Abstract
Individual differences are a conspicuous feature of color vision and arise from many sources, in both the observer and the world. These differences have important practical implications for comparing and correcting perception and performance, and important theoretical implications for understanding the design principles underlying color coding. Color percepts within and between individuals often vary less than the variations in spectral sensitivity might predict. This stability is achieved by a variety of processes that compensate perception for the sensitivity limits of the eye and brain. Yet judgments of color between individuals can also vary widely, and in ways that are not readily explained by differences in sensitivity or the environment. These differences are uncorrelated across different color categories, and could reflect how these categories are learned or represented.
Introduction
Sensory processing varies widely from one individual to the next. These variations are not restricted to clinical deficits or anomalies, and more broadly represent a natural and inherent property of all sensory systems that affect all aspects of coding, from sensitivity to conscious experience. As a result, the notion of a “standard observer” (a widely-used metric in colorimetry [1]) belies the fact that individual differences are the standard, and that an average function characterizes the behavior of few if any actual observers. Studies of individual differences are thus important for describing the distribution of percepts and abilities on different sensory tasks. However, they are also increasingly recognized as an important source of information about the underlying causes for these differences [2–4]. Systematic variation arises from systematic differences in the mechanisms and computations mediating perception, and thus the specific patterns of variation provide clues about the nature and function of these latent processes. Here we briefly review insights from individual differences about how and to what extent the brain forms a consistent representation of color. We highlight two striking features of this representation. First, despite enormous variations both within and across observers, the visual system often maintains a highly stable perceptual experience of color. Second, despite this stability or constancy, some aspects of color experience nevertheless vary markedly across observers. These patterns point in surprising ways to the nature of the perceptual architecture of color appearance.
Sources of variation in color vision
Systematic variability in color vision arises at all levels of the visual system, beginning even before light reaches the receptors. The lens and macular pigments selectively absorb short-wavelength light, and variations in their density strongly bias spectral sensitivity [5,6]. The spectral sensitivities of the cone receptors can also vary because of common polymorphisms in the genes encoding the cone opsins, leading to small but reliable differences in the wavelength of peak sensitivity [7]. Differences in the concentration or optical density of the photopigment also varies the bandwidth of the cone sensitivities [8]. More pronounced genetic alterations of the long- or medium-wavelength photopigments (L or M) underlie common forms of color deficiencies, affecting ~8% of Caucasian males [9]. These can range from alterations of the sensitivities (anomalous trichromacy) to a complete loss of one cone class (dichromacy). Because these genes are coded on the X chromosome, they may potentially also lead to an extra dimension of color vision (tetrachromacy) in some female carriers of color deficiencies [10,11], though the link between the number of photopigments and the dimensionality of color vision is not simple [12].
Together these peripheral factors strongly influence color matches, or which physical spectra lead to equal quantum catches in the cones and are thus indistinguishable or metameric to the observer [5,6]. Consequently, stimuli that look identical to one observer will appear different to another, so that we each live in unique perceptual worlds. Metamer differences across observers may become more pronounced with the introduction of narrow-band light sources in wide gamut lighting and displays [13], prompting interest in developing color profiles for individual observers to try to partially adjust images for these differences. In color research, analogous corrections are routinely applied to adjust for the luminance sensitivity of the individual [14,15]. This sensitivity depends primarily on the combined responses of the L and M cones, though in complex ways [16]. The L:M cone ratio can be measured in vivo with adaptive optics [17,18] and shows a dramatic range of normal variation, from 1:1 to 16:1 [19].
We know much less about the sources and nature of normal variation in postreceptoral color mechanisms. However, it seems likely that neural variability is pronounced at all processing stages, and for example, post-receptoral limits on chromatic sensitivity also vary widely among individuals (e.g. [20,21]). As a result, we each view the world through a unique visual apparatus. Moreover, it is important to emphasize that this physiological variation can be equally dramatic within the individual, across both time and space. The visual system undergoes enormous changes during normal development or aging or with the progression of disease [20,22,23]. Similarly, visual processing varies both quantitatively and qualitatively from the center of gaze to the periphery [24]. Thus even an individual observer “sees” the world through a visual system that is very different at different times and locations.
Stability despite variation
If vision did not adjust for these sensitivity variations, we would each experience color very differently - uniform surfaces would appear with steep color gradients, and the world would seem yellower and lower contrast as we age. Yet sensory systems exhibit a remarkable capacity to compensate for their sensitivity limits in order to maintain a constant or stable representation of physical properties of the world. An example is filling-in of information in the blind spot or scotomas [25]. Similarly, color perception rests on a range of adjustments that correct for the idiosyncratic spectral sensitivity of the observer. Thus the stimulus that appears white or as a particular hue shows little variation with age [26–28], and color percepts across the visual field change much less than predicted by the spatial variations in spectral sensitivity [29]. These constancies are sometimes complete, but not always [30], and how they succeed or fail can provide insights into the limits and mechanisms of neural compensation, and perhaps into fundamental strategies in perceptual processing.
While many of these strategies remain unknown, it is apparent that compensation for variations in the individual involves multiple processes and levels, similar to the many adjustments and heuristics that support color constancy with variations in the stimulus (e.g. allowing stable color of surfaces despite changes in the lighting) [31,32]. One simple mechanism is adaptation to the average stimulus spectrum, which can reciprocally reweight sensitivity to discount a sensitivity bias. If these adjustments are local they could operate across the visual field to maintain a constant white balance in the response [33]. However, the visual system does more than adjust to the mean. Hue percepts remain more consistent between the fovea and near periphery than predicted by simply rescaling the cone sensitivities [34,35], and they may also compensate for the sensitivity biases that are introduced by changing the spectral bandwidth and thus saturation of the stimulus [36]. Adaptation could also adjust the contrast gain of the system, in order to maintain a constant perceptual gamut [37]. Another class of adjustments may involve learning. For example, prominent color percepts could reflect the stimulus properties of the world rather than the physiological processing of the observer. For example, pure blue and yellow lie close to the axis of natural daylight variation and thus have a clear environmental analog [38], while a neural substrate for these special hues has proven elusive [39]. Learning has also been invoked to explain how color percepts could remain invariant across different retinal locations [40].
These types of adjustments could also support “inter-observer” constancy. As long as the stimulus characteristics of the visual environment are more consistent than the physiological characteristics of the observers, then adaptation to – or learning about – a common world should tend to converge observers toward common color experiences. For example, inter-observer variation in achromatic settings is much less than expected from the wide natural range of human spectral sensitivity [33,41]. Another potential illustration is the color experience of anomalous trichromats. Their altered cone sensitivities predict very weakened L vs. M cone responses (roughly reddish-greenish sensations). Yet their perceptual reports of color suggest that in some of these observers the L vs. M signal is amplified so that they may experience visible colors more like normal trichromats, even if their thresholds for detecting color differences are much higher [42]. This amplification is consistent with an adaptation that – like normal trichromats - matches the gain of their neural coding to the same range of color contrasts in their environment [43].
A further line of inquiry pointing to stable inter-observer color experience derives from cross-cultural studies of color naming. Berlin and Kay’s World Color Survey revealed consistent patterns of linguistic color categories across different populations [44]. For example, most languages have a basic color term for “red” that labels a similar region of color space. The link between naming and appearance is complex, and despite strong universal trends there are also clear examples of linguistic relativity in color categories and perceptual performance. As such the interpretation of these naming patterns continues to evolve [45–48]. However, the similarities in color categorization suggest that something – in the world or the brain – is again consistent enough to maintain shared properties across observers in at least some aspects of color perception and communication.
Variation despite stability
While the foregoing emphasizes the potential similarities in color percepts both within and between observers, in other regards measures of color appearance are striking for the dissimilarities they suggest, a point dramatically illustrated by the image of the blue-black or white-gold dress [49–51]. Differences in color appearance could again arise from many factors. For example, the same mechanisms that calibrate different visual systems for the same environment should drive individuals toward divergent percepts when it is the environment that varies [52]. The color statistics of the environment can vary widely (e.g. between lush or arid habitats, or natural or carpentered). Thus any given environment may hold its inhabitants in very different states of adaptation [53]. Notably the range of variation this predicts (though not the specific pattern) is comparable to the range observed in average color naming across different cultures [54]. Even in the same environment, color perception could cycle with the seasons [55], tracking the annual variations in the color characteristics of the world [56].
Differences in color naming are also surprisingly large. In a reanalysis of the World Color Survey, Lindsey and Brown showed that there is often more similarity in the color naming of respondents from two different languages than among speakers of the same language [57]. These patterns suggest that different individuals tend to adopt different strategies or motifs that are themselves universal in that they are deployed across languages. Similarly, within the English language many studies have now documented the reported color percepts of individuals by measuring the unique hues (pure sensations of red, green, blue or yellow). The focal stimuli corresponding to these hues vary widely and consistently across observers [58]. In some cases these variations are predictable from individual differences in spectral sensitivity (e.g. the wavelength that appears unique green has been found to be correlated with both macular pigment density and L:M cone ratio) [59,60]. Such results are important because they would implicate a relatively fixed neural readout for the unique hues, as assumed by conventional color-opponent theory. However, more often it has been difficult to show an association between unique hues and sensitivity. For example, unique yellow settings appear unaffected by very different L:M cone ratios [61], and more generally, the range of variation in each of the hues is inconsistent with the distribution expected from normal variations in spectral sensitivity [62].
A further surprising and important characteristic of hue percepts is that the individual variations across different hues are largely independent [62]. That is, how observers differ in unique red is uncorrelated with the differences in unique yellow. Even more surprising, these differences are also uncorrelated with the intermediate binary hues (e.g. orange or purple) [63]. Thus knowing an individual’s red and yellow settings does not predict their orange setting, even though according to color-opponent theory, orange is encoded only implicitly by the underlying mechanisms representing red-green and blue-yellow sensations. This independence is also not predicted from most peripheral sources of sensitivity variation, which should instead lead to more broadband and thus correlated changes in the different hues [62].
Analyses of such correlations provide a powerful and widely-used tool in the study of individual differences. Measurements that covary (or are independent) are likely to reflect the influence of common (or independent) underlying processes. Statistical approaches such as factor analysis or principal components analysis are designed to extract these processes, and in vision, factor analysis has been applied to a variety of data sets to try to estimate the number and characteristics of the mechanisms mediating different visual tasks [64]. Importantly, many visual judgments are precise enough (yet vary enough across observers) to provide precise quantitative information about these mechanisms and how they differ among the subjects [6].
Recently Emery et al. applied factor analysis to measurements of hue-scaling data, in which different colors are described in terms of their perceived proportion of red vs. green or blue vs. yellow [65,66]. Even though observers were explicitly instructed to decompose their color percepts in terms of these four primaries, the resulting factor pattern instead revealed roughly seven distinct factors, each narrowly tuned to a different region of color space. While the basis for this finding remains uncertain, one possible interpretation is that observers learn to partition or otherwise encode the color plane into many color categories (e.g. the four unique hues and their binary combinations), and that the stimuli encompassing each category are learned or encoded independently. That is, each individual may separately represent the stimuli they classify as yellow or orange or red. A further implication is that while the stimulus for color can be described in a two-dimensional metrical space, the perceptual representation of color may be non-metrical, with different colors coded as qualitatively different categories rather than quantitatively different vectors [66]. Whatever their cause, hue percepts appear remarkably constant within the observer, yet surprisingly different across observers.
Conclusion
The representation of color remains highly stable within the individual despite many factors that bias spectral sensitivity. Some aspects of color percepts may also remain relatively stable across observers, because of physiological or environmental constraints. Yet in other ways color appearance manifests as a private experience, so that, for example, the stimuli for unique hues are unique to the individual. The neural or environmental bases for these variations have yet to be revealed, but may point to fundamental principles in the visual construction of color.
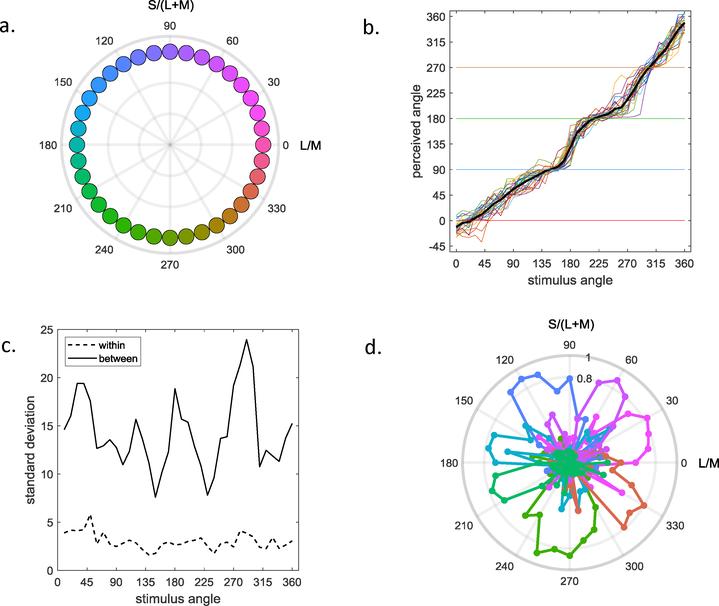
Figure 1.
Individual differences in hue scaling (after [61]). a) 26 observers rated the perceived proportion of red, green, blue, or yellow in 36 stimuli spanning the cone-opponent plane. b) Hue scaling functions derived by converting the RGBY percents into a perceptual angle in a blue-yellow vs red-green plane, as a function of stimulus angle in the cone-opponent plane. Color lines are for for individual observers; black line plots the mean for all observers. c) Standard deviations across trials within an observer (dashed lines), or for the mean settings between observers (solid line). Between-subject differences are consistently larger suggesting they reflect real inter-observer differences in hue scaling rather than measurement noise. d) Factor analysis of the individual differences in the hue scaling functions identified 7 systematic factors, each accounting for the variance over a different narrow range of hues.
Highlights.
Individual differences in color sensitivity and color appearance are large and reliable
Differences in sensitivity often fail to predict differences in appearance
Color perception remains remarkably stable despite sensitivity variations within the observer
Variations across different hues are uncorrelated, suggesting hue categories are learned or encoded independently
Acknowledgments
Supported by NIH EY-010834.
Footnotes
Conflict of Interest None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brainard DH, Stockman A: Colorimetry. In OSA Handbook of Optics. Edited by Bass M; 2010:10–11. [Google Scholar]
- 2.Mollon JD, Bosten JM, Peterzell DH, Webster MA: Individual differences in visual science: What can be learned and what is good experimental practice? Vision Res 2017, 141:4–15.* A review of the advantages and methodological challenges in individual differences research
- 3.Wilmer JB: How to use individual differences to isolate functional organization, biology, and utility of visual functions; with illustrative proposals for stereopsis. Spat Vis 2008, 21:561–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedge C, Powell G, Sumner P: The reliability paradox: Why robust cognitive tasks do not produce reliable individual differences. Behav Res Methods 2018, 50:1166–1186.** An analysis showing that the strength of some cognitive assessments in producing consistent effects is is a weakness for revealing individual differences in cognition
- 5.Asano Y, Fairchild MD, Blonde L: Individual Colorimetric Observer Model. PLoS One 2016, 11:e0145671.* A recent analysis of sources of variation in color matching functions and how these variations can be incorporated into colorimetry
- 6.Webster MA, MacLeod DI: Factors underlying individual differences in the color matches of normal observers. J Opt Soc Am A 1988, 5:1722–1735. [DOI] [PubMed] [Google Scholar]
- 7.Winderickx J, Lindsey DT, Sanocki E, Teller DY, Motulsky AG, Deeb SS: Polymorphism in red photopigment underlies variation in colour matching. Nature 1992, 356:431–433. [DOI] [PubMed] [Google Scholar]
- 8.Neitz J, Neitz M, He JC, Shevell SK: Trichromatic color vision with only two spectrally distinct photopigments. Nat Neurosci 1999, 2:884–888. [DOI] [PubMed] [Google Scholar]
- 9.Neitz J, Neitz M: The genetics of normal and defective color vision. Vision Res 2011, 51:633–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan G, Deeb SS, Bosten JM, Mollon JD: The dimensionality of color vision in carriers of anomalous trichromacy. J Vis 2010, 10:12. [DOI] [PubMed] [Google Scholar]
- 11.Bochko VA, Jameson KA: Investigating Potential Human Tetrachromacy in Individuals with Tetrachromat Genotypes Using Multispectral Techniques. Electronic Imaging 2018:1–12. [Google Scholar]
- 12.Jacobs GH: Photopigments and the dimensionality of animal color vision. Neurosci Biobehav Rev 2018, 86:108–130. [DOI] [PubMed] [Google Scholar]
- 13.David A, Whitehead LA, 19(3), 169–181.: LED-based white light. Comptes Rendus Physique 2018, 19:169–181. [Google Scholar]
- 14.Kaiser PK: Sensation luminance: a new name to distinguish CIE luminance from luminance dependent on an individual’s spectral sensitivity. Vision Res 1988, 28:455–456. [DOI] [PubMed] [Google Scholar]
- 15.Lennie P, Pokorny J, Smith VC: Luminance. J Opt Soc Am A 1993, 10:1283–1293. [DOI] [PubMed] [Google Scholar]
- 16.Stockman A, Henning GB, Anwar S, Starba R, Rider AT: Delayed cone-opponent signals in the luminance pathway. J Vis 2018, 18:6. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, Kurokawa K, Lassoued A, Crowell JA, Miller DT: Classifying cone photoreceptors in the living human eye using their unique phase response to light. Ophthalmic Technologies XXIX. International Society for Optics and Photonics 2019, 10858:10858K. [Google Scholar]
- 18.Roorda A, Duncan JL: AOSLO and Eye Disease. Annual Review of Vision Science 2015, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofer H, Carroll J, Neitz J, Neitz M, Williams DR: Organization of the human trichromatic cone mosaic. J Neurosci 2005, 25:9669–9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werner JS: The Verriest Lecture: Short-wave-sensitive cone pathways across the life span. J Opt Soc Am A Opt Image Sci Vis 2016, 33:A104–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott SL, Werner JS, Webster MA: Individual and age-related variation in chromatic contrast adaptation. J Vis 2012, 12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owsley C: Aging and vision. Vision Res 2011, 51:1610–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braddick O, Atkinson J: Development of human visual function. Vision Res 2011, 51:1588–1609. [DOI] [PubMed] [Google Scholar]
- 24.Rosenholtz R: Capabilities and Limitations of Peripheral Vision. Annu Rev Vis Sci 2016, 2:437–457. [DOI] [PubMed] [Google Scholar]
- 25.Komatsu H: The neural mechanisms of perceptual filling-in. Nat Rev Neurosci 2006, 7:220–231. [DOI] [PubMed] [Google Scholar]
- 26.Schefrin BE, Werner JS: Loci of spectral unique hues throughout the life span. J Opt Soc Am A 1990, 7:305–311. [DOI] [PubMed] [Google Scholar]
- 27.Werner JS, Schefrin BE: Loci of achromatic points throughout the life span. Journal of the Optical Society of America A 1993, 10:1509–1516. [DOI] [PubMed] [Google Scholar]
- 28.Wuerger SM: Colour constancy across the life span: Evidence for compensatory mechanisms. PLoS ONE 2013, 8(5):e63921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyler CW: Peripheral color vision and motion processing. Electronic Imaging 2016:1–5. [Google Scholar]
- 30.Murray IJ, Parry NR, McKeefry DJ: Cone opponency in the near peripheral retina. Vis Neurosci 2006, 23:503–507. [DOI] [PubMed] [Google Scholar]
- 31.Foster DH: Color constancy. Vision Res 2011, 51:674–700. [DOI] [PubMed] [Google Scholar]
- 32.Smithson HE: Sensory, computational and cognitive components of human colour constancy. Philos Trans R Soc Lond B Biol Sci 2005, 360:1329–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webster MA, Leonard D: Adaptation and perceptual norms in color vision. Journal of the Optical Society of America A 2008, 25:2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webster MA, Halen K, Meyers AJ, Winkler P, Werner JS: Colour appearance and compensation in the near periphery. Proceedings of the Royal Society B-Biological Sciences 2010, 277:1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bompas A, Powell G, Sumner P: Systematic biases in adult color perception persist despite lifelong information sufficient to calibrate them. J Vis 2013, 13. [DOI] [PubMed] [Google Scholar]
- 36.O’Neil SF, McDermott KC, Mizokami Y, Werner JS, Crognale MA, Webster MA: Tests of a functional account of the Abney effect. J Opt Soc Am A Opt Image Sci Vis 2012, 29:A165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon M, Legge GE, Fang F, Cheong AM, He S: Adaptive changes in visual cortex following prolonged contrast reduction. J Vis 2009, 9:20 21–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mollon JD: Monge (The Verriest Lecture). Visual Neuroscience 2006, 23:297–309. [DOI] [PubMed] [Google Scholar]
- 39.Forder L, Bosten J, He X, Franklin A: A neural signature of the unique hues. Sci Rep 2017, 7:42364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Regan JK, Noe A: A sensorimotor account of vision and visual consciousness. Behav Brain Sci 2001, 24:939–973; discussion 973–1031. [DOI] [PubMed] [Google Scholar]
- 41.Bosten JM, Beer RD, MacLeod DI: What is white? J Vis 2015, 15:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boehm AE, MacLeod DI, Bosten JM: Compensation for red-green contrast loss in anomalous trichromats. J Vis 2014, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webster MA, Juricevic I, McDermott KC: Simulations of adaptation and color appearance in observers with varying spectral sensitivity. Ophthalmic Physiol Opt 2010, 30:602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Regier T, Kay P, Cook RS: Focal colors are universal after all. Proc Natl Acad Sci U S A 2005, 102:8386–8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park J, Tauber S, Jameson KA, Narens L: The Evolution of Shared Concepts in Changing Populations. . Review of Philosophy and Psychology 2018:1–20.30881528* A recent example of modeling color communication to account for shared systems of color categorization
- 46.Lindsey DT, Brown AM, Brainard DH, Apicella CL: Hunter-Gatherer Color Naming Provides New Insight into the Evolution of Color Terms. Curr Biol 2015, 25:2441–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibson E, Futrell R, Jara-Ettinger J, Mahowald K, Bergen L, Ratnasingam S, Gibson M, Piantadosi ST,Conway BR: Color naming across languages reflects color use. Proc Natl Acad Sci U S A 2017, 114:10785–10790.* Empirical measurements and model accounting for the emergence of different color categories in terms of the color statistics of objects
- 48.Zaslavsky N, Kemp C, Regier T, Tishby NEcicnaie: Efficient compression in color naming and its evolution. Proceedings of the National Academy of Sciences 2018, 115:7937–7942.* Computational model of the evolution of color categories and differences across languages
- 49.Lafer-Sousa R, Hermann KL, Conway BR: Striking individual differences in color perception uncovered by ‘the dress’ photograph. Curr Biol 2015, 25:R545–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gegenfurtner KR, Bloj M, Toscani M: The many colours of ‘the dress’. Curr Biol 2015, 25:R543–544. [DOI] [PubMed] [Google Scholar]
- 51.Winkler AD, Spillmann L, Werner JS, Webster MA: Asymmetries in blue-yellow color perception and in the color of ‘the dress’. Curr Biol 2015, 25:R547–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Webster MA: Visual adaptation. Annual Review of Vision Science 2015, 1:547–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Webster MA, Mollon JD: Adaptation and the color statistics of natural images. Vision Research 1997, 37:3283–3298. [DOI] [PubMed] [Google Scholar]
- 54.Webster MA: Probing the functions of contextual modulation by adapting images rather than observers. Vision Res 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welbourne LE, Morland AB, Wade AR: Human colour perception changes between seasons. Curr Biol 2015, 25:R646–647. [DOI] [PubMed] [Google Scholar]
- 56.Webster MA, Mizokami Y, Webster SM: Seasonal variations in the color statistics of natural images. Network 2007, 18:213–233. [DOI] [PubMed] [Google Scholar]
- 57.Lindsey DT, Brown AM: World Color Survey color naming reveals universal motifs and their within-language diversity. Proc Natl Acad Sci U S A 2009, 106:19785–19790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuehni RG: Variability in unique hue selection: a surprising phenomenon. Color Research and Application 2004, 29:158–162. [Google Scholar]
- 59.Schmidt BP, Neitz M, Neitz J: Neurobiological hypothesis of color appearance and hue perception. Journal of the Optical Society of America A 2014, 31:A195–A207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Welbourne LE, Thompson PG, Wade AR, Morland AB: The distribution of unique green wavelengths and its relationship to macular pigment density. J Vis 2013, 13. [DOI] [PubMed] [Google Scholar]
- 61.Brainard DH, Roorda A, Yamauchi Y, Calderone JB, Metha A, Neitz M, Neitz J, Williams DR, Jacobs GH: Functional consequences of the relative numbers of L and M cones. J Opt Soc Am A Opt Image Sci Vis 2000, 17:607–614. [DOI] [PubMed] [Google Scholar]
- 62.Webster MA, Miyahara E, Malkoc G, Raker VE: Variations in normal color vision. II. Unique hues. J Opt Soc Am A Opt Image Sci Vis 2000, 17:1545–1555. [DOI] [PubMed] [Google Scholar]
- 63.Malkoc G, Kay P, Webster MA: Variations in normal color vision. IV. Binary hues and hue scaling. J Opt Soc Am A Opt Image Sci Vis 2005, 22:2154–2168. [DOI] [PubMed] [Google Scholar]
- 64.Peterzell DH: Discovering sensory processes using individual differences: A review and factor analytic manifesto. Electronic Imaging 2016, 2016:1–11.* A review of applications of factor analysis for characterizing visual mechanisms
- 65.Emery KJ, Volbrecht VJ, Peterzell DH, Webster MA: Variations in normal color vision. VII. Relationships between color naming and hue scaling. Vision Res 2017, 141:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Emery KJ, Volbrecht VJ, Peterzell DH, Webster MA: Variations in normal color vision. VI. Factors underlying individual differences in hue scaling and their implications for models of color appearance. Vision Res 2017, 141:51–65.* Analysis of individual differences in color appearance suggesting that hue is encoded in terms of narrowly tuned categories that vary independently of each other.