Abstract
Importance: Occupational therapy can play a role in primary care management of chronic diseases among older adults.
Objective: To assess the feasibility of delivering a primary care occupation-focused intervention (Integrated PRimary care and Occupational therapy for Aging and Chronic disease Treatment to preserve Independence and Functioning, or i-PROACTIF) for older adults with chronic disease.
Design: Feasibility study comparing i-PROACTIF with complex care management using a two-group randomized controlled trial design with data gathered at baseline and during and after the 8-wk intervention.
Setting: Family medicine clinic serving an urban, low-income, working-class community.
Outcomes and Measures: Feasibility indicators were recruitment, retention, utility of clinical assessments, and acceptability of interventions assessed through feedback surveys completed by patients and primary care providers (PCPs). Patient outcomes, including perspectives on chronic illness care, occupational performance, and overall well-being, were collected using standardized, validated measures and analyzed descriptively.
Participants: Eighteen adult volunteers, ages ≥50 yr, with heart disease, arthritis, and uncontrolled diabetes completed the study. Ten PCPs completed feedback surveys.
Intervention: i-PROACTIF focuses on preserving functional independence, is based on the Person–Environment–Occupation framework, and consists of two assessment sessions and six weekly treatment sessions.
Results: Recruitment goals were achieved, with an 86% retention rate. Clinical measures unearthed deficits in areas that were unreported or underreported by patients. Participants reported being extremely satisfied with the intervention. Physicians and nurses also supported the intervention. Both groups showed improved scores on most outcomes.
Conclusion and Relevance: Delivering and evaluating i-PROACTIF was feasible and acceptable. Future efficacy trials are needed before it can be used in clinical settings.
What This Article Adds: The results of this study can inform future occupational therapy interventions and clinical trials in primary care for older adults with chronic conditions.
The United States is facing a growing population of older adults with chronic diseases. Chronic illnesses and secondary physical decline are associated with a higher risk of hospitalization and long-term institutionalization as well as a near doubling of health care spending (Centers for Disease Control and Prevention, 2013). Thus, improved primary care management of chronic diseases among older adults is a critical health care priority (National Center for Health Statistics, 2014). Integrating occupational therapy into primary care settings offers new options to achieve this goal (Muir, 2012).
Aging and Chronic Illness
By 2030, one in five U.S. residents will be of retirement age (U.S. Census Bureau, 2018), with most managing more than one chronic disease such as diabetes, heart disease, or arthritis (Ward & Schiller, 2013). These conditions, when not appropriately managed, result in decreased ability to carry out daily life activities (Klijs et al., 2011). Limitations in basic activities of daily living (BADLs) increase with the number of chronic conditions (Ralph et al., 2013). Decline in functional independence diminishes quality of life and hinders self-management of one’s condition, triggering a vicious cycle of worsening disease and further decline (Cramm et al., 2014).
Current Problems With Clinical Management of Chronic Disease
In the United States, chronic diseases are usually managed in primary care settings. However, standard primary care appointments are often spent managing acute medical problems. This leaves insufficient time to address patients’ complex needs, which might require managing secondary complications and coordinating home and community care (Rich et al., 2012). Patients have reported that they are dissatisfied with primary care services because of the limited time that health care providers spend with them and poor understanding of recommendations for day-to-day disease management (Freed et al., 2013).
Although occupational therapy practitioners can address these gaps, their services are typically available only in specialized rehabilitation settings. Early access to occupational therapy services would require physicians to recognize signs of functional decline and make appropriate referrals. However, primary care physicians lack requisite training in identification of preclinical functional decline and, even when aware, do not routinely monitor functional status (Rich et al., 2012). Therefore, referrals are usually made only after serious decline and secondary complications arise. The current fee-for-service payment system also deters early identification and referral of patients. Physician fees do not cover the extra costs of comprehensive assessments or time spent on specialist referral and interprofessional care coordination (Rich et al., 2012).
Evidence for Occupational Therapy’s Role in Primary Care
The evidence supporting occupational therapy interventions in primary care is limited to non–U.S. studies. One Canadian randomized controlled trial (RCT) found that patients with chronic illnesses who received a combined occupational and physical therapy intervention at a primary care center reported significantly higher satisfaction with services and had significantly fewer planned hospital days than those who received routine rehabilitation services in the community (Richardson et al., 2010).
A subsequent study investigated an intervention that included a function-based individual assessment, a 5-wk self-management program, and an online personal record system to promote self-monitoring of functional health. Using a quasi-experimental design with a matched control group, Richardson et al. (2012) found significant differences in patients’ self-reported physical functioning and objective measures of physical performance.
Similarly, an Irish RCT found that a 6-wk occupational therapist–led self-management support program for older adults with multiple chronic conditions demonstrated significant improvement in frequency of activity participation, goal achievement, perceived activity performance and satisfaction, self-efficacy, independence in daily activities, and quality of life (Garvey et al., 2015).
Existing literature provides preliminary evidence that occupational therapy can be beneficial for older adults with chronic conditions in primary care settings. However, these studies are not directly applicable to the U.S. health care context, which makes it difficult to justify integration of occupational therapy practitioners into U.S.-based primary care settings (Killian et al., 2015).
Our study aimed to develop and deliver an occupation-focused intervention for preserving functional independence among older adults with chronic diseases in a primary care setting. The feasibility study compared i-PROACTIF (Integrated PRimary care and Occupational therapy for Aging and Chronic disease Treatment to preserve Independence and Functioning) with an occupational therapy–informed complex care management (CCM) protocol. Our objectives were to
Recruit and retain 18–20 patients, with an enrollment rate of ≥50% of eligible patients and with 80% of enrolled patients expected to complete intervention sessions and follow-up assessments (Chhatre al., 2018);
Explore the utility of clinical measures for screening and assessment;
Assess the acceptability of i-PROACTIF and CCM from the perspective of patients and primary care providers (PCPs), with >85% of participants expected to rate the interventions as satisfactory; and
Explore the potential of i-PROACTIF and CCM to improve patient outcomes.
Method
Study Design
A two-group RCT design was used. The intervention group received the i-PROACTIF intervention, and the comparison group received the CCM protocol, which was adapted to patients’ occupational goals.
Intervention and Comparison Condition
We based the i-PROACTIF intervention on the premise of “catching” patients with preclinical functional decline and preventing the deconditioning associated with aging and chronic disease. It is informed by the Person–Environment–Occupation Model (PEO; Law et al., 1996) and involves eight weekly sessions. The first two sessions focus on goal planning and clinical assessment in the three focus areas of the PEO model: (1) physical functioning (i.e., person factors; Session 1), (2) home safety and accessibility (i.e., environmental factors; Session 2), and (3) occupational competence and performance in context (i.e., occupational factors; Session 2). Information in these areas was used by the interventionist to collaboratively identify three patient-centered goals. To facilitate this process, we developed an agenda-mapping script and a goal-planning worksheet based on principles of motivational interviewing. Agenda mapping is a motivational interviewing technique intended to facilitate collaborative goal setting and problem solving between the patient and provider (Sanders et al., 2013; see Supplemental Figure 1 for examples of the goal-planning worksheet, available online at https://ajot.aota.org; navigate to this article, and click on “Supplemental”).
Goal planning was followed by three in-person treatment sessions of 30 min duration held at the study clinic (Sessions 3–5) and three weekly phone follow-ups that lasted 15–20 min (Sessions 6–8). Treatment sessions focused on chronic disease education, recommendations for embedding physical activity in everyday tasks, and environmental modifications or activity adaptations to increase functional independence. The goal-planning worksheet was revisited at every session to ensure patient-centeredness. Thus, although the intervention was manualized, it also included built-in flexibility for individualized patient goals and treatment plans.
The CCM intervention was based on guidelines from the Blue Cross Blue Shield Initiative (BCBSI), which supports CCM at primary care clinics across the nation. At the time of the study, these guidelines were the standard of care for patients with chronic diseases at the study clinic. This intervention involved eight weekly phone counseling sessions focusing on five topics: diet, symptom management, medication management, community resources, and referral management. The interventionist was expected to identify occupation-focused goals but could address these goals only by providing counseling in these five areas. The existing BCBSI protocol did not specify any schedule or duration for patient contact and did not include a protocol manual with information on the five focus areas. The ad hoc BCBSI guidelines were manualized to ensure consistency across patients and to structure the intervention into weekly sessions spread across 8 wk. These steps were necessary to establish equivalence between the two interventions and thereby enhance the internal validity of comparisons.
The interventionist for both interventions was a licensed occupational therapist (Maureen Gecht-Silver) with 20 yr of clinical experience with older adults in various settings. She was already working at the study clinic as a care coordinator and was aware of the study aims. The principal investigator (Mansha Mirza) or a research assistant (RA) observed three i-PROACTIF and three CCM sessions and completed a fidelity checklist for each observation to identify departures from the prescribed protocol.
Sampling, Recruitment, and Randomization
Participants included patients and PCPs at a family medicine clinic, located on an urban public university campus, that serves a primarily low-income, working-class catchment area. Eligible PCPs were those employed at the clinic during the study duration. They were kept apprised of study progress through a series of four formal presentations at regularly scheduled staff meetings. Presentations included intervention description, case studies, and preliminary findings. At the final presentation, PCPs were asked to complete a feedback survey.
Eligible patients had at least one clinic visit in the previous year, were >50 yr old, and had a primary diagnosis of at least one of three conditions—arthritis (osteoarthritis or rheumatoid), heart disease (coronary artery disease, ischemic heart disease, hypertension), or uncontrolled diabetes (hemoglobin A1c ≥10). In addition, they showed risk of functional decline (score ≥3) on the 11-item Brief Risk Identification of Geriatric Health Tool (BRIGHT; Kerse et al., 2008); had self-identified a need for chronic disease management support; or had medical records in the past 12 mo suggesting a history of hospitalization, fall, or fracture or indicating three or more emergency department visits. Patients were excluded if they were not proficient in English; resided in a long-term care facility; were participating in other clinical trials involving a rehabilitation or health management component; or scored ≥5 on the AD8 Dementia Screening Interview (Carpenter et al., 2011), indicating severe cognitive impairment.
Recruitment was conducted in three ways: (1) Eligible patients were identified using the clinic’s electronic medical records (EMRs) and received an invitation via phone call from a member of the study team, (2) patients were identified and directly referred by PCPs at the clinic, and (3) flyers instructing interested patients to contact the study team were posted at the clinic. Prospective participants completed a phone screening for eligibility. Eligible participants were enrolled after providing written consent.
The randomization sequence was generated using a random number generator by the senior author (Anders Kottorp), who was not involved in recruitment or data collection. Sealed envelopes containing group assignment were prepared and sequentially numbered for each diagnostic condition (e.g., A1–A9 for arthritis; D1–D9 for diabetes, H1–H9 for heart disease). Each patient recruited into the study was matched with the successively numbered envelope for their primary diagnosis. For example, the first patient recruited with a primary diagnosis of arthritis was matched with envelope A1, the second patient recruited with a primary diagnosis of diabetes was matched with envelope D2, and so on. This was done to balance diagnoses across the two groups. After baseline assessment, the RA opened the envelope for each participant, revealing the participant’s group assignment.
Measures and Data Collection
Recruitment and Retention Indicator
We kept records of the number of participants screened, enrolled, and randomized. Reasons for refusal and dropout were also noted.
Clinical Measures
Four clinical assessments were performed by the interventionist with i-PROACTIF participants only. The assessments included the Physical Performance Test (PPT; Reuben & Siu, 1990), Timed Up and Go Test (TUG; Podsiadlo & Richardson, 1991), Performance Assessment of Self-Care Skills–Home (PASS–Home; Chisholm et al., 2014), and Home Falls and Accidents Screening Tool (HOME FAST; Vu & Mackenzie, 2012).
The PPT includes nine tasks that simulate daily living activities of various degrees of difficulty. The measure has shown high interrater reliability (r = .99) and high internal consistency (α = .87) in samples of older adults (Reuben & Siu, 1990).
The TUG assesses functional mobility and balance. The participant is asked to rise from a regular chair with arms, walk 3 m at a comfortable pace, turn, walk back to the chair, and sit down. The TUG has shown high test–retest reliability (intraclass correlation coefficient [ICC] = .99) and interrater reliability (ICC = .99) among older adults and correlates well with other measures of balance and mobility (Podsiadlo & Richardson, 1991).
The PASS–Home is a performance-based, criterion-referenced assessment of performance of daily living tasks. The assessment has demonstrated high interrater reliability in well-elderly samples (91%–97% agreement among raters across domains; Chisholm et al., 2014). It includes 26 tasks, categorized under functional mobility, BADLs, instrumental activities of daily living (IADLs) with a physical emphasis, and IADLs with a cognitive emphasis. Tasks are scored on safety, independence, and adequacy. For this study, one or two PASS–Home tasks most relevant to the participant’s occupational goals were selected for observation.
The HOME FAST is a 25-item tool designed to identify fall hazards. The tool can predict fall risk in community-dwelling older people and has good interrater reliability (ICC = .82) and good test–retest reliability (ICC = .77; Vu & Mackenzie, 2012).
The PPT and TUG were conducted at the study clinic during Session 1; the PASS–Home and HOME FAST were conducted in the patient’s home during Session 2. Both sets of assessments took approximately 60 min.
Acceptability Measures
Separate satisfaction surveys were created for the i-PROACTIF and CCM interventions. Questions were adapted from an evaluation form developed by the World Health Organization (2008). Participants in each treatment condition were asked to rate overall satisfaction with the program, delivery method, and resources provided and to give feedback on the length and duration of contacts. Open-ended comments were also solicited and were recorded in handwritten notes and typed immediately after into a word-processing file.
After all patients had completed the program, feedback was also sought from PCPs at the study clinic. The PCPs were asked to complete a 6-item survey assessing their understanding of how chronic conditions can affect a patient’s physical functioning and occupational performance as well as their perceptions of the value and benefits of integrating occupational therapy into chronic disease management. Response categories ranged from 1 (strongly disagree) to 5 (strongly agree). Three open-ended questions were also included and asked about benefits, drawbacks, and suggestions for integrating occupational therapy services within primary care.
Outcome Measures
Outcomes were assessed for CCM and i-PROACTIF participants at baseline and postintervention. Trained RAs who were blind to group assignment administered assessments in person during an hour-long interview conducted in a private research office. The time between baseline and postintervention assessments ranged from 8 to 16 wk (mean [M] = 79 days). The Patient Assessment of Chronic Illness Care (Glasgow et al., 2005) is a 20-item measure of patient perspectives on aspects of health care delivery outlined in the Chronic Care Model (Wagner, 1998). It has five subscales: Patient Activation, Delivery System Design/Decision Support, Goal Setting, Problem Solving/Contextual Counseling, and Follow-Up/Coordination. Previous research has shown reasonable levels of internal consistency (α = .68–.90) for the subscales and moderate 3-mo test–retest reliability (r = .47–.68; Glasgow et al., 2005).
The Occupational Self-Assessment (Version 2.2; OSA; Baron et al., 2006) assesses perceived value of and competence in 21 areas of occupational performance and participation and generates a comparable occupation-based measure across individuals. Among older adults, the OSA has demonstrated adequate correlation with other measures of occupational performance and satisfaction (r = .41–.54; Stuber & Nelson, 2010).
The Patient-Reported Outcomes Measurement Information System Global Health Short Form (PROMIS; Hays et al., 2009) is a 10-item assessment of health-related quality of life based on patient self-report. The instrument provides separate summary scores for physical and mental health. In a national internet panel comparable to 2000 U.S. Census data, the measure has demonstrated excellent internal consistency and good construct validity for both physical components (α = .81, r = .76) and mental health components (α = .86, r = .71; Hays et al., 2009).
Data Analysis
Descriptive statistics were used to analyze sociodemographic and diagnostic characteristics of participants. Independent-samples t tests and Fisher’s exact tests were used to assess whether randomization procedures were successful in creating two groups with baseline equivalence on these characteristics. Quantitative acceptability data from patients and PCPs were analyzed descriptively using frequency counts. Open-ended comments were reproduced verbatim and organized according to the corresponding question asked on the feedback survey. These comments were not subject to qualitative content analysis because in-depth thematic analysis was beyond the scope of this study. For exploratory efficacy analyses, mean change scores for outcome measures, and corresponding effect sizes, were computed for both groups.
Results
Recruitment and Retention
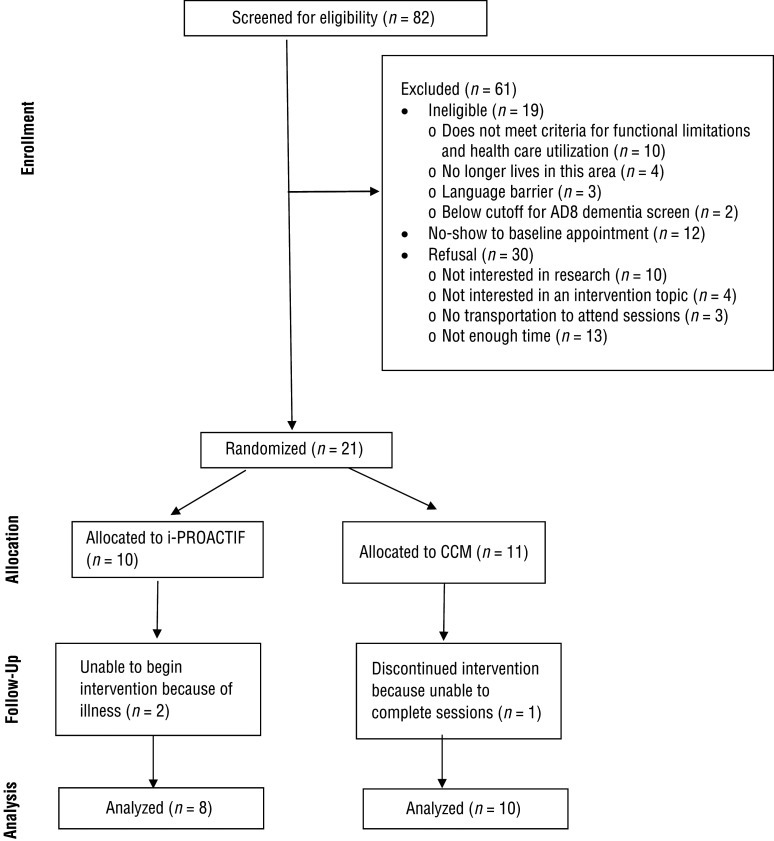
Eighty-two individuals were screened. Nineteen were deemed ineligible, 12 did not show at the baseline appointment, and 30 declined to participate. Lack of time (n = 13) and lack of interest in research (n = 10) were common reasons for refusal. Of the 21 patients who were enrolled and randomized (34% of eligible participants), 2 were identified using EMRs, 1 was self-referred, and the remaining were referred by physicians or nurse practitioners. Of 10 patients randomized to the i-PROACTIF group, 2 were unable to begin the intervention because of illness. Of 11 patients randomized to the CCM group, 1 was lost to follow-up. Therefore, the retention rate was 86% (Figure 1).
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram for study participants.
Note. CCM = complex care management; i-PROACTIF = Integrated PRimary care and Occupational therapy for Aging and Chronic disease Treatment to preserve Independence and Functioning.
Flow diagram format adapted from “The CONSORT Statement: Revised Recommendations for Improving the Quality of Reports of Parallel-Group Randomized Trials,” by D. Moher, K. F. Schulz, & D. G. Altman; CONSORT Group, 2001, JAMA, 285, p. 1990. https://doi.org/10.1001/jama.285.15.1987
Participant Characteristics
The final analyzed sample (N = 18) included 7 men and 11 women aged 50–78 yr (M = 61.8, standard deviation = 7.3). The groups did not significantly differ on sociodemographic and diagnostic characteristics at baseline (Table 1). At baseline, 1 participant (i-PROACTIF) was receiving occupational therapy outside this study, averaging up to 4 hr a month, and 3 (2 i-PROACTIF, 1 CCM) were receiving physical therapy, averaging 4–6 hr a month.
Table 1.
Participant Characteristics
| Characteristic | i-PROACTIF Group (n = 8), n or M (SD) | CCM Group (n = 10), n or M (SD) |
| Primary chronic condition | ||
| Diabetes | 5 | 4 |
| Heart disease | 0 | 3 |
| Arthritis | 3 | 3 |
| >1 chronic condition | 4 | 6 |
| Gender | 4 | 3 |
| Female | 4 | 7 |
| Male | 4 | 3 |
| Age, yr | 62.1 (7.95) | 61.5 (7.15) |
| Education | ||
| <High school | 3 | 2 |
| High school or GED | 2 | 4 |
| >High school | 3 | 4 |
| Race/ethnicity | ||
| Non-Hispanic White | 0 | 1 |
| Non-Hispanic Black | 6 | 6 |
| Hispanic/Latino | 1 | 2 |
| Native Hawaiian or Pacific Islander | 0 | 1 |
| Mixed race | 1 | 0 |
Note. CCM = complex care management; GED = general education diploma; i-PROACTIF = Integrated PRimary care and Occupational Therapy for Aging and Chronic disease Treatment to Preserve Independence and Functioning.
Utility of Clinical Measures
Clinical measures were easily implemented and did not require a dedicated space, special equipment, or setup. Regarding person factors, the performance-based assessments unearthed deficits in areas that were unreported or underreported by patients. Five of 8 i-PROACTIF participants performed below the cutoff score on the PPT; 7 participants demonstrated unacceptable, dependent, or unsafe performance on at least one PASS–Home task; and 4 participants were identified on the TUG as high fall risk. The TUG was not performed with 1 participant because of safety concerns identified on the PPT. Of 8 participants, 4 did not meet the BRIGHT’s cutoff score, indicating risk of functional decline. Yet, these participants were identified as having deficits on one or more of the three performance-based assessments. This finding suggests the likelihood of false-negative results on self-report screeners of functional limitations.
Regarding environmental factors, the HOME FAST was found to be useful for identifying hazards in the home environment. Common hazards included lack of grab rails near the shower or bath (72.2%), lack of ability to switch a light on easily from bed (44.4%), lack of slip-resistant mats in the bathroom (38.9%), and presence of loose mats on the floor (38.9%).
Regarding occupational goals, 63 priority areas were identified on the OSA across both groups. Common priorities included physical functioning (n = 26; e.g., climbing stairs, walking), activities of daily living or IADLs (14/63; e.g., bathing, dressing, financial management, meal preparation), health management (n = 9; e.g., exercise, medication management, taking care of oneself), goal setting and decision making (n = 6), social interactions (n = 5; e.g., getting along with others, communicating with others), and personal fulfillment (n = 3; e.g., relaxing and enjoying oneself, having a satisfying routine).
Acceptability of the Interventions
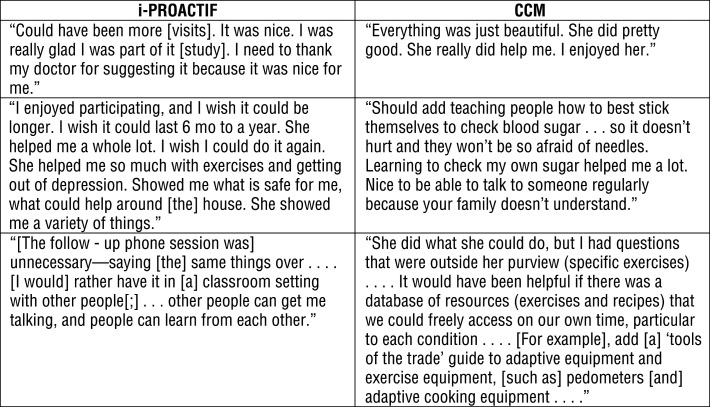
All i-PROACTIF participants reported being extremely satisfied with the intervention and found the resources to be helpful. Seven found the service delivery model to be engaging, and 1 found it to be somewhat engaging. Participants were also satisfied with the length and number of visits. Nine of the 10 CCM participants reported being extremely satisfied with the intervention, whereas 1 was somewhat satisfied. All 10 found that the provided resources were helpful. Examples of open-ended comments from participants in both groups are presented in Figure 2.
Table 2.
Change From Baseline to Postintervention
| Outcome Measure/Domain | i-PROACTIF (n = 8) | CCM (n = 10) | ||
| Change, M (SD) | Effect Size (Cohen’s d) | Change, M (SD) | Effect Size (Cohen’s d) | |
| PACIC/Patient Activation | −0.67 (0.76) | 0.88 | −0.40 (1.19) | 0.33 |
| PACIC/Delivery System Design/Decision Support | −0.42 (1.21) | 0.35 | −0.47 (0.86) | 0.54 |
| PACIC/Goal Setting | −0.30 (0.94) | 0.32 | −0.32 (0.93) | 0.34 |
| PACIC/Problem Solving/Contextual Counseling | 0.03 (1.15) | 0.03 | −0.70 (0.99) | 0.71 |
| PACIC/Follow-Up/Coordination | −0.20 (0.95) | 0.21 | −0.25 (1.19) | 0.21 |
| OSA | −0.63 (12.27) | 0.05 | −2.30 (7.78) | 0.30 |
| PROMIS/Physical | −7.54 (9.83) | 0.77 | −4.90 (4.55) | 1.08 |
| PROMIS/Mental | −2.21 (4.08) | 0.54 | −3.15 (7.41) | 0.43 |
Note. Negative values indicate improvement. CCM = complex care management; i-PROACTIF = Integrated PRimary care and Occupational therapy for Aging and Chronic disease Treatment to preserve Independence and Functioning; OSA = Occupational Self-Assessment; PACIC = Patient Assessment of Chronic Illness Care; PROMIS = Patient-Reported Outcomes Measurement Information System.
Figure 2.
i-PROACTIF and CCM participant feedback comments.
Note. CCM = complex care management; i-PROACTIF = Integrated PRimary care and Occupational therapy for Aging and Chronic disease Treatment to preserve Independence and Functioning.
Ten PCPs (9 physicians and 1 nurse practitioner) completed feedback surveys. Not all had referred patients to the study, but all were aware of the intervention offered. Nine providers agreed or strongly agreed that after learning about i-PROACTIF, they
Understood that chronic conditions affect a patient’s physical functioning and ability to perform daily living tasks,
Understood the role of occupational therapy in chronic disease management and in improving patients’ functional independence in carrying out daily living tasks,
Believed that offering occupational therapy services at the study clinic would be beneficial for patients with chronic health conditions, and
Were interested in learning more about what occupational therapy could offer their patients with chronic health conditions.
One provider strongly disagreed with all items. However, these ratings were not aligned with this provider’s open-ended comments, which were mostly positive. Open-ended comments about the perceived benefits of integrating occupational therapy services into primary care acknowledged the role of occupational therapy in providing “holistic care [and] recognizing [the] importance of function and independence”; “address[ing] multiple issues that [affect] health, mobility, [and so forth]”; and “elicit[ing] self-identified goals, [providing] resources [and] improv[ing the] home environment for self-care.”
Concerns were also raised about making referrals for occupational therapy. Specifically, respondents wanted more clarity about “what diagnoses qualify patients for OT [occupational therapy] referral” and what procedural changes would be needed to “[easily] connect OT for an assessment . . . during a clinic session,” to implement “regular hours and scheduled times for OT,” and to “bill for these services.”
Exploratory Efficacy Analyses
On most outcomes, both groups showed improvement from baseline to postintervention, with the biggest improvement shown on the PROMIS subscales (Table 2).
Discussion
The results of this feasibility study can be used to inform future occupational therapy clinical trials in primary care. The slow pace of recruitment affected study randomization procedures. Our original plan was to use a permuted block design and randomize participants in blocks of six within strata defined by primary diagnosis. This plan would have ensured an even number of participants with arthritis, diabetes, and heart disease across treatment groups. However, recruitment challenges made it difficult to accrue and stratify participants in these blocks before group assignment. Therefore, we switched to a simple randomization scheme within strata as we enrolled participants one at a time. This change resulted in an imbalanced representation of participants with heart disease in the treatment groups. Future trials may benefit from alternative randomization procedures such as covariate adaptive randomization (Kang et al., 2008), in which participants are sequentially assigned to treatment groups using an algorithm that includes covariates of previously allocated participants.
As has previous research (Chhatre et al., 2018), we found that PCP referral was a more effective recruitment strategy than patient identification using EMRs and phone calls. EMRs did not always include up-to-date information, for example, for patients who had moved or changed their primary care clinic. In addition, securing buy-in from PCPs and having them refer patients helped cultivate trust in the study and the research team, thereby facilitating recruitment. Outreach to physicians and other health care providers is considered among the most useful recruitment strategies (Chhatre et al., 2018). This strategy would be especially important for studies involving occupational therapy interventions in primary care because many PCPs have limited knowledge of occupational therapy’s role and scope of practice (Donnelly et al., 2013).
Feedback from PCPs suggests that learning about i-PROACTIF helped them understand occupational therapy’s role in chronic disease management. PCP comments also indicated a new awareness of occupational therapy practitioners’ unique skill set for assessing functional independence and addressing patient-identified goals that is beyond the scope of generally short PCP visits.
Despite their overall support for i-PROACTIF, PCPs expressed a need for greater clarity regarding screening and referral of patients for occupational therapy services. It is therefore necessary to develop validated screening tools that can be administered by a range of clinical staff to rapidly and accurately screen patients in primary care settings.
We used the 11-item BRIGHT, a brief self-report, which can be easily completed by patients while waiting to see the PCP. This screening tool has established cutoff scores that can be used to identify patients for occupational therapy referral. However, we identified false-negative results on the BRIGHT; that is, some patients who did not meet the BRIGHT criteria for risk of functional decline demonstrated deficits on one or more performance-based assessments. This finding underscores the need for future research in the form of systematic reviews comparing sensitivity and specificity of screening tools for risk of functional decline.
The i-PROACTIF intervention was well received by patients and feasible to implement in the primary care setting. An important feature of i-PROACTIF is the combination of a standardized protocol and customization options for each patient’s needs. Previous literature has recommended a generalist role for occupational therapy practitioners working in primary care given the wide-ranging caseload (Killian et al., 2015; Muir, 2012). However, the imperative to treat patients with a variety of conditions, and to adapt treatments accordingly, makes it difficult to standardize procedures (Koverman et al., 2017), an essential condition for efficacy testing in clinical trials. These competing demands were addressed by developing the i-PROACTIF manual to serve as a toolbox from which the interventionist could select tools specific to each patient’s needs. Inclusion of the OSA to identify patient-centered goals allowed further customization of the intervention. In addition, consistency of implementation was ensured through the use of agenda-mapping scripts and goal-planning worksheets with all patients.
This study also included occupational goal setting in the CCM protocol. CCM was the standard of care for patients with chronic diseases at the study clinic and was delivered by an occupational therapist working as a care coordinator. According to the available literature, to build a role for occupational therapy in primary care, practitioners must capitalize on job positions not specific to occupational therapy, such as care coordinators and case managers (Halle et al., 2018). Therefore, we used this study as an opportunity to modify the existing CCM protocol so that it is informed by the core values of occupational therapy. This decision served a dual purpose. From a practice perspective, we were able to demonstrate that occupational goal setting did not derail or hamper existing care coordination practice at the clinic, thereby consolidating the role of the occupational therapist as care coordinator. From a research perspective, it allowed us to compare an occupational therapy–focused intervention in a primary care setting (i-PROACTIF) with an occupational therapy–informed primary care intervention (CCM).
Patient comments indicated that CCM participants would have benefited from key components of i-PROACTIF, such as individualized patient education, recommendations for embedding physical activity in daily life, and prescriptions for adaptive equipment. Unlike CCM guidelines, which included only generic health-management information provided exclusively by phone, the i-PROACTIF protocol offered opportunities for direct observation of patient performance, thereby allowing customized strategies and adaptations for meeting occupational goals.
Even so, further research, in the form of a full-scale trial, is needed to isolate and characterize the active ingredients of i-PROACTIF and compare them with standard care that is not informed by occupational therapy. Research is also needed on the role of client and contextual factors in mediating the effects of i-PROACTIF among primary care patients. On the basis of our study’s findings, client factors such as gender and cardiovascular comorbidities and contextual factors such as caregiver support might be important mediators to consider in future research.
Future clinical trials in primary care need to select outcome measures that are appropriate for this population and sensitive to change. Primary care patients with preclinical functional decline are likely to be higher functioning than patients seen in traditional rehabilitation settings. Therefore, it is important to consider the possibility of ceiling effects when selecting outcome measures that have been previously validated with samples of traditional rehabilitation patients. For example, the OSA, one of the main outcome measures in this study, has demonstrated some ceiling effects for higher functioning people (Baron et al., 2006) and needs to be further validated with primary care patients.
Lack of established cutoffs for minimal detectable change (MDC) in comparable samples also limits the ability to interpret whether change scores on outcome measures represent true clinical change beyond measurement error. Finally, if occupational therapy services in primary care are intended to prevent, decelerate, or stall functional decline in patients with a variety of conditions, patient-centered measures of activity participation with MDC cutoffs derived from a variety of clinical samples, such as the Patient-Specific Functional Scale (Stratford et al., 1995), might be a good choice.
Study Limitations
The small sample size and absence of a control group limited our ability to detect Group × Time changes. Because of scheduling difficulties, not all patients could complete the intervention and postintervention assessments in 8 wk, which could have contributed to differences in effects among participants. In addition, participants may have had differences at baseline that were not measured for the study, and these differences could have influenced study findings. Long-term benefits of i-PROACTIF and its cost effectiveness and related billing and payment issues could not be examined within the scope of this study.
Implications for Occupational Therapy Practice
Our primary purpose was to explore feasibility of the study design and implementation of i-PROACTIF in a primary care setting. The intervention may have potential to benefit patients, provided larger studies show positive results. The results of this study have the following implications for occupational therapy practice:
Practitioners interested in establishing services in primary care settings are encouraged to educate PCPs about the content and scope of occupational therapy interventions for patients with chronic conditions. Similar to our study, previous work in primary care has found that direct PCP outreach and orientation are critical for establishing clinical programs in primary care and sourcing referrals for occupational therapy services (Koverman et al., 2017).
Clinical implementation of the i-PROACTIF intervention requires that practitioners carefully document treatment content and closely monitor client progress.
Practitioners need to be cognizant of client factors such as gender, caregiver needs, and comorbidities that can influence treatment outcomes, thereby warranting adaptation of treatment intensity and delivery.
Conclusion
The i-PROACTIF intervention, an occupation-focused intervention for preserving functional independence among older adults with chronic diseases, can feasibly be delivered in a primary care setting. The intervention was acceptable to patients and appreciated by PCPs. Future efficacy trials are needed before the intervention can be used in clinical settings.
Supplementary Material
Acknowledgments
This research was funded by an Intervention Research Grant from the American Occupational Therapy Foundation (AOTF). Statistical analysis for this project was partly supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant UL1TR002003. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the AOTF or NIH. Special thanks to John Hickner for his support; to Sherry Muir for reviewing the intervention protocol; and to research assistants Amy Morton, Elizabeth Harrison, and Rachel Luangdilok.
Contributor Information
Mansha Mirza, Mansha Mirza, PhD, OTR/L, MSHSOR, is Associate Professor, Department of Occupational Therapy, University of Illinois at Chicago; mmirza2@uic.edu.
Maureen Gecht-Silver, Maureen Gecht-Silver, OTD, MPH, OTR/L, is Assistant Professor, Clinical Family Medicine and Clinical Occupational Therapy, and Associate Director, Medical Student Education, Department of Family Medicine, University of Illinois at Chicago..
Emily Keating, Emily Keating, OTD, OTR/L, is Occupational Therapist, Chicago Public Schools, Chicago..
Amy Krischer, Amy Krischer, OTD, OTR/L, is Occupational Therapist and Independent Early Intervention Contractor, Chicago..
Hajwa Kim, Hajwa Kim, MS, is Associate Director, Biostatistics Core, Center for Clinical and Translational Science, University of Illinois at Chicago..
Anders Kottorp, Anders Kottorp, PhD, OT Reg, is Professor and Dean, Health and Society, University of Malmö, Malmö, Sweden..
References
- Baron K., Kielhofner G., Iyenger A., Goldhammer V., & Wolenski J. (2006). The Occupational Self-Assessment (Version 2.2). Chicago: Model of Human Occupation Clearinghouse, Department of Occupational Therapy, College of Applied Health Sciences, University of Illinois at Chicago. [Google Scholar]
- Carpenter C. R., DesPain B., Keeling T. N., Shah M., & Rothenberger M. (2011). The Six-Item Screener and AD8 for the detection of cognitive impairment in geriatric emergency department patients. Annals of Emergency Medicine, 57, 653–661. 10.1016/j.annemergmed.2010.06.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2013). The state of aging and health in America 2013. Atlanta: Author.
- Chhatre S., Jefferson A., Cook R., Meeker C. R., Kim J. H., Hartz K. M., . . . Jayadevappa, R., (2018). Patient-centered recruitment and retention for a randomized controlled study. Trials, 19, 205 10.1186/s13063-018-2578-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm D., Toto P., Raina K., Holm M., & Rogers J. (2014). Evaluating capacity to live independently and safely in the community: Performance Assessment of Self-Care Skills. British Journal of Occupational Therapy, 77, 59–63. 10.4276/030802214X13916969447038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramm J. M., Twisk J., & Nieboer A. P. (2014). Self-management abilities and frailty are important for healthy aging among community-dwelling older people: A cross-sectional study. BMC Geriatrics, 14, 28 10.1186/1471-2318-14-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly C., Brenchley C., Crawford C., & Letts L. (2013). The integration of occupational therapy into primary care: A multiple case study design. BMC Family Practice, 14, 60 10.1186/1471-2296-14-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed C. R., Hansberry S. T., & Arrieta M. I. (2013). Structural and hidden barriers to a local primary health care infrastructure: Autonomy, decisions about primary health care, and the centrality and significance of power. Research in the Sociology of Health Care, 31, 57–81. 10.1108/S0275-4959(2013)0000031006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey J., Connolly D., Boland F., & Smith S. M. (2015). OPTIMAL, an occupational therapy led self-management support programme for people with multimorbidity in primary care: A randomized controlled trial. BMC Family Practice, 16, 59 10.1186/s12875-015-0267-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow R. E., Wagner E. H., Schaefer J., Mahoney L. D., Reid R. J., & Greene S. M. (2005). Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Medical Care, 43, 436–444. 10.1097/01.mlr.0000160375.47920.8c [DOI] [PubMed] [Google Scholar]
- Halle A. D., Mroz T. M., Fogelberg D. J., & Leland N. E. (2018). Occupational therapy and primary care: Updates and trends. American Journal of Occupational Therapy, 72, 7203090010. 10.5014/ajot.2018.723001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays R. D., Bjorner J. B., Revicki D. A., Spritzer K. L., & Cella D. (2009). Development of physical and mental health summary scores from the Patient-Reported Outcomes Measurement Information System (PROMIS) global items. Quality of Life Research, 18, 873–880. 10.1007/s11136-009-9496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M., Ragan B. G., & Park J. H. (2008). Issues in outcomes research: An overview of randomization techniques for clinical trials. Journal of Athletic Training, 43, 215–221. 10.4085/1062-6050-43.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerse N., Boyd M., McLean C., Koziol-McLain J., & Robb G. (2008). The BRIGHT tool. Age and Ageing, 37, 553–588. 10.1093/ageing/afn145 [DOI] [PubMed] [Google Scholar]
- Killian C., Fisher G., & Muir S. (2015). Primary care: A new context for the Scholarship of Practice model. Occupational Therapy in Health Care, 29, 383–396. 10.3109/07380577.2015.1050713 [DOI] [PubMed] [Google Scholar]
- Klijs B., Nusselder W. J., Looman C. W., & Mackenbach J. P. (2011). Contribution of chronic disease to the burden of disability. PLoS One, 6, e25325 10.1371/journal.pone.0025325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koverman B., Royeen L., & Stoykov M. (2017). Occupational therapy in primary care: Structures and processes that support integration. Open Journal of Occupational Therapy, 5 10.15453/2168-6408.1376 [DOI] [Google Scholar]
- Law M., Cooper B., Strong S., Stewart D., Rigby P., & Letts L. (1996). The Person– Environment–Occupation model: A transactive approach to occupational performance. Canadian Journal of Occupational Therapy, 63, 9–23. 10.1177/000841749606300103 [DOI] [PubMed] [Google Scholar]
- Muir S. (2012). Occupational therapy in primary health care: We should be there. American Journal of Occupational Therapy, 66, 506–510. 10.5014/ajot.2012.665001 [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. (2014). Healthy People 2020. Retrieved from https://www.cdc.gov/nchs/healthy_people/hp2020.htm
- Podsiadlo D., & Richardson S. (1991). The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society, 39, 142–148. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- Ralph N. L., Mielenz T. J., Parton H., Flatley A. M., & Thorpe L. E. (2013). Multiple chronic conditions and limitations in activities of daily living in a community-based sample of older adults in New York City, 2009. Preventing Chronic Disease, 10, E199 10.5888/pcd10.130159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben D. B., & Siu A. L. (1990). An objective measure of physical function of elderly outpatients: The Physical Performance Test. Journal of the American Geriatrics Society, 38, 1105–1112. 10.1111/j.1532-5415.1990.tb01373.x [DOI] [PubMed] [Google Scholar]
- Rich E., Lipson D., Libersky J., & Parchman M. (2012). Coordinating care for adults with complex care needs in the patient-centered medical home: Challenges and solutions [White paper]. Rockville, MD: Agency for Healthcare Research and Quality. [DOI] [PMC free article] [PubMed]
- Richardson J., Letts L., Chan D., Officer A., Wojkowski S., Oliver D., . . . Kinzie, S. (2012). Monitoring physical functioning as the sixth vital sign: Evaluating patient and practice engagement in chronic illness care in a primary care setting—A quasi-experimental design. BioMed Central, 13, 29 10.1186/1471-2296-13-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J., Letts L., Chan D., Stratford P., Hand C., Price D., . . . Law, M. (2010). Rehabilitation in a primary care setting for persons with chronic illness: A randomized controlled trial. Primary Health Care Research and Development, 11, 382–395. 10.1017/S1463423610000113 [DOI] [Google Scholar]
- Sanders K. A., Whited A., & Martino S. (2013). Motivational interviewing for patients with chronic kidney disease. Seminars in Dialysis, 26, 175–179. 10.1111/sdi.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford P., Gill C., Westaway M., & Binkley J. (1995). Assessing disability and change on individual patients: A report of a patient specific measure. Physiotherapy Canada, 47, 258–263. [Google Scholar]
- Stuber C. J., & Nelson D. L. (2010). Convergent validity of three occupational self-assessments. Physical and Occupational Therapy in Geriatrics, 28, 13–21. 10.3109/02703180903189260 [DOI] [Google Scholar]
- U.S. Census Bureau. (2018). Older people projected to outnumber children for first time in U.S. history. Retrieved from https://www.census.gov/newsroom/press-releases/2018/cb18-41-population-projections.html
- Vu T.-V., & Mackenzie L. (2012). The interrater and test–retest reliability of the Home Falls and Accidents Screening Tool. Australian Occupational Therapy Journal, 59, 235–242. 10.1111/j.1440-1630.2012.01012.x [DOI] [PubMed] [Google Scholar]
- Wagner E. H. (1998). Chronic disease management: What will it take to improve care for chronic illness? Effective Clinical Practice, 1, 2–4. [PubMed]
- Ward B. W., & Schiller J. S. (2013). Prevalence of multiple chronic conditions among US adults: Estimates from the National Health Interview Survey, 2010. Preventing Chronic Disease, 10, E65 10.5888/pcd10.120203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2008). Training modules and instructions for healthcare providers. Retrieved from https://www.who.int/ceh/capacity/training_modules/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.