Study 2 replicated Study 1, showing that higher scores on the inflammatory composite were associated with lower rsFC within the emotion regulation network, controlling for sex, age and pubertal status, and found a similar pattern for rsFC within a central executive network. Study 2 also found that higher numbers of classical monocytes were associated with lower rsFC within both the emotion regulation and central executive networks. There was no relationship between rsFC in the anterior salience or default mode networks with inflammation in either study.
Conclusions
These findings document relationships between peripheral inflammation and rsFC within an emotion regulation and central executive network, and replicate these associations with the emotion regulation network across two independent samples.
Keywords: Inflammation, neuroscience, fMRI, resting state, mental health, physical health
Introduction
Growing evidence documents bidirectional signaling between the brain and immune system in the pathogenesis of emotional and physical health problems(1–4). For example, animal research implicates neuroimmune signaling in the acquisition and expression of behaviors related to anxiety(5,6) and antidepressants diminish stress-induced inflammation and corresponding anxiety and depressive behaviors(7–9). Neuroimmune signaling is amplified and dysregulated in animal models of multiple psychiatric disorders, ranging from depression and anxiety to schizophrenia and substance use(3,5,10–12).
Despite the strength of animal findings, there are only a handful of studies examining brain functioning and inflammatory signaling in humans. This research indicates that healthy adults subjected to immunologic challenges (with endotoxin, vaccines, or interferon-α) display disrupted neural activity in regions that subserve emotion processing and regulation, as well executive control(13–17). This work also suggests that heightened tonic inflammation is associated with alterations in brain functioning that have implications for health(18–22).
The present paper extends this work by examining the relationship between tonic inflammatory signaling and resting-state functional connectivity (rsFC) in large-scale brain networks, as measured by fMRI. We focus on tonic activity because chronic low-grade inflammation is implicated in a heterogeneous set of mental and physical illnesses(1–4,23–24). The foundation for rsFC analyses is the discovery that there is a functional architecture to the brain’s activity when a person is not engaged in a task(25,26). This intrinsic activity is coordinated by a set of functional networks anchored to anatomically distributed nodes. This synchrony of functional activity during periods of rest is referred to as rsFC. An advantage of rsFC is that it allows researchers to examine large-scale brain networks that are not constrained by the parameters of a task(26). Recent work highlights the stability of these functional brain networks, suggesting we can use rsFC to measure s` traits within individuals(27). Individual differences in rsFC are apparent by late childhood(28).
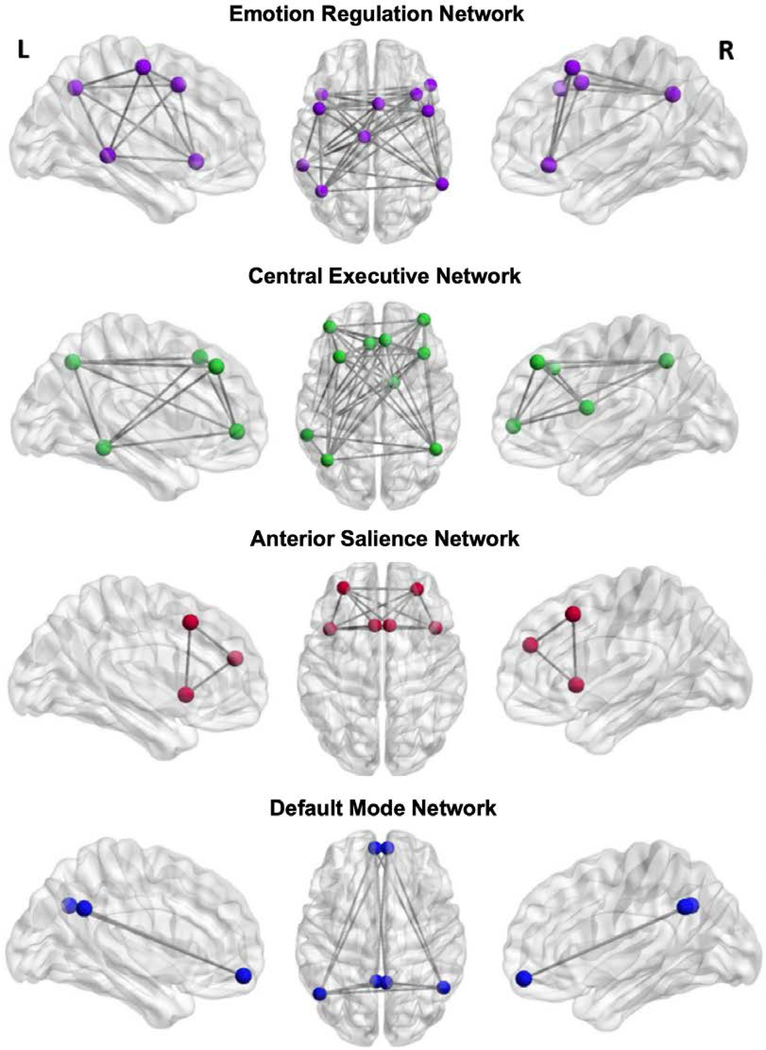
We focus on four rsFC networks implicated in emotion processing, regulation, and other cognitive functions given their involvement in psychiatric illnesses(29). The first is the emotion regulation network (ERN), which supports the conscious, voluntary, and cognitive regulation of emotion(30)(31,32). The ERN is anchored in the inferior frontal gyrus, middle temporal gyrus, and precentral gyrus, and plays a key role in regulating limbic circuitry, including the amygdala (31). Complementing the ERN is the frontoparietal central executive network (CEN), which connects areas of the dorsolateral prefrontal cortex and posterior parietal cortex to support the cognitive regulation of emotion, behavior, and attention(26,33–35). Young adults experiencing depression or anxiety display aberrant connectivity in both the ERN and CEN(29,31). The anterior salience network (aSN) is anchored in the anterior insula and dorsal anterior cingulate cortex and is important for monitoring the salience of external inputs and internal brain events(36). Finally, the default mode network (DMN) is anchored in the posterior cingulate cortex and medial prefrontal cortex and is implicated in self-related cognitive activity and mental simulation(37).
We present results from two distinct studies of African-American youth (12–14 years old) and young adults (25 years old). Compared with Whites, African-Americans have a similar (or lower) prevalence of most psychiatric disorders that involve inflammation(38,39). However, there are racial disparities in the course of many psychiatric disorders, with African-Americans experiencing more severe, disabling, and chronic manifestations(40–42). These disparities may stem from Black’s higher exposure to inflammation-triggering stressors including childhood adversity, racial discrimination, and economic hardship(43–45). Also, the vast majority of human brain imaging research has been on Caucasians, with minimal attention given to racial/ethnic variation. Studying both youth and young adults allows us to assess neuroinflammatory signaling across a developmental period associated with neural maturation(46), increases in immune system competence(47), and elevated risk for psychiatric disorder onset(48).
Study 1 examined the relationship between rsFC and inflammatory biomarkers, as quantified by inflammatory cytokines and C-reactive protein (CRP), among rural African American young adults. Peripheral cytokines can access the brain through active transport or enter at circumventricular organs or leaky regions of the blood-brain barrier(2,49). Study 2 had two aims, the first of which was to replicate the analyses of Study 1 in an independent sample and determine whether they generalize to younger African American individuals living in an urban setting. Study 2 then enumerated leukocyte sub-populations to examine a novel immune-brain pathway recently identified in animal studies(5). Although inflammatory cytokines are a principal channel for immune-to-brain communication(10–12,54), recent preclinical studies reveal another mechanism for such crosstalk, which involves monocytes(5). This research shows that when mice are subjected to chronic social stress, a population of immature monocytes is mobilized from bone marrow into circulation(6,50). These cells traffic to the blood vessels supplying the brain, and acting in concert with resident microglia, increase neuroinflammatory signaling in stress-sensitive regions like the prefrontal cortex, amygdala, and hippocampus. (It is unclear whether these monocytes migrate into the brain parenchyma, or just signal microglia which are present there.) Regardless, this chain-of-events is critical to the emergence of anxiety: if immature monocytes are prevented from trafficking into the brain, stressed mice show minimal evidence of anxiety-like behavior(9). These immature monocytes are defined as Ly-6chigh in mice; their homologue in humans is the classical monocyte, defined as CD14++/CD16-. To date, no human studies have considered how these cells relate to brain function. Accordingly, we extended the animal research by examining, for the first time, the relationship between rsFC and tonic levels of classical monocytes in humans. To evaluate the specificity of any such association, we also enumerated other leukocyte sub-populations, and evaluated their association with rsFC.1
Our predictions varied across rsFC networks. We hypothesized that higher inflammatory signaling (i.e., inflammatory biomarkers and classical monocytes) would be associated with lower rsFC in both the ERN and CEN, because in both animal and human studies, systemic inflammation diminishes self-regulation and executive-control by modulating PFC structure, function, and development(11,15,16,51–53). By contrast, the aSN monitors the salience of stimuli and is implicated in threat processing(36). We hypothesized that more connectivity among aSN nodes constitutes a vulnerability for inflammation. We based this prediction on experimental studies showing that activation of threat circuitry primes immune cells to show larger cytokine responses to microbial stimuli(1,49), which, over time, should accumulate to produce systemic inflammation. The DMN is involved in self-referential cognition(37). It was not apparent how variations in such processes would relate to inflammation, and thus we made a null prediction for the DMN.
Study 1
Methods and Materials
Participants
A total of 119 right-handed rural African-Americans, age 25 years, were recruited from a larger longitudinal study(54). Participants grew up in rural Georgia in households characterized as working poor; primary caregivers worked an average of 39.4 hours per week, yet 46.3% of the sample lived below federal poverty standards. Participants were right handed and screened for MRI contraindications. Participants were free of any psychiatric medications for at least one month before participating. Subsequent analyses excluded 28 participants because of excessive movement (n=23) and other technical problems (n=6). Thus, the final analytic sample was 90 (52% female). Participants provided written informed consent.
Procedure
We assessed rsFC and inflammatory biomarkers on the same day using procedures outlined below.
MRI Acquisition
Imaging data were collected at the University of Georgia using a GE Signa HDx 3-Tesla scanner. Structural imaging consisted of a high-resolution T1-weighted, fast spoiled gradient echo scan (TR=7.8ms, TE=3.1ms, flip angle=20°; FOV=25.6cm, matrix = 256×256, 160 contiguous 1mm axial slices, voxel size=1mm3). Whole-brain functional images were acquired using T2* echoplanar imaging with a single-shot gradient echo pulse sequence (TR=2000ms; TE=25ms; flip angle, 90°; FOV=225×225mm; matrix=64×64; 38 contiguous 3.5mm axial slices; voxel size=3.5mm3). The Study 1 resting state paradigm consisted of two 4-minute imaging runs of 120 brain volumes each.
MRI Image Processing
fMRI data preprocessing was conducted using Analysis of Functional Neuroimages software(55). Functional data were despiked, slice time shift corrected, and aligned to T1 data before being registered into Montreal Neurological Institute standardized space. The first four volumes of each run were removed to allow the MR signal to reach steady state. Volumes with greater than 25% of voxels identified as outliers (AFNI based version of DVARS) or intervolume movement greater than 0.2mm along any axis were censored(56,57). Bandpass filtering was applied to remove low and high frequency noise (.01 to .08hz), and motion correction was accomplished by including the six standard (de-meaned) motion parameters and their temporal derivatives as regressors of no interest. Data were spatially smoothed using a 6mm full-width half-maximum Gaussian filter.
Resting State Functional Connectivity Analyses
For each region-of-interest (i.e., node) within a network, we placed a 5mm sphere around the coordinates of peak activation for each discrete cluster separately within the left and right hemisphere masks. Raw time series data for each voxel were de-meaned and converted to percent-signal-change scores to reduce variability between subjects. We calculated the region-of-interest (ROI) seed data as the average percent-signal-change for all voxels contained in a given region. rsFC was quantified using the correlation of the average time series in each ROI with the average time series in all other ROIs in the network using Pearson’s r. Next, we converted r values to Z-scores using Fischer’s r to Z transformation. Finally, we averaged the Z-scores of all possible connections to compute a total network value reflecting the connectivity of all possible nodes within the network. We a priori selected the ERN ROIs from an activation likelihood estimation meta-analysis(31), which identified an emotion regulation network across 23 studies/479 participants. We defined the CEN and aSN ROIs utilizing a publicly available atlas of resting state networks derived through an independent components analysis(58). DMN ROIs were defined based on a meta-analysis of default mode network connectivity(59). Table 1 presents the coordinates and labels for each ROI, and Figure 1 presents axial and sagittal views of the ROIs for each rsFC network.
Table 1.
Regions-of-Interest for the Resting State Networks.
| Network | MNI Coordinates (x, y, z) | Label |
|---|---|---|
| Emotion Regulation Network (ERN) | −06 14 58 −42 22 −06 −44 10 46 −58 −38 −02 −42 −60 44 06 14 58 50 30 −08 48 08 48 38 22 44 60 −54 40 |
L somatomotor area L inferior frontal gyrus L precentral gyrus L middle temporal gyrus L angular gyrus R somatomotor area R inferior frontal gyrus R precentral gyrus R middle temporal gyrus R angular gyrus |
| Central Executive Network (CEN) | −42 −63 46 −32 23 49 −40 48 −01 −59 −42 −12 −07 34 43 38 26 42 48 −54 47 38 54 01 13 02 14 06 37 46 |
L inferior parietal lobule L middle frontal gyrus L middle frontal gyrus L middle temporal gyrus L medial frontal gyrus R middle frontal gyrus R inferior parietal lobule R middle frontal gyrus R caudate R medial frontal gyrus |
| Anterior Salience Network (aSN) | −06 17 47 −31 47 22 −42 14 −03 06 17 47 28 46 26 −42 14 −03 |
L dorsal anterior cingulate cortex L middle frontal gyrus L anterior insula R dorsal anterior cingulate cortex R middle frontal gyrus R anterior insula |
| Default Mode Network (DMN) | −04 −52 32 −05 55 −13 −49 −62 34 04 −53 35 05 55 −13 50 −57 36 |
L posterior cingulate cortex L ventromedial prefrontal cortex L temporoparietal junction R posterior cingulate cortex R ventromedial prefrontal cortex R temporoparietal junction |
Note: L = left. R = right. MNI = Montreal Neurological Institute standardized space. For each region-of-interest (i.e., node) within a network, we placed a 5mm sphere around the coordinates of peak activation for each discrete cluster separately within the left and right hemisphere masks.
Figure 1.
Axial and both left and right hemisphere sagittal views of regions-of-interests (ROIs) for the resting state functional connectivity networks. For each region-of-interest (i.e., node) within a network, we placed a 5mm sphere around the coordinates of peak activation for each discrete cluster separately within the left and right hemisphere masks.
Inflammation biomarkers
From antecubital blood, we quantified serum levels of CRP, interleukin-6, interleukin-10 and tumor necrosis factor-α (TNF-α).2 CRP was measured by high-sensitivity immunoturbidimetric assay on a Roche/Hitachi cobas c502 analyzer (lower limit of detection, 0.2mg/L). The average intra- and inter-assay coefficients of variation were 2.5% and 5.6%. The cytokines were measured in duplicate by electrochemiluminescence on a SECTOR Imager 2400A (MesoScale Discovery) with a Human Pro-Inflammatory Ultra-Sensitive assay (MesoScale Discovery), following the manufacturer’s instructions. The kit’s lower limits of detection range from 0.19pg/mL (IL-6) to 0.57pg/mL (IL-10). Across runs, the intra-assay coefficients of variation for duplicate pairs were 4.01% (IL-6), 4.59% (IL-10), and 3.80% (TNF-α). Following previous work(60), we z-scored the values of each biomarker and then summed them to form a composite inflammatory biomarker score. A higher score on this composite reflects higher systemic inflammation.3
Results
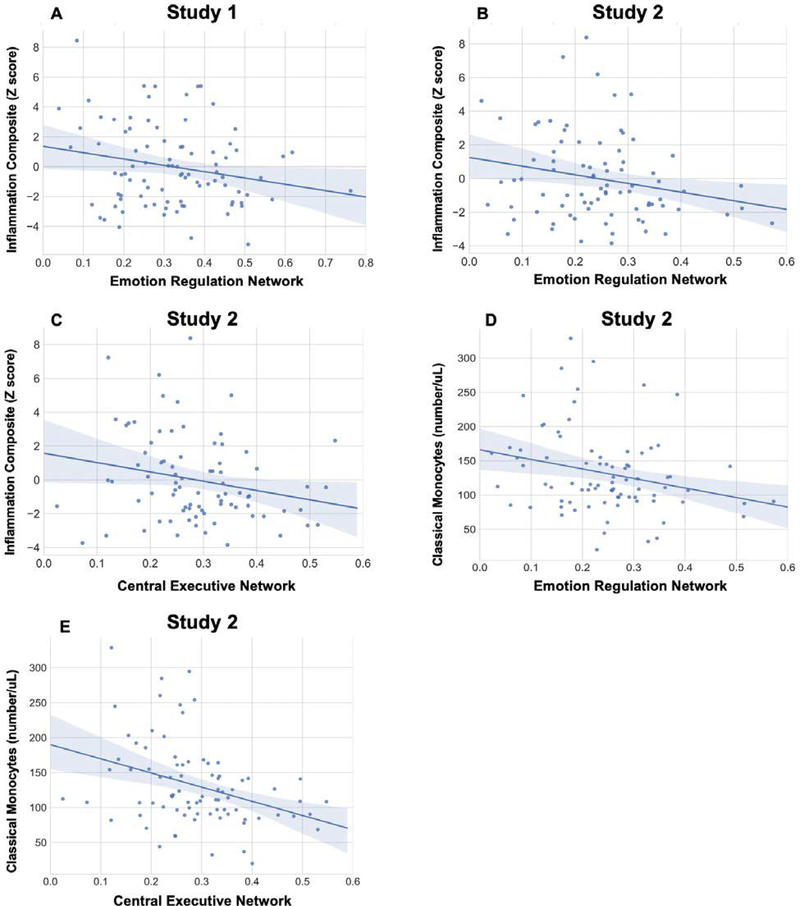
We regressed the composite inflammation score on to each of the rsFC networks in four separate hierarchical multiple regression analyses. We statistically controlled for sex in all models and we present the results of these adjusted analyses in Table 2.4 In line with predictions, higher scores on the inflammatory biomarker composite were associated with lower rsFC in the ERN, (B = −.21, t = −2.01, p = .05; Figure 2A). There were no significant associations between the inflammation composite and the other rsFC networks, (p’s>.15). (Supplemental Table S1 presents the relationships between rsFC in all networks and each separate inflammatory biomarker. Supplemental Figure S1 presents relationships between the inflammatory composite score and specific node-to-node associations within the ERN).
Table 2.
Hierarchical multiple regression analyses of the relationship between resting state functional connectivity (rsFC) and the composite inflammation score (CRP, IL6, IL10, TNFα) controlling for sex in Study 1.
| Inflammation Composite | |||
|---|---|---|---|
| B | t- score | p | |
| Emotion Regulation Network (ERN) | −.21 | −2.00 | .05 |
| Central Executive Network (CEN) | −.14 | −1.3 | .20 |
| Anterior Salience Network (aSN) | −.15 | −1.44 | .15 |
| Default Mode Network (DMN) | .07 | .63 | .53 |
Note: Separate regression analyses were conducted to examine the relationship between composite inflammation score and each of the resting state networks.
Figure 2.
Relationships between resting state functional connectivity (rsFC) in the emotion regulation and central executive networks with composite inflammation score (CRP, IL6, IL10, TNFα) and classical monocytes. Confidence intervals are 95%.
Study 2
Although inflammatory cytokines are a principal channel for immune-to-brain communication(10–12,61), recent preclinical studies reveal another mechanism for such crosstalk, which involves monocytes(5). This research shows that when mice are subjected to chronic social stress, a population of immature monocytes is mobilized into circulation(6,50). These cells traffic to the blood vessels supplying brain, and acting in concert with resident microglia, increase neuro-inflammatory signaling in stress-sensitive regions like the prefrontal cortex, amygdala, and hippocampus. (It is unclear whether these monocytes migrate into the brain parenchyma, or just signal microglia which are present there.) Regardless, this chain-of-events is critical to the emergence of anxiety: if immature monocytes are prevented from trafficking into the brain, stressed mice show minimal evidence of anxiety-like behavior(9). These immature monocytes are defined as Ly-6chigh in mice; their homologue in humans is the classical monocyte, defined as CD14++/CD16-.
We had three aims for Study 2. First, we sought to replicate the results of Study 1 in an independent sample, and determine if they generalize to younger African-Americans living in an urban setting. Second, we extended the animal research outlined above to humans, and assessed whether on a tonic basis, higher numbers of classical monocytes were associated with lower rsFC. Finally, to evaluate the specificity of any such association, we enumerated other leukocyte sub-populations, and evaluated their association with rsFC. Based on Study 1 and animal findings, we predicted that higher levels of inflammatory biomarkers and classical monocytes would relate to lower rsFC, but that other leukocyte populations would not.
Methods and Materials
Participants
Data were collected from 106 African-American youth from Chicago, Illinois. Participants were in eighth grade, English-speaking, and in good health, defined as being without a history of chronic medical or psychiatric illness, free of prescription medications during the past three months, and without acute infectious disease in the two weeks before participating. Participants were right handed and free of MRI contraindications. Fourteen participants did not have rsFC data because they could be not be scheduled, or were too obese or too anxious to get into the scanner. Ten additional participants were excluded because of poor quality MRI data, leaving an analytic sample of 82. Youth in the analytic sample had a mean age of 13.9 (range 12–14) and 55 of them were female (67.1%). 24 of them were in early or middle stages of puberty (29.3%) and the others were late (42 or 51.2%) or post-pubertal (16 or 19.5%). In terms of socioeconomic conditions, 22% of youth resided in households whose income was below the federal poverty threshold (i.e., an income-to-poverty ratio ≤ .99). Another 35.4% had income-poverty ratios from 1.00–1.99, a category typically described as low income. Participants with complete and missing data were similar on age, gender, and pubertal stage (p’s ranging from .10 to .85).
Procedure
We assessed rsFC and inflammatory variables at two separate laboratory visits using procedures outlined below.
MRI Acquisition
Imaging data were collected at Northwestern University using a Siemens Prisma 3 Tesla scanner with a 64 phased-array head coil. Structural imaging consisted of a high-resolution navigated multiecho magnetization prepared rapid acquisition gradient echo (MEMPR) sequence (TR=2300ms, TE=1.86, 3.78, ms, flip angle=7°; FOV=256×256; matrix = 320×320, 208 slices, voxel size=0.8mm3). Whole-brain functional images were acquired using T2* echoplanar imaging with a fast TR sequence (TR=555ms; TE=22ms; flip angle, 47°; FOV = 208×208mm; voxel size=2.0mm3; multiband factor=8; partial Fourier Factor=6/8). The Study 2 resting state paradigm consisted of one 10-minute imaging run involving 1,110 brain volumes(62).
MRI Image Processing
Data were processed using Northwestern University Neuroimaging Data Archive (NUNDA)(63) in-house pipelines. We modified Study 2’s processing pipeline to accommodate its multiband sequence and youth sample (see Supplemental Materials for details). Functional data were despiked and aligned to T1 data. Data were registered to Montreal Neurological Institute standardized space using a non-linear transformation(64). The first ten volumes were removed to allow the MR signal to reach steady state. Volumes with framewise displacement (FD) > 0.5mm or whole-brain changes in BOLD signal (DVARS) > 0.9% were regressed from the dataset(56), as were white matter and CSF. Participants needed to have 436 useable volumes (i.e., 4 minutes) to be included in analyses. Bandpass filtering was applied to remove low and high frequency noise (.01 to .08 hz). Data were spatially smoothed using a 6mm full-width half-maximum Gaussian filter.
Resting State Functional Connectivity Analyses
Network connectivity values were computed, and network ROIs were defined, using procedures identical to Study 1.
Inflammation Biomarkers and Leukocyte Phenotyping
Antecubital blood was collected between 8:00 and 10:00am, after an overnight fast, to minimize the influence of dietary intake and circadian variation. Serum levels of inflammatory biomarkers (CRP, IL-6, IL-10, TNF-α) were quantified using procedures and reagents that were identical to Study 1. The mean intra-assay coefficient of variation for duplicate pairs were 3.71% (IL-6), 3.42% (IL-10), and 3.57% (TNF-α).5
From the same blood draw, major leukocyte subsets (granulocytes, monocytes, lymphocytes) were enumerated with an automated hematology analyzer (AcT 5Diff, Beckman-Coulter). A standardized flow cytometry protocol was used to enumerate populations of classical and non-classical monocytes(65). Briefly, antecubital blood was drawn into Sodium-Heparin Vacutainers (Becton-Dickinson). After red blood cells had been removed (Pharm Lyse, Becton-Dickinson), the pelleted cells were washed, blocked with normal human serum, and stained with mouse, anti-human monoclonal antibodies against CD14(FITC), CD16(PE), HLA-DR (PerCPCy5.5), and CD45(APC), all purchased from Becton-Dickinson. Following a 20-minute incubation, the cells were washed and fixed (CytoFix/CytoPerm, Becton-Dickinson), and incubated for another 20 minutes. Data were acquired on a Guava 6HT2L (Millipore), with 30,000 events collected per specimen, and analyzed using FlowJo software. Following previous work(65), populations of classical (CD14++/CD16-) and non-classical (CD14+/CD16++) monocytes were defined by a sequential gating procedure.
Results
Using a series of hierarchical regression analyses, we regressed the inflammatory variables on to each of the rsFC networks. We statistically controlled for sex, age, and pubertal status in all models and present the results of these analyses in Table 3 Replicating the results of Study 1, higher scores on the inflammatory biomarker composite were associated with lower rsFC in the ERN, (B = −.23, t = −2.07, p = .04; Figure 2B). Higher scores on this composite also were associated with lower rsFC in the CEN, (B = −.27, t = −2.42, p = .02; Figure 2C). (Supplemental Table S2 presents the relationships between rsFC in all networks with each separate inflammatory biomarker).
Table 3.
Hierarchical multiple regression analyses of the relationship between resting state functional connectivity (rsFC) and inflammatory variables controlling for sex, age and pubertal status in Study 2.
| Inflammation Composite | Classical Monocytes | Non-Classical Monocytes | |||||||
| B | t-score | p | B | t-score | p | B | t-score | p | |
| ERN | −.23 | −2.07 | .04 | −.25 | −2.20 | .03 | −.16 | −1.40 | .17 |
| CEN | −.27 | −2.42 | .02 | −.37 | −3.38 | .001 | −.19 | −1.68 | .10 |
| aSN | .09 | .72 | .48 | −.17 | −1.56 | .12 | −.02 | −.15 | .88 |
| DMN | −.09 | −.74 | .46 | −.18 | −1.62 | .11 | −.05 | −.47 | .64 |
| Lymphocytes | Total White Blood Cells | ||||||||
| B | t−score | p | B | t−score | p | ||||
| ERN | −.04 | −.39 | .70 | .03 | .22 | .82 | |||
| CEN | −.09 | −.86 | .39 | −.08 | −.70 | .49 | |||
| aSN | −.10 | −.90 | .37 | −.08 | −.75 | .46 | |||
| DMN | −.02 | −.19 | .85 | .02 | .14 | .89 |
Note: Separate regression analyses were conducted to examine the relationship between inflammatory variables and each of the resting state networks. ERN = emotion regulation network; CEN = central executive network; aSN = anterior salience network; DMN = default mode network.
Turning to cellular phenotyping data, our results paralleled findings from animal research on the role of classical monocytes in immune-brain communication. Specifically, higher counts of classical monocytes were associated with lower rsFC in both the ERN, (B = −.25, t = −2.20, p = .03; Figure 2D) and CEN, (B = −.37, t = −3.38, p = .001; Figure 2E). Also paralleling the preclinical literature, these associations were specific to the classical monocyte population – there were no significant associations between the ERN and CEN with any other leukocyte subpopulations considered (p’s>.11). Finally, rsFC in the aSN and the DMN were not significantly related to any of the inflammatory variables (p’s>.10). (Supplemental Figure S1 presents relationships between inflammatory variables and specific node-to-node associations within the ERN and CEN).
Discussion
This is the first investigation of the relationship between peripheral inflammatory signaling and functional connectivity of intrinsic brain networks in humans. Consistent with predictions, Study 1 found evidence that higher scores on an inflammatory biomarker composite (CRP, IL6, IL10, TNFα) were associated with lower rsFC within the ERN among rural African-American young adults. Study 2 replicated this finding in an independent sample of African-American youth, and also found evidence that higher scores on the inflammatory biomarker composite were associated with lower rsFC in the CEN. Study 2 also found that higher counts of classical monocytes, a key leukocyte sub-population involved in immune-brain signaling, were associated with lower rsFC within both the ERN and CEN. These relationships were maintained after adjusting for sex, age, and pubertal status. There was no relationship between rsFC in the aSN or DMN with inflammatory signaling in either study.
The ERN and CEN support the cognitive regulation of emotion, attention, and behavior(26,31–33,35,58). The engagement of these regulatory processes has been associated with better mental and physical health(66–68). There is growing evidence, however, that chronic inflammation modulates the structure, function, and development of the prefrontal cortex (11,15,16,51–53). Here, and in our neuroimmune network model(1), we propose that by altering prefrontal processes, inflammation weakens the regulatory influence that the ERN and CEN have on limbic reactivity, thus heightening negative, and lowering positive affect. In line with this view is evidence that lower rsFC in the ERN and CEN are associated with dysphoria, depression, and anxiety(29,31). We next propose that reduced regulatory strength predisposes individuals to high-risk, proinflammatory behaviors like smoking, drug use, and high fat diets, in part, to self-medicate dysphoria. If the inflammation triggered by these behaviors spreads to the brain, it could establish a positive-feedback circuit, whereby reduced regulatory strength facilitates proinflammatory behaviors, which, in turn, further reduces prefrontal regulatory strength. When combined with evidence that inflammation elevates threat-, and reduces reward-related brain function(13–17), this positive-feedback circuit could overtime engender vulnerability for both emotional and physical health problems.
Study 2 extends animal research by examining the relationship between rsFC and tonic levels of classical monocytes. Although inflammatory cytokines are a principal channel for immune-to-brain signaling(10–12,61), recent work in rodents highlights classical monocytes as a key leukocyte sub-population in this crosstalk(5). To date, no studies have examined how these cells relate to human brain function. In line with prediction, higher counts of classical monocytes were associated with lower rsFC in both the ERN and CEN. Paralleling the animal literature, these associations were specific to the classical monocyte population, as there were no significant associations between other leukocyte subpopulations and rsFC in the ERN and CEN. Importantly, classical monocytes are mobilized into circulation when mice are subjected to chronic social stress, and amplify inflammatory signaling in brain regions involved in emotion processing and regulation(6,50). This chain-of-events appears to be critical to the emergence of anxiety, as stressed mice show minimal anxiety-like behavior if classical monocytes are prevented from trafficking into the brain(6,50). Taken together, this suggests that the trafficking of classical monocytes to the brain, and in particular to prefrontal regulatory systems, may be involved in the pathogenesis of anxiety and stress-related disorders in humans. Future research is needed to test this claim.
The present paper replicates the association between higher scores on the inflammatory biomarker composite and lower rsFC in the ERN across two independent samples of African-American youth (12–14 years old) and young adults (25 years old). This suggests the linkage between inflammatory signaling and ERN activity is stable across development, and may reflect a preclinical biomarker for psychiatric symptoms which frequently emerge during adolescence(48). It is noteworthy that CEN activity was only associated with inflammatory signaling in Study 2. While the ERN and CEN share some common regulatory processes, there are distinctions across these networks. For example, while the CEN involves connections from the prefrontal cortex to posterior portions of the parietal cortex subserving higher-order attention, the ERN projects to the somatomotor area and precentral gyrus, which are implicated in behavior inhibition and motor control(31,69). One possibility is that ERN, as compared to CEN, regulatory processes are more reliably associated with inflammatory signaling across development (i.e., youth to young adulthood). A second possibility is that methodological differences account for the inconsistent results. Due to financial and feasibility constraints, Study 1 did not implement an overnight fast prior to blood draw, or restrict blood draws to a set time of day, both of which affect the reliability of inflammatory markers(70,71). Thus, further research is needed to better understand the reliability of CEN-inflammation associations across development.
Contrary to prediction, there was no relationship between inflammation and rsFC in the aSN. Task-based fMRI paradigms may be required to provoke the required variation in salience processing to assess its relationship with inflammatory signaling. Future research should test this possibility. We predicted the null association between inflammatory signaling and DMN activity because it was not apparent how variations in self-referential cognition might relate to inflammation. We analyzed DMN connectivity to assess for specificity between inflammatory signaling and other rsFC networks. Collectively, our findings suggest that inflammation most strongly relates to intrinsic brain networks implicated in emotion regulation and executive control. However, given that DMN abnormalities are common in neuropsychiatric disorders such as depression(29), future research should examine the relationship between DMN connectivity and inflammatory signaling in clinical samples.
The studies in this paper should be interpreted in the context of their limitations. First, the cross-sectional, observational nature of their designs preclude inferences about causality. A longitudinal study tracking rsFC and inflammatory signaling across development is needed. A study like this could test for the presence of the proposed positive-feedback circuit between inflammation and prefrontal regulatory strength, and answer mechanistic questions about how such a circuit might develop. Next, the present studies examined neuroimmune signaling in participants who, for the most part, were in good health. This is important for identifying neuroimmune profiles that predate illness onset and medication regimens. This restrictive eligibility criteria, however, precludes our ability to examine the relationship between neuroimmune signaling and emotional and physical health problems, which should be examined in future research. Finally, the present studies focused exclusively on African-American participants given blacks’ higher exposure to inflammation-triggering stressors, including childhood adversity, racial discrimination, and economic hardship(43–45). Future research should examine whether our results extend to non-African-American participants.
Meanwhile, the present studies advance knowledge on neuroimmune signaling in humans. In particular, these studies report that higher inflammation, as measured at multiple levels of analysis (inflammatory biomarkers, classical monocytes), is associated with lower functional connectivity in intrinsic brain networks implicated in emotion regulation and executive control. These findings have implications for understanding the pathogenesis of emotional and physical health problems, and the generation of neuroimmunological interventions for targeting these problems.
Supplementary Material
Acknowledgements and Disclosures
Preparation of this article was supported by grants from the National Institute on Drug Abuse (P30 DA027827), National Heart, Lung, and Blood Institute (R01 HL122328), National Institute of Mental Health (R01 MH100117), the National Institute of Child Health and Human Development (R01 HD030588), the Ryan Licht Sang Bipolar Foundation, and the Chauncey and Marion D. McCormick Foundation.
Footnotes
Study 1 did not have the infrastructure to enumerate leukocyte sub-populations in order to assess the relationship between classical monocytes and rsFC.
Although IL-10 functionally is an anti-inflammatory cytokine, it is only expressed under conditions of inflammation. Thus, statistically, it behaves like the other inflammatory cytokines such that higher levels reflect more inflammatory activity.
In Study 1, the mean and standard deviation for each biomarker in pg/ml were 3.07 and 4.38 for CRP, 2.02 and 2.12 for IL-6, 1.42 and 2.32 for IL-10, and 3.69 and 1.24 for TNF-α. The composite inflammatory biomarker score was significantly associated with each of the individual inflammatory biomarkers at p <.001 (IL-6, r = .72; IL-10, r = .61; TNFα, r = .69; CRP, r = .69).
We did not statistically control for age in Study 1 because all participants were approximately 25 years old (mean age = 24.92, SD = .57).
In Study 2, the mean and standard deviation for each biomarker in pg/ml were 1.27 and 2.43 for CRP, 0.9 and 2.05 for IL-6, 2.71 and 8.43 for IL-10, and 2.43 and .048 for TNF-α. The composite inflammatory biomarker score was significantly associated with each of the individual inflammatory biomarkers at p <.001 (IL-6, r = .64; IL-10, r = .59; TNFα, r = .44; CRP, r = .77).
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Nusslock R, Miller GE (2016): Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biol Psychiatry. 80:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haroon E, Raison CL, Miller AH (2012): Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 37:137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller AH, Raison CL (2016): The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 16:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felger JC, Treadway MT (2017): Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology. 42:216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber MD, Godbout JP, Sheridan JF (2017): Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal. Neuropsychopharmacology. 42:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wohleb ES, Powell ND, Godbout JP, Sheridan JF (2013): Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 33:13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez K, Niraula A, Sheridan JF (2016): GABAergic modulation with classical benzodiazepines prevent stress-induced neuro-immune dysregulation and behavioral alterations. Brain Behav Immun. 51:154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramirez K, Shea DT, McKim DB, Reader BF, Sheridan JF (2015): Imipramine attenuates neuroinflammatory signaling and reverses stress-induced social avoidance. Brain Behav Immun. 46:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. (2011): beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 31:6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, et al. (2014): Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 10:643–660. [DOI] [PubMed] [Google Scholar]
- 11.Meyer U (2013): Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 42:20–34. [DOI] [PubMed] [Google Scholar]
- 12.Hutchinson MR, Watkins LR (2014): Why is neuroimmunopharmacology crucial for the future of addiction research? Neuropharmacology. 76:218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI (2012): Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 59:3222–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR (2010): Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 68:748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD (2009): Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological psychiatry. 66:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, et al. (2012): Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 69:1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasselin J, Treadway MT, Lacourt TE, Soop A, Olsson MJ, Karshikoff B, et al. (2017): Lipopolysaccharide Alters Motivated Behavior in a Monetary Reward Task: a Randomized Trial. Neuropsychopharmacology. 42:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, et al. (2016): Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatr. 21:1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muscatell KA, Eisenberger NI, Dutcher JM, Cole SW, Bower JE (2016): Links between inflammation, amygdala reactivity, and social support in breast cancer survivors. Brain Behavior and Immunity. 53:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, et al. (2016): Neural mechanisms linking social status and inflammatory responses to social stress. Soc Cogn Affect Neur. 11:915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, et al. (2015): Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behavior and Immunity. 43:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tawakol A, Ishai A, Takx RAP, Figueroa AL, Ali A, Kaiser Y, et al. (2017): Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 389:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotamisligil GS (2006): Inflammation and metabolic disorders. Nature. 444:860–867. [DOI] [PubMed] [Google Scholar]
- 24.Odegaard JI, Chawla A (2013): Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 339:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raichle ME (2015): The restless brain: how intrinsic activity organizes brain function. Phil Trans R Soc B. 370:20140172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bressler SL, Menon V (2010): Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 14:277–290. [DOI] [PubMed] [Google Scholar]
- 27.Gratton C, Laumann TO, Nielsen AN, Greene DJ, Gordon EM, Gilmore AW, et al. (2018): Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron. 98:439–452. e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grayson DS, Fair DA (2017): Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. Neuroimage. 160:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menon V (2011): Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 15:483–506. [DOI] [PubMed] [Google Scholar]
- 30.Ochsner KN, Silvers JA, Buhle JT (2012): Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 1251:E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U (2014): Neural network of cognitive emotion regulation--an ALE meta-analysis and MACM analysis. Neuroimage. 87:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. (2014): Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 24:2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gagnepain P, Hulbert J, Anderson MC (2017): Parallel Regulation of Memory and Emotion Supports the Suppression of Intrusive Memories. J Neurosci. 37:6423–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD (2012): Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wessing I, Rehbein MA, Postert C, Furniss T, Junghofer M (2013): The neural basis of cognitive change: reappraisal of emotional faces modulates neural source activity in a frontoparietal attention network. Neuroimage. 81:15–25. [DOI] [PubMed] [Google Scholar]
- 36.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raichle ME (2015): The brain’s default mode network. Annu Rev Neurosci. 38:433–447. [DOI] [PubMed] [Google Scholar]
- 38.Williams DR, Gonzalez HM, Neighbors H, Nesse R, Abelson JM, Sweetman J, et al. (2007): Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National Survey of American Life. Archives of general psychiatry. 64:305–315. [DOI] [PubMed] [Google Scholar]
- 39.Gibbs TA, Okuda M, Oquendo MA, Lawson WB, Wang S, Thomas YF, et al. (2013): Mental health of African Americans and Caribbean blacks in the United States: results from the National Epidemiological Survey on Alcohol and Related Conditions. Am J Public Health. 103:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hankerson SH, Fenton MC, Geier TJ, Keyes KM, Weissman MM, Hasin DS (2011): Racial differences in symptoms, comorbidity, and treatment for major depressive disorder among black and white adults. Journal of the National Medical Association. 103:576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zapolski TC, Pedersen SL, McCarthy DM, Smith GT (2014): Less drinking, yet more problems: understanding African American drinking and related problems. Psychological bulletin. 140:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banks DE, Rowe AT, Mpofu P, Zapolski TC (2017): Trends in typologies of concurrent alcohol, marijuana, and cigarette use among US adolescents: An ecological examination by sex and race/ethnicity. Drug and alcohol dependence. 179:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acevedo-Garcia D, Osypuk TL, McArdle N, Williams DR (2008): Toward a policy-relevant analysis of geographic and racial/ethnic disparities in child health. Health Aff (Millwood). 27:321–333. [DOI] [PubMed] [Google Scholar]
- 44.Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, et al. (2010): Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosom Med. 72:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.U.S. Census Bureau (2014): U.S. poverty report.
- 46.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. (2004): Dynamic mapping of human cortical development during childhood through early adulthood. P Natl Acad Sci USA. 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coe CL (2010): All roads lead to psychoneuroimmunology Handbook of health psychology and behavioral medicine, pp 182–199. [Google Scholar]
- 48.Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB (2007): Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry. 20:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irwin MR, Cole SW (2011): Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 11:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, et al. (2013): Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 110:16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, Verstynen TD (2013): Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cereb Cortex. 23:2058–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR (2008): Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 64:484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C (2012): Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology. 78:720–727. [DOI] [PubMed] [Google Scholar]
- 54.Brody GH, Yu T, Chen YF, Kogan SM, Evans GW, Beach SR, et al. (2013): Cumulative socioeconomic status risk, allostatic load, and adjustment: a prospective latent profile analysis with contextual and genetic protective factors. Dev Psychol. 49:913–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cox RW (1996): AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical research. 29:162–173. [DOI] [PubMed] [Google Scholar]
- 56.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. (2013): An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirer W, Ryali S, Rykhlevskaia E, Menon V, Greicius MD (2012): Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral cortex. 22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D (2011): Connectivity gradients between the default mode and attention control networks. Brain connectivity. 1:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller GE, Brody GH, Yu T, Chen E (2014): A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proc Natl Acad Sci U S A. 111:11287–11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller AH, Haroon E, Raison CL, Felger JC (2013): Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 30:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu J, Moeller S, Auerbach EJ, Strupp J, Smith SM, Feinberg DA, et al. (2013): Evaluation of slice accelerations using multiband echo planar imaging at 3 T. Neuroimage. 83:991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alpert K, Kogan A, Parrish T, Marcus D, Wang L (2016): The Northwestern University Neuroimaging Data Archive (NUNDA). Neuroimage. 124:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andersson JL, Jenkinson M, Smith S (2007): Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford. 2:1–21. [Google Scholar]
- 65.Heimbeck I, Hofer TP, Eder C, Wright AK, Frankenberger M, Marei A, et al. (2010): Standardized single-platform assay for human monocyte subpopulations: Lower CD14+CD16++ monocytes in females. Cytometry A. 77:823–830. [DOI] [PubMed] [Google Scholar]
- 66.Hu T, Zhang D, Wang J, Mistry R, Ran G, Wang X (2014): Relation between emotion regulation and mental health: a meta-analysis review. Psychological reports. 114:341–362. [DOI] [PubMed] [Google Scholar]
- 67.Gross JJ (2015): Emotion regulation: Current status and future prospects. Psychological Inquiry. 26:1–26. [Google Scholar]
- 68.Appleton AA, Buka SL, Loucks EB, Gilman SE, Kubzansky LD (2013): Divergent associations of adaptive and maladaptive emotion regulation strategies with inflammation. Health Psychol. 32:748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN (2008): Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 59:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nilsonne G, Lekander M, Akerstedt T, Axelsson J, Ingre M (2016): Diurnal Variation of Circulating Interleukin-6 in Humans: A Meta-Analysis. PLoS One. 11:e0165799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fogarty CL, Nieminen JK, Peräneva L, Lassenius MI, Ahola AJ, Taskinen M-R, et al. (2015): High-fat meals induce systemic cytokine release without evidence of endotoxemia-mediated cytokine production from circulating monocytes or myeloid dendritic cells. Acta diabetologica. 52:315–322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.