Abstract
Antibodies produced in response to a foreign antigen are characterized by polyclonality, not only in the diverse epitopes to which their variable domains bind, but also with respect to the various effector molecules to which their constant regions engage. Thus, the antibody’s Fc domain mediates diverse effector activities by engaging two distinct classes of Fc-receptors (Type-I and Type-II FcRs) based on the two dominant conformational states that the Fc domain may adopt. These conformational states are regulated by the amino acid differences among antibody subclasses and by the complex, biantennary Fc-associated N-linked glycan. The diverse downstream pro-inflammatory, anti-inflammatory, and immunomodulatory consequences of Type-I and Type-II FcR engagement are discussed in the context of infectious, autoimmune, and neoplastic disorders.
Antibodies are the key components linking the innate and adaptive branches of immunity and are normally produced in response to a foreign antigen to mediate host protection. A defining characteristic of the antibody response is its polyclonality; antibodies have the capacity to target a seemingly limitless array of antigens through almost unlimited diversification of their Fab domains by somatic recombination and mutation. However, it is becoming increasingly clear that polyclonality of the antibody response applies as well to the effector molecules that are engaged by the antibody-antigen complex. Thus, while Fab-antigen interactions are crucial to the specificity of the antibody response, there is a crucial role for the Fc domain in mediating the diverse effector properties triggered by antigen recognition, even for processes attributed solely to Fab recognition, like toxin and virus neutralization1, 2. Specific interactions of the IgG Fc domain with distinct receptors expressed by diverse leukocyte cell types result in pleiotropic IgG effector functions, including the clearance of pathogens and toxins, lysis and removal of infected or malignant cells, modulation of the innate and adaptive branches of immunity to shape an immune response, or initiation of anti-inflammatory pathways that actively suppress immunity3, 4.
As with every aspect of the immune system, multiple layers of regulation exist to finely tune the interaction of an IgG Fc domain with its cognate receptors to eliminate any potential for self-destructive autoimmunity and uncontrolled inflammation. More specifically, the capacity of an IgG molecule to engage the various Fc-receptors (FcRs) is a dynamic and tightly-regulated process that is primarily controlled by the intrinsic structure and heterogeneity of the Fc domain of IgG. While traditionally considered to be the invariant region of an IgG molecule, the Fc domain displays considerable heterogeneity, arising from the amino acid differences among the four human subclasses (IgG1, IgG2, IgG3 and IgG4) and their Gm allotypes and from the complex, biantennary N-linked glycan attached at Asn 297; a site conserved in all the different IgG subclasses5 and species examined to date. The net result of this Fc heterogeneity translates into hundreds of different Fc structures that can associate with any one variable region. Ultimately, this substantial structural heterogeneity of the Fc domain allows for the extrinsic modulation of Fc conformation resulting in selective engagement of particular classes of FcRs with distinct effector activities. Thus, for any single Fab a diversity of Fc structures is possible, resulting in distinct effector responses for any given antigen binding activity.
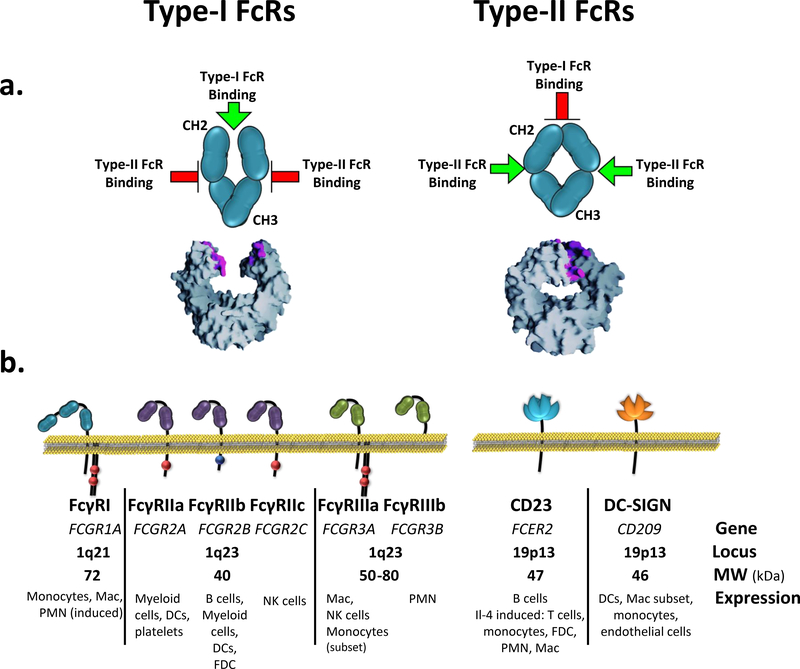
Based on the two dominant conformational states that the Fc domain can adopt two structurally distinct sets of IgG FcRs are now recognized, with selective capacities to engage each of these conformational states. Type-I Fc-receptors (Type-I FcRs) belong to the Ig receptor superfamily (IgSF) and are represented by the canonical FcγRs, including the activating FcRs (FcγRI, FcγRIIa/c, and FcγRIIIa/b) and the inhibitory FcR (FcγRIIb). Each of these receptors bind Fc domains in the open conformation near the hinge-proximal region6 (Fig. 1) in a 1:1 complex. On the other hand, Type-II Fc receptors (Type-II FcRs), represented by the family of C-type lectin receptors (CLRs), including DC-SIGN (homologous to SIGN-R1 in mice) and CD23, specifically bind Fc domains in the closed conformation at the CH2-CH3 interface7 in a 2:1 complex. Given the capacity of each receptor family to initiate distinct effector and immunomodulatory pathways, the conformational diversity of the IgG Fc domain serves as a general strategy to shift receptor specificity in order to actively affect different immunological outcomes.
Figure 1. Reciprocal engagement of Type-I and Type-II receptors by IgG Fc domain.
a) The Fc domain alternates between open and closed conformations depending on the sialylation status of the Fc glycan. Non-sialylated Fc adopts an open conformation capable of binding Type-I receptors near the hinge-proximal surface, whereas the binding site for Type-II receptors remains inaccessible. Upon sialic acid conjugation, the Fc acquires a closed conformation that occludes the Type-I receptor binding site and reveals a binding site for Type-II receptors. (PDB file:3AVE, for non-sialylated Fc structure). b) Schematic and expression profile of the human Type-I and Type-II receptors known to bind the Fc domain of IgG. Type-I and Type-II receptors form part of the Ig-family and C-type lectin receptors, respectively. Mac, macrophage; PMN, polymorphonuclear leukocytes; NK, natural killer; FDC, follicular dendritic cell; DC, dendritic cell.
The well-known roles of Type-I FcRs in mediating antibody-triggered inflammation, as seen, for example, in autoimmune diseases have been extensively reviewed8, 9, 10, 11, 12, 13. In this review, we discuss new observations regarding the structural heterogeneity of the IgG Fc domain that highlight the intrinsic flexibility of this domain to adapt distinct conformational states (open or closed) with preferential binding to either Type-I and Type-II FcRs. The functional consequences of the Fc domain’s interaction with these two types of IgG FcRs as well as the molecular events that are initiated upon receptor engagement are also discussed in the context of recent studies of infectious, autoimmune, and neoplastic disorders. Defining the molecular and structural determinants that govern the selection of Fc-mediated effector pathways will provide greater understanding of how antibodies regulate an immune response, as well as inform on the development of therapeutic antibodies with specific effector properties.
IgG Fc domain structural heterogeneity regulates interactions with Type-I and Type-II FcRs
Structural determinants of IgG
IgG molecules consist of two identical pairs of polypeptide chains; each polypeptide chain is organized into modular units of approximately 110 amino acids characterized by genetically variable (V) or constant (C) domain sequences. These domains adopt a common tertiary structural motif, the immunoglobulin fold (Ig-fold), which is defined by antiparallel β-sheets stabilized by a disulfide bond and hydrophobic core interactions14. Within the Fab domain, the Ig-fold serves as a robust protein scaffold to accommodate highly variable loop regions that link the strands of the β-sheets. Antigen recognition, as determined by crystal structures of antigen-antibody complexes, involves contacts with the complementarity-determining regions (CDRs) formed by these hyper-variable loops within the VLight and VHeavy domains15. Variation in the amino acid sequence of CDRs functions as the structural basis for the broad repertoire of antigen-specific antibodies by providing a heterogeneous protein-binding surface capable of interacting with diverse ligands.
In contrast to the observed sequence variability of the Fab fragment, the C-terminal constant domains of both heavy chains (CH2 and CH3) form the Fc fragment, which assumes a familiar horseshoe-like topology where both CH3 domains stay tightly associated and the CH2 domains remain further apart. In the cleft between the CH2 domains lies an Fc-conjugated carbohydrate structure that shields the hydrophobic core of the Fc from solvent (Fig. 2a). Despite a nomenclature that traditionally emphasizes the invariant nature of the Fc, careful analysis of structural and functional data reveals that the Fc domain also displays considerable heterogeneity arising, in part, from differences in amino acid sequences among multiple IgG subclasses16, heterogeneity in the glycan composition, as well as through highly dynamic regions encompassing the hinge proximal surface and the flexible loops within the Ig-fold of the CH2 domains17, 18.
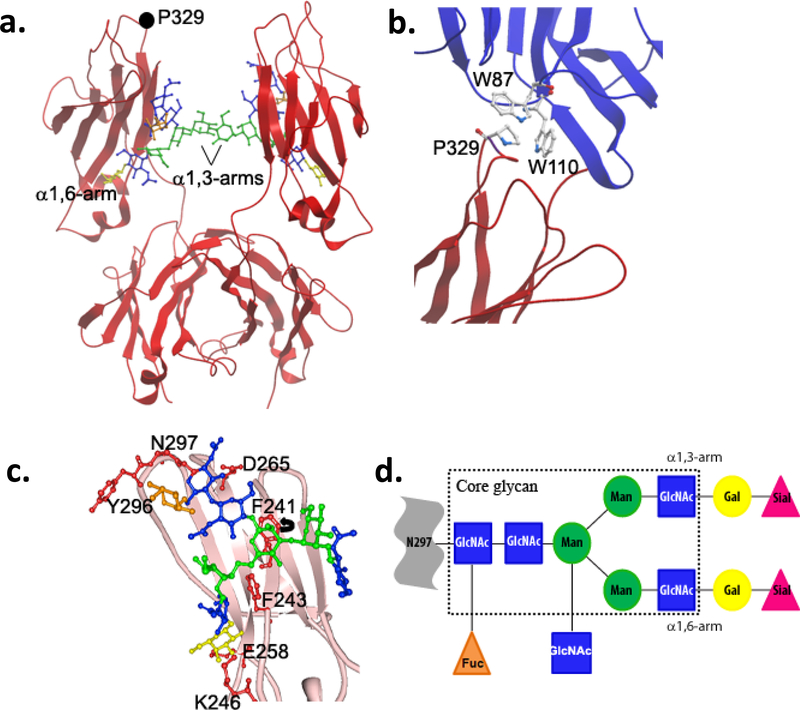
Figure 2. Glycan-dependent modulation of Fc structure.
a) Crystal structure of Fc fragment of human IgG1 (PDB 1H3Y) depicting the orientation of the glycan arms with respect to the CH2 domains of the two heavy chains. The interaction between the α1,3-arms maintains the Fc in the appropriate conformation for FcγR binding. b) The proline sandwich configuration represents a key contact point between P329 on the Fc (red) and 2 tryptophan residues on FcγRIII (blue; PBD 1E4K). c) Side-chain interactions of glycan residues with their respective amino acids. Individual glycan:amino acid interactions are indicated in red. The ring structure of F241 rotates ~90° in the structure of a sialylated Fc. d) Schematic view of the principal glycosylation structures attached to Asn 297 of the Fc. The core glycan structure within the rectangle shows the invariable heptasaccharide group conjugated to the Fc. Sugars beyond the core are attached with varying frequencies. GlcNAc, N-Acetylglucosamine; Fuc, Fucose; Man, Mannose; Gal, Galactose; Sial, N-Acetylneuraminic Acid (sialic acid).
The hinge proximal region of the Fc serves as a consensus binding site for Type-I FcRs19, 20. Crystallographic analysis of several IgG1 Fc structures consistently shows the hinge proximal region in a disordered state, though with relatively minor variability in overall CH2 domain orientation. However, rather than lacking defined structure, the hinge proximal region and the flexible loops involved in receptor binding likely alternate between multiple conformational states with certain conformers preferentially interacting with individual ligands. In support of this model, Davies et al. recently solved two high-resolution structures of human IgG4 Fc, which, when compared to previous high-resolution structures of human IgG1 Fc, reveal unique conformational folds of the flexible loops of the CH2 domain21. More specifically, the FG-loop in IgG4 flips away from the CH2 domain to prevent Pro 329 from intercalating between Trp residues in Type-I FcRs in the so-called “proline sandwich” configuration necessary for receptor binding20. Such conformational differences correspond to the specialized effector functions attributed to both IgG subclasses with IgG1 demonstrating greater ADCC activity over IgG4 as a result of enhanced binding affinity for activating Type-I FcRs22.
Fc Glycosylation
In recent years, several studies have demonstrated that differential glycosylation of the Fc fragment modulates the effector function of IgG23, 24, 25, establishing the Fc-associated glycan as a critical regulatory determinant for antibody activity. The complex, bi-antennary Fc glycan, which consists of a core heptasaccharide structure conjugated to a highly-conserved glycosylation site at Asn 297, shows considerable heterogeneity in sugar composition due to the variable addition of fucose, galactose, bisecting N-acetylglucosamine, or sialic acid (Fig. 2a). Interestingly, the glycoform profile of serum IgG varies predictably between health and disease states with autoantibodies produced during autoimmune or inflammatory reactions preferentially lacking galactose and sialic acid residues relative to bulk antibodies present in the steady state23, 26, 27. These differences are observed in both mouse models of autoimmunity, such as K/BxN and MRL/lpr strains that develop rheumatoid arthritis-like disease, as well as patient cohorts with rheumatoid arthritis, Crohn’s disease28, systemic lupus erythematosus29, and other conditions. Differential Fc-glycosylation results in altered binding affinity towards FcγRs and complement factors, which ultimately influences the effector pathways elicited by the Fc domain. For example, fucosylation and sialylation represent two extensively investigated Fc-glycan modifications examined to date which reduce affinity specifically for FcγRIIIa30 or all Type-I FcRs23, respectively. Sialylation results in increased conformational flexibility of the CH2 domain such that the conformational states that preferentially engage Type-II receptors are more frequently sampled in the population (Ahmed A, Giddens J, Wang LX, Bjorkman P; personal communication).
Binding to any Type-I or Type-II FcR requires, at minimum, the presence of the core glycan group on the Fc despite crystallographic analysis demonstrating that the glycan does not directly interact with receptors20. This suggests that the glycan modulates receptor binding affinity by determining the conformational state of the Fc. In support of this view, crystal structures of aglycosylated Fc fragments typically resolve the Fc in a “closed” conformation, where the CH2 domains collapse together to occlude the Type-I FcR binding site31. Consequently, aglycosylated antibodies fail to bind Type-I FcRs and lack any effector function in vivo32. Thus, a key role for the Fc glycan is to stabilize the Fc in an, “open” conformation, accessible to Type-I FcR interactions. Due to the bi-antennary construction of the glycan, one branch (called the α1,6-arm) folds along the protein backbone of the CH2 domain making several non-covalent contacts with amino acid side chains; whereas, the second branch (the α1,3-arm) extends into the cavity between the CH2 domains where it interacts with the other α1,3-arm conjugated to the opposite heavy chain (Fig. 2b). By occupying this space, the α1,3-arms limit the inter-domain flexibility of the CH2 domains to maintain the Fc in the appropriate “open” conformation for binding Type-I FcRs.
Crystal structures of Fc fragments yield carbohydrate branches with well-defined electron density suggesting that protein-sugar interactions significantly restrain mobility of the Fc glycan. However, NMR analysis indicates that the glycan arms exhibit greater motion than previously assumed33. This suggests that modifying the Fc glycan with various sugar moieties may not only alter protein-glycan interactions, but also influence glycan mobility as a means of fine-tuning Fc conformation. For example, addition of terminal galactose to the α1,6-arm more firmly anchored this branch to the Fc protein backbone34, though an unbound state also exists33. In contrast, the α1,3-arm remains highly dynamic with the addition of sialic acid further increasing branch mobility. Such glycan dynamics may be a critical parameter because the position of the glycan arms may determine the spacing between the CH2 domains and receptor specificity.
Intrinsic Flexibility of the Fc Domain
Several groups have demonstrated that sialylation of the Fc glycan switches the in vivo activity of IgG23, 35, 36, 37. Whereas non-sialylated IgG stimulates pro-inflammatory pathways upon engagement of activating Type-I FcRs, sialylated IgG suppresses inflammation associated with autoimmunity. We found that this change in antibody effector properties coincides with a change in receptor binding affinity, where sialylated Fc preferentially binds Type-II FcRs, such as DC-SIGN and CD23, but not Type-I FcRs7, 36. Though initially surprising, it now appears as if the reciprocal engagement of disparate classes of receptors is a general property of antibodies. For example, like IgG, IgE also binds to Type-I (FcεRI) and Type-II FcRs (CD23) resulting in either inflammatory or immunosuppressive responses, respectively. This has been attributed to the intrinsic flexibility of the Cε3 domain on the Fc fragment of IgE38, which in co-crystal structures complexed to CD23 or FcεRI reveals two mutually exclusive Fc conformations: a closed (CD23-bound) or an open (FcεRI-bound) one25, 39. The CH2 domain of IgG lacks the intrinsic flexibility of the Cε3 domain of IgE in the absence of sialylation. However, upon sialic acid attachment, we observe structural perturbations in the Fc associated with the sialylated Fc fragment acquiring greater flexibility compared to the non-sialylated Fc7. Molecular models that simulate sialylated Fc binding to DC-SIGN predict that the Fc may adopt a closed conformation as a result of the sialylated α1,3-arms moving out of the internal cavity. Consistent with this model, Ahmed et al. recently solved a crystal structure of a fully sialylated Fc preparation in which the Fc in the crystal is found in both “open” and “closed” states (Ahmed A, Giddens J, Wang LX, Bjorkman P; personal communication). Though the α1,3-arms are poorly resolved in this structure, an intriguing finding is that the aromatic ring of Phe 241, which normally forms a hydrophobic stacking interaction with a mannose residue at the base of the α1,3-arm, rotates away from the glycan (Fig. 2c). Potentially, the rotation of Phe 241 severs its interaction with the glycan and provides a structural link to the greater motion of α1,3-arm measured by NMR and predicted by molecular modeling.
Fc glycosylation in disease
Regulatory mechanisms controlling Fc glycan composition are not well understood. With respect to sialylation of the Fc glycan, numerous studies in humans demonstrate findings consistent with strict control of ST6Gal1, the glycosyltransferase responsible for terminal Fc glycan sialylation. For example, modulation in sialylated Fc abundance on antigen-specific IgGs following immunization suggests active regulation of ST6Gal1 over the course of a vaccine response40. Further, patients with rheumatoid arthritis or Wegener’s granulomatosis are observed to have specific modulations in abundance of sialylation on antigen-specific IgG Fcs that correspond with the severity of clinical disease; anti-citrullinated protein antibodies (ACPA) in rheumatoid arthritis and anti-proteinase 3 (PR3) antibodies in Wegener’s granulomatosis have been observed to decrease in abundance of sialylated Fc during disease flares, whereas sialylation is elevated during periods of clinical remission27, 41. Whether low sialylation on Fc glycans of ACPA or PR3 antibodies plays a role in the pathogenesis of either disease remains to be determined. These and other observations in the literature suggest that precise regulation of ST6Gal1 activity (and likely other glycosyltransferases that act on the Fc glycan) is a fundamental homeostatic process; this remains an open area for investigation.
Immunomodulatory function of Type-I and Type-II FcRs
Upon exposure to antigens, specific IgGs in the peripheral repertoire or generated early in the antibody response result in the formation of immune complexes, which, in turn, depending on their Fc conformations, interact either with Type-I or Type-II FcRs on effector cells and on B cells to modulate both humoral and innate immune processes. Balanced positive and negative signaling through Type-I and Type-II FcRs are essential for the development of appropriate immune responses to soluble protein antigens or microorganisms.
As described above, the specific composition of IgG subclasses and Fc glycans within immune complexes dictates the degree to which Type-I or Type-II FcRs are engaged. The IgG subclass distribution is regulated by both the cytokine milieu and the biochemical nature of the antigen. Determinants of Fc glycan composition on IgGs elicited during an active immune response are not well understood, but data suggest that antigen exposure can induce glycan modifications on antigen-specific IgGs and that those modifications may have a role in shaping an ongoing antibody response37, 40, 42. Profound immunomodulation through immune complex signaling is seen in the regulation of vaccine responses, antigen presentation by dendritic cells (DCs), B cell selection, and in the exacerbation of infection by some pathogens; we will consider each of these processes separately.
Vaccine responses
Type-I FcR-dependent immunomodulation through vaccination with immune complexes can affect resulting IgG titer and/or avidity for antigen. Activating Type-I FcRs on DCs, follicular dendritic cells (FDCs), and macrophages are most relevant in adaptive immune responses, and bone marrow chimera experiments have shown that DCs and macrophages have the greatest contribution to priming antibody responses43. Type-I FcR expression critically regulates T cell responses generated by immune complex-primed DCs by influenxing DC maturation and antigen presentation44. Signaling through the FcR γ-chain ITAM motifs matures DCs and upregulates MHC and co-stimulatory molecules, a process required for their function as antigen presenting cells (APCs). Immune complexes have also been demonstrated to polarize macrophages towards the M2b phenotype, which have enhanced antigen presentation activity through increased expression of co-stimulatory molecules45 (Fig. 3a). Signaling through the inhibitory FcγRIIb ITIM pathway regulates IgG-dependent maturation of both DCs and macrophages; FcγRIIb-mediated inhibition can be attenuated by the presence of Toll-like receptor (TLR) signaling in conjunction with activating Type-I FcR signaling. Importantly, DCs that do not receive FcγRIIb inhibitory signaling undergo spontaneous maturation, demonstrating the critical function of this receptor in regulating immune activation44,46, 47. A level of control over immune cell activation may also occur through inhibitory ITAM signaling, induced by low-valency targeting of activating Type-I FcRs48.
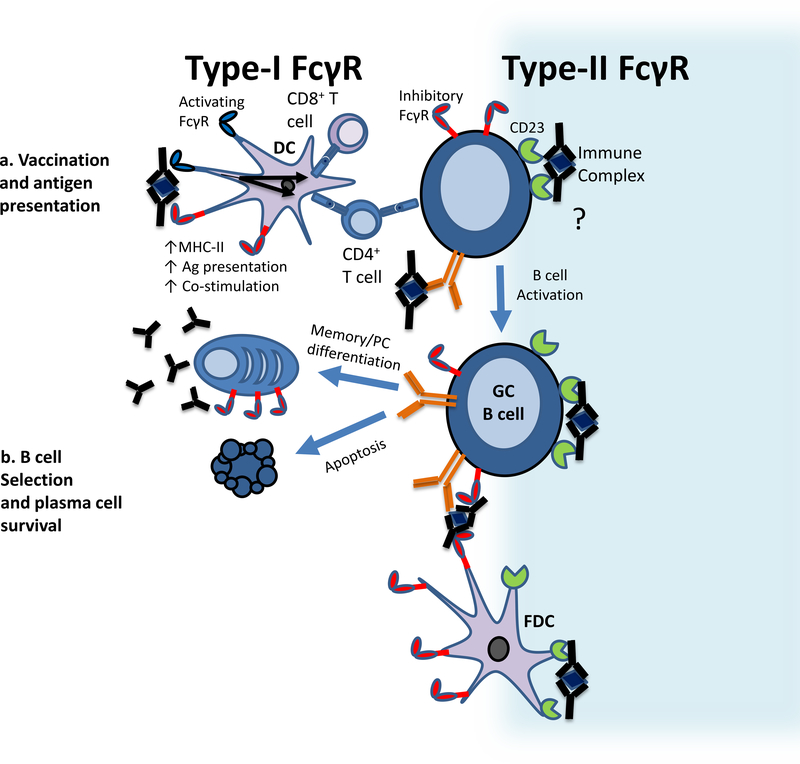
Figure 3. Immunomodulatory functions of Type-I and Type-II FcRs.
a) Antigen presentation. Antibodies bind to soluble antigens to form immune complexes. Recognition of immune complexes by Type-I activating FcyRs expressed on DCs results in the engulfment of the complex by endocytosis and DC maturation, including the upregulation of MHC costimulatory molecules. The internalized antigens are processed and presented on MHC class I and MHC class II molecules to CD4+ and CD8+ T cells, resulting in their activation to mediate specific effector functions. b) B cell selection. Selection of B cells with high affinity B cell receptors occurs in the germinal center. FDCs express the inhibitory receptor FcyRIIB, which binds immune complexes and presents them to germinal center B cells. The Type-I Fc-receptor FcyRIIB and the Type-II Fc-receptor CD23 are also expressed on the cell surface of GC B cells, facilitating immune complex binding. Preferential antigen binding by the B cell receptor results in the positive selection of the respective B cells, enabling them to further differentiate into antibody producing plasma cells or memory B cells. By contrast, exclusive binding of the presented immune complex via the inhibitory FcyRIIB leads to the induction of apoptosis, thereby setting a threshold for the selection of B cells with high affinity B cell receptors. Further, since sialylated immune complexes may also interact with CD23 on GC B cells, regulation of Fc glycosylation may provide a means to modulate cell activation and/or affinity maturation in an as-yet uncharacterized novel pathway.
Antigen presentation
A well-defined consequence of activating Type-I FcR engagement is uptake of immune complexes by endocytosis or phagocytosis49, 50. DCs internalize immune complexes through Type-I FcR-mediated pathways and efficiently process and present the antigen on both MHC class I and MHC class II molecule in a process central to induction of adaptive cellular immune responses (Fig. 3a). Activation of DCs and subsequent priming of both CD4+ and CD8+ T cell-mediated immune responses are significantly enhanced when antigen is internalized as immune complexes through activating Type-I FcRs51, 52, 53. Signaling in cis through the inhibitory FcγRIIb on DCs negatively regulates antigen presentation, as DCs derived from FcγRIIb-deficient mice are more potent inducers of T cell activation both in vitro and in vivo44, 54. Other innate effector cell types also demonstrate increased uptake of antigens that are present as immune complexes. Type-I activating FcγR pathways on granulocytes, monocytes, and macrophages triggers degradation of antigens in lysosomal compartments and production of pro-inflammatory chemokines and cytokines45, 55. This activity, as with DCs, is moderated by FcγRIIb signaling. It is important to note that macrophage and DC subsets can differ in FcR expression patterns, a topic that has recently been reviewed56.
B cell selection
B cells express a single Type-I FcR throughout development, FcγRIIb, while the Type-II FcR, CD23, is also expressed at variable levels during B cell maturation57, 58. FcγRIIB on B cells is a key regulator of affinity maturation and B cell repertoire. Exclusive engagement of FcγRIIB on B cells is pro-apoptotic; however, ligation by immune complexes of FcγRIIB in conjunction with the B cell receptor results in attenuation of apoptotic signaling in a process mediated by SHIP59. In this way, B cells with higher affinity for antigen are selected for survival, while those with receptors of irrelevant specificity or low affinity are more likely to undergo apoptosis.
In the germinal center, somatically mutated B cells with receptors of higher affinity for antigen are selected for against immune complexes retained on a specialized stromal cell type, the FDCs (Fig. 3b). As with B cells, FDCs express FcγRIIB and complement receptors, both of which are involved in retention of immune complexes. This has been shown in studies of mice with selective deficiency of FcγRIIB on either FDCs or B cells; mice with a selective FcγRIIB deficiency on FDCs generate higher avidity antibody responses, likely resulting from the lack of competition for B cell FcγRIIB binding60, 61. In contrast, when only B cells lack FcγRIIB expression, mice generate antibodies of lower avidity, presumably due to an absence of B cell selection based on specificity/avidity of the receptor62. Since sialylated immune complexes may also interact with CD237 on GC B cells, it is likely that regulation of Fc glycosylation provides a means to modulate cell activation and/or affinity maturation in a novel pathway that remains to be characterized (Fig. 3b).
Plasma cells express little or no B cell receptor, and display elevated levels of FcγRIIB; they are therefore subject to unopposed pro-apoptotic FcγRIIB signaling, which is likely to be involved in the homeostasis of long-lived plasma cells. During an ongoing immune response, immune complexes can induce FcγRIIB-dependent apoptosis in existing long-lived plasma cells, suggesting a possible mechanism for maintaining a reservoir of relatively consistent size that allows for addition of new cells with different antibody specificities63, 64.
Antibody-dependent enhancement of infection and/or disease
A long appreciated, yet not fully understood immunomodulatory property of Type-I FcRs is their occasional ability to mediate enhanced infection and/or clinical disease. Perhaps the best studied example of antibody-dependent enhancement (ADE) is secondary dengue infection, which represents a case of both increased viral infection and associated sequelae: while primary dengue infection in humans is often asymptomatic or mild in presentation, subsequent infection with a distinct dengue strain can cause exponentially higher viral titers along with severe and even fatal disease65, 66. This enhanced disease phenotype during secondary infection is thought to be caused by low avidity, cross-reactive, non-neutralizing antibodies generated during the primary infection that mediate increased infection of monocytes and macrophages through Type-I FcRs (Fig. 4a). Elevated virus replication in these cells results in a massive release of cytokines that can cause gastrointestinal hemorrhage and vascular leakage resulting in the clinical phenotypes of dengue hemorrhagic fever or shock syndrome67.
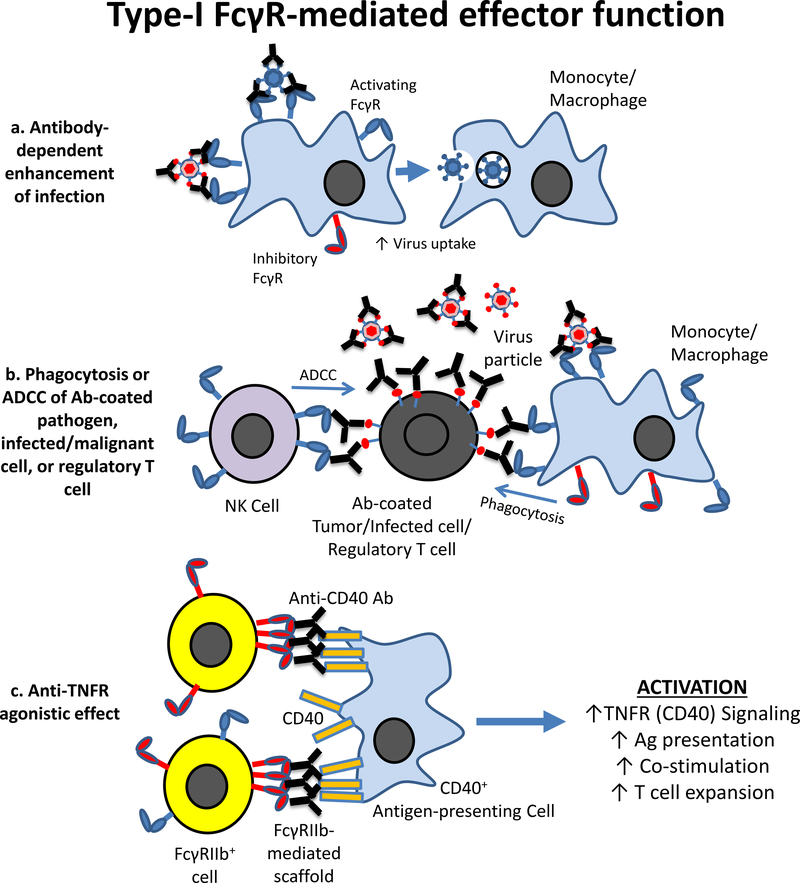
Figure 4. Type-I FcR-mediated effector functions.
a) Antibody-dependent enhancement of infection and/or disease. Pre-existing sub-neutralizing antibodies present from a primary infection (e.g., dengue) bind to viral particles during a secondary infection with a different viral serotype. These immune complexes are bound by Type-I activating FcγR expressed on monocytes and macrophages, mediating increased virus uptake and replication that results in the enhancement of infection. b) Clearance/cytotoxicity of antibody-coated pathogen, infected/neoplastic cells, or regulatory T cells. Antibody-opsonized pathogens interact with Type-I activating FcγRs on monocytes/macrophages, leading to phagocytosis and clearance. Antibody-coated malignant, virally-infected, or regulatory T cells also engage monocytes/macrophages or NK cells, resulting in phagocytosis or cell-mediated cytotoxicity (ADCC) of the target cell. Removal of infected/tumor cells leads to clearance of disease, while removal of regulatory T cells leads to enhanced cellular immunity. c) Anti-TNFR family agonistic effect requires Type-I FcγRIIb expression. Anti-TNFR family antibody (e.g., anti-CD40) binds CD40 on antigen-presenting cells. FcγRIIb+ cells bind CD40-bound antibody in trans, thereby acting as a scaffold to provide the clustering of TNFR molecules on the membrane to mimic the effect of multimeric ligand engagement and activate TNFR-related pathways (CD40 signaling and cellular activation).
An example of ADE of infection may have been present in the recent STEP HIV vaccine trial in which pre-existing antibody against the adenovirus vector used to deliver HIV antigens correlated significantly with increased risk of HIV infection68. Examples of ADE of infectious disease, not secondary to increased infection/replication, have occurred during outbreaks of RSV or measles in humans previously vaccinated with formalin-inactivated viral proteins and in some severe pandemic influenza virus infections. Disease enhancement in these situations was thought to occur when immune complexes formed from non-neutralizing IgGs deposited in tissues, or formed with viral antigens expressed on host cells resulting in cytotoxicity and/or complement deposition and inflammation69, 70, 71.
Why some microbes cause enhanced infection and/or clinical disease through antibody opsonization is not well understood. Adaptation to productive replication in monocytes or macrophages may be one determinant of cytokine-associated disease enhancement. A second determinant might be antigenic variability due to multiple serotypes, as with dengue viruses, or due to continuous selection of novel variants, as with influenza viruses; such antigenic variability predisposes the host to generation of cross-reactive, non-neutralizing antibodies that may mediate enhanced uptake and infection through FcRs or, in rare circumstances, may form insoluble immune complexes that cause disease secondary to Type-I FcR engagement and inflammation or direct cytotoxicity.
Type-I Fc-receptors regulate the function of anti-viral, anti-tumor, and immunomodulatory antibodies in vivo
Type-I FcRs play crucial roles during humoral immune responses against foreign pathogens or epitopes expressed by malignant cells during tumor immunity. IgG antibodies serve to bridge the target, which recognized by the antibody’s Fab region, and the activating Type-I FcRs that are expressed by NK cells, monocytes, macrophages, and other innate immune effector cells that mediate the antibody’s activity. Upon activating Type-I FcR crosslinking and ITAM phosphorylation, effector cells mediate cytotoxicity through the release of cytokines, or are activated to induce phagocytosis of the opsonized target3. Here, we discuss the roles of Type-I FcRs during the effects carried out by anti-viral, anti-tumor, and immunomodulatory antibodies in vivo.
Type-I FcRs during immunity against infectious disease
The important function that Type-I activating FcRs play during immune responses is exemplified by antibodies that engage antigens expressed by various infectious agents. While in some instances results from in vitro viral neutralization assays correlate with in vivo protection by a neutralizing antibody, other times the in vivo protective capabilities of an antibody are significantly greater than their in vitro effects. This disparity can be explained by the protective contributions FcR-expressing effector cells. Thus, in influenza, interactions between anti-HA antibodies and activating Type-I FcRs have been implicated during in vivo protection from influenza virus infections by antibodies targeting antigens including the viral M2 protein72 or HA2, 73. While dispensible for in vitro neutralization, Fc-FcR interactions are required for in vivo protection mediated by broadly-neutralizing anti-influenza antibodies directed at the HA stalk domain2. In vivo protection required Fc-FcR interactions after viral entry into target cells, suggesting that effector cells mediated ADCC of infected cells expressing HA on their surface. Similarly, anti-HIV neutralizing antibodies mutated to abrogate Type-I FcR engagement showed an impaired ability to protect macaques from SHIV infection74, suggesting that HIV antibodies also recruit FcR-mediated effector function to mediate their activity in vivo. Further, in vivo protection from Bacillus anthracis infection by anti-protective antigen antibodies absolutely requires activating Type-I FcR engagement1, 75. Anti-pathogen antibodies can even be Fc-engineered to enhance interactions with activating Type-I FcRs to augment their protective abilities in vivo1, 2. In each of these models, FcR-expressing effector cells may function at one or both of two stages: the phagocytosis/cytotoxicity of the antibody-coated pathogen itself, or the phagocytosis/cytotoxicity of infected cells (Fig. 4b). Thus, multiple classes of pathogen-specific antibodies interact with Type-I activating FcRs on innate effector cells in order to mediate their in vivo protective effects.
Type-I FcRs recruited by anti-tumor antibodies
Numerous therapeutic antibodies against tumor-specific antigens are currently being investigated or used in the clinical setting, including those that directly induce cell death, block survival signals, neutralize growth-supporting ligands, or directly engage Type-I FcR-expressing effector cells. Rituximab, the first therapeutic monoclonal antibody approved for the treatment of cancer, is widely used to treat different classes of CD20+ lymphomas and leukemias. The mechanism of rituximab has been intensively investigated, and while the induction of apoptosis and activation of the complement cascade have been proposed as possible modes of action, the recruitment of FcR+ effector cells dominates in vivo76 (Fig. 4b). Thus, human FcγRIIa and FcγRIIIa functional polymorphisms correlate with efficacy of CD20+ cell depletion in lymphoma and autoimmune disease77, 78. Animal studies using various murine models have clearly demonstrated that anti-CD20 antibodies absolutely require interactions with activating Type-I FcRs expressed by monocytes/macrophages for the depletion of CD20+ cells79, 80. A similar mechanism governs the function anti-HER2/neu antibody (Trastuzumab), since the human FcγRIIIa V158F polymorphism that affects receptor affinity for IgG1 correlate with antibody efficacy81, 82 and in vivo animal models require activating FcγR expression to prevent HER2+ tumor growth79. FcγRIIa and /FcγRIIIa alleles are also predictive of efficacy during anti-EGFR antibody (Cetuximab) therapy of colorectal cancer83. Further, modulating the ability of antibodies to engage activating versus inhibitory Type-I FcRs on effector cells, either through genetic deletion of the inhibitory FcγRIIb or Fc-engineering the IgG Fc to selectively engage activating Type-I FcRs, modulates the cytotoxic potential of anti-tumor antibodies for increased ADCC and tumor clearance79, 84. A striking example of this importance of Type-I FcR engagement by anti-tumor antibodies is provided by the recently approved anti-CD20 mAb enhanced for FcRIIIA binding (Obinutuzumab) that extends survival by a year in CLL patients when directly compared to an unmodified anti-CD20 antibody (Rituximab)85. Thus, numerous anti-tumor antibodies require interactions with activating Type-I FcRs on innate effector cells to activate ADCC and mediate their therapeutic effects on malignant cells. Optimization of this pathway is thus critical to the clinical efficacy of this broad class of therapeutics.
Type-I Fc-receptor engagement for optimal anti-tumor activity of immunomodulatory antibodies
Antibodies targeting immune regulatory receptors, such as T cell checkpoints or APC maturation signals, have received intense interest from the cancer immunotherapy field. In order to enhance an anti-tumor immune response, immunomodulatory antibodies targeting cell-surface immune receptors can act as agonists or antagonists to either stimulate or block, respectively, a target antigen resulting in enhanced T cell-mediated responses. Since these antibodies modulate the signaling pathways triggered by their targets, it was generally accepted that they would act without the need of effector function or to engage FcR-expressing cells. However, the surprising contribution of different members of the Type-I FcR family to the activities of immunomodulatory antibodies has recently been described.
Interactions with the inhibitory FcγRIIB is required for the agonistic activity of antibodies targeting different members of the TNFR superfamily. For example, an absolute requirement for FcγRIIb has been described for the in vivo immunostimulatory and anti-tumor activities of agonistic anti-CD40 antibodies86, 87. T cell expansion and activation induced by anti-CD40 antibodies were observed in mice lacking all activating Type-I FcRs (Fcer1g−/− mice) but not in mice deficient for FcγRIIb (Fcgr2b−/−). Moreover, dramatically enhanced anti-tumor responses mediated by CD8+ T cells were observed using anti-CD40 IgG antibodies that were Fc-engineered to augment interactions with FcγRIIb86, 87. Studies using antibodies targeting other members of the TNFR superfamily have described a general requirement for FcγRIIb engagement by this class of agonistic antibodies. Animal models using FcR-deficient mice or comparing Fc variants with distinct FcR-binding capabilities were used to demonstrate that toxicity triggered by agonistic anti-Fas and anti-death receptor DR4 and DR5 antibodies show a common requirement for FcγRIIb co-engagement for their optimal therapeutic effects88, 89, 90, 91. The general mechanistic basis for these anti-TNFR therapeutics has recently been described92. First, the agonistic antibodies function in trans (i.e., engage FcγRIIb on a distinct cell other than the antibody-bound target cell). Second, this trans co-engagement is independent of FcγRIIb downstream signaling. Third, distinct FcγRIIb-expressing cellular populations are required for the activity of the various anti-TNFR antibodies92. Taken together, the Type-I inhibitory FcγRIIb acts as a scaffold to mediate the clustering of TNFR molecules on the membrane and thereby mimic the effect of multimeric ligands engaging these receptors (Fig. 4c).
Therapeutic immunomodulatory antibodies targeting cell-surface immunoregulatory molecules, such as the inhibitory receptor CTLA-4, were initially thought to function solely through antagonizing signals mediated by their targets. However, recent reports have demonstrated a requirement for activating Type-I FcRs during the anti-tumor therapeutic effects of anti-CTLA-4 and anti-GITR immunomodulatory antibodies93, 94. Antibodies against the immune checkpoint receptor CTLA-4 significantly lose their anti-tumor activity in mice lacking activating FcRs in colon and B16 melanoma tumor models. The anti-tumor efficacy of anti-CTLA-4 antibody was found to be associated with FcR-mediated depletion of intratumoral (but not peripheral) regulatory T cells (Treg)94 (Fig. 4b), thereby calling into question the common mechanistic paradigm that antagonist antibodies function by blocking inhibitory signaling. Similarly, intratumoral Treg depletion was observed after in vivo administration of anti-GITR antibodies in tumor models93. Both CTLA-4 and GITR are also expressed on effector T cells (Teff), which are essential for the anti-tumor immune response. However, the elevated expression of these immune receptors on the surface of Treg compared to Teff and the presence of activated FcR-expressing effector cells in the tumor microenvironment contributes to the increased Teff/Treg ratio after Treg depletion within the tumor microenvironment, thereby leading to effective anti-tumor immunity. Ipilimumab, the anti-CTLA-4 therapeutic being used in the clinic, has an IgG1 Fc isotype and therefore has the potential to engage effector cells to mediate Treg depletion, but it remains unknown whether Treg depletion also occurs in patients treated with anti-CTLA-4. Considering the new paradigms of Type-I FcR requirements for the optimal activity of immunmodulatory antibodies, the development of future antibody-based therapeutics should incorporate Fc domains that interact specifically with the appropriate FcRs.
Both Type-I and Type-II Fc-receptors mediate the anti-inflammatory effect of sialylated IgG
Intravenous immunoglobulin (IVIG) consists of polyclonal IgG molecules purified from thousands of blood donors. IVIG therapy was initially developed as a replacement therapy for immune deficient patients with impaired antibody production capabilities. However, in the 1980s, Imbach and colleagues95 reported that administration of high doses of IVIG restored platelet levels in patients suffering from immunothrombocytopenia (ITP), an antibody-mediated autoimmune disease in which the immune system depletes platelets from the blood. Ever since, IVIG administration has been included as an approved therapy of many chronic autoimmune diseases, such as Guillain-Barrè syndrome, Kawasaki disease, and chronic inflammatory demyelinating polyneuropathy (CIDP).
The mode of action of IVIG has been the subject of numerous studies and reviews4, 96, 97. Three key components of the pathway triggered by IVIG infusion have been discovered to be integral for its immunomodulatory activity in multiple systems: 1) the sialylated Fc fraction of the immunoglobulins present in the IVIG preparation35, 98, 2) the Type-II FcRs, such as DC-SIGN/CD209 (SIGN-R1 in mice)36, and 3) expression of the Type-I inhibitory FcγRIIb99. Thus, the immunoinhibitory effect of IVIG was recapitulated in a murine model of rheumatoid arthritis by employing an in vitro galactosylated and sialylated recombinant IgG-Fc fragment35. Since sialylation of the Fc glycan results in reduced affinity for Type-I FcRs, an additional receptor triggered by this ligand must be responsible for the anti-inflammatory activity. Mouse studies with IVIG demonstrated that the sialylated IgG fraction binds to murine SIGN-R1 (the orthologue of human DC-SIGN) expressed on regulatory macrophages, an interaction necessary for its inhibitory activity in vivo36. Multiple studies have also demonstrated that FcγRIIb is required for the immunoinhibitory activity of IVIG during autoimmune disease models35, 99, 100, 101, since FcγRIIb-deficient mice fail to respond to IVIG or sialyalted IgG-Fc treatment in K/BxN arthritis, ITP, nephrotoxic nephritis (NTN), and epidermolysis bullosa acquisita (EBA). In addition, IVIG has been shown to upregulate FcγRIIb expression on monocytes and B cells among clinically responding CIDP patients102, and functional polymorphisms of both CD209 (DC-SIGN) and FCGR2B genes are associated with the clinical response to IVIG therapy among Kawasaki disease patients103.
A detailed mechanism of how sialylated Fc, the Type-II FcR DC-SIGN, and the inhibitory Type-I FcγRIIb function in tandem to regulate inflammation was described in the K/BxN serum-induced joint inflammation model. The engagement of Fc-sialylated IgG by the Type-II FcR SIGN-R1 on SIGN-R1-expressing regulatory macrophages induces the secretion of IL-33, a potent Th2-polarizing cytokine known for its pleiotropic effects on various immune effector cells. IL-33 secretion subsequently signals basophils to release IL-4 at sites of inflammation, thereby inducing increased expression of the inhibitory Type-I FcR, FcγRIIb, on effector inflammatory monocytes (Figure 5) (7). This upregulation of FcγRIIB increases the threshold of activation for inflammatory effector macrophages, thereby decreasing IgG-mediated inflammation.
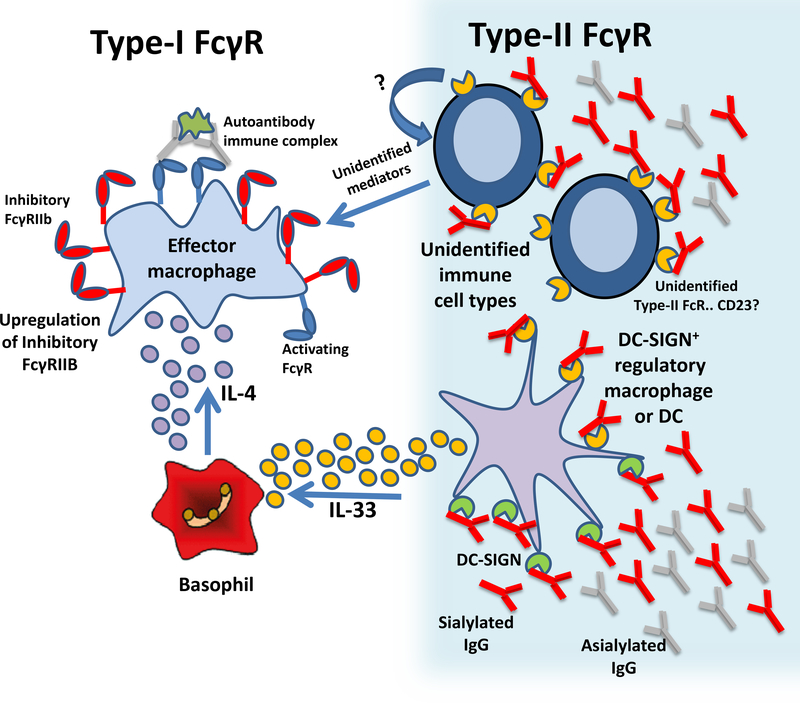
Figure 5. Both Type-I and Type-II Fc-receptors mediate the anti-inflammatory effects of IVIG or sialylated IgG.
Immune complexes between autoantibodies and autoantigens crosslink Type-I activating FcγRs promoting the activation of macrophages and inflammatory autoimmune disease. Sialylated Fcs engage DC-SIGN/SIGN-RI+ macrophages or DCs, promoting IL-33 expression. This IL-33 signlas activated FcɛRI+ innate leukocytes (basophils) to produce IL-4. IL-4, in turn, promotes upregulation of FcγRIIB on effector macrophages, thereby increasing the activation threshold required to trigger inflammation. Alternatively, as-yet unidentified Type-II FcRs expressed by various cell types may also mediate the anti-inflammatory effects of IVIG or sialylated IgG through unidentified pathways, depending on the type and location of the inflammation.WWW
New data also suggests that other sialylated IgG-mediated mechanisms may be at play to regulate inflammation during autoimmunity (Fig. 5). For example, IL-4 and IL-33 are not involved in the IVIG-dependent suppression of ITP104, 105. This observation is consistent with the recent finding that SIGN-R1, although crucial for IVIG activity during the preventive treatment of ITP, was dispensable for IVIG activity during the therapy of ITP and during the late phase in EBA and inflammatory arthritis models99. Thus, the presence of additional receptors for sialylated IgG that act during the later phases of inflammation is likely. Sialylated IgG also engages the Type-II FcR, CD237. CD23 exists in two isoforms; CD23a, which is present on mature B cells, and CD23b, which requires induction by IL-4 for expression on T cells, monocytes, Langerhans cells, eosinophils, and macrophages. While early studies indicated that B cells are dispensable for IVIG activity, only the cytokine production aspect of B cells was assessed. Thus, the effect of CD23 ligation by sialylated IgG on antibody and autoantibody production by B cells, as well as the engagement of CD23 on other innate cellular populations during autoimmunity remains to be determined.
Conclusions
IgG Fc structural diversity and conformational flexibility, controlled by the amino acid sequence of the Fc isotypes and Fc glycan composition, are essential for regulating antibody effector functions through differential engagement of particular members of the Type-I and Type-II FcR families. Thus, for any individual antibody Fab, a diversity of Fc effector functions are possible. It is clear that antibody Fc isotype and glycosylation, and thus effector function, are tightly regulated during immune responses and potentially dysregulated during autoimmunity. However, the signals that dictate IgG Fc structure and effector function in vivo during the development of an immune response remain poorly understood. Antibody therapeutics for the treatment of autoimmune disorders, infectious disease, or tumors require multiple respective effector functions and must consider not only target specificity, but also which downstream effector functions will be required for optimal therapeutic efficacy. Thus, optimal interactions with either activating or inhibitory Type-I FcRs, or Type-II FcRs must be manipulated during new vaccination strategies and engineered into next-generation antibody therapeutics.
References
- 1.Bournazos S, Chow SK, Abboud N, Casadevall A, Ravetch JV. Human IgG Fc domain engineering enhances antitoxin neutralizing antibody activity. The Journal of clinical investigation 2014, 124(2): 725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nature medicine 2014, 20(2): 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nature reviews Immunology 2008, 8(1): 34–47. [DOI] [PubMed] [Google Scholar]
- 4.Nimmerjahn F, Ravetch JV. Antibody-mediated modulation of immune responses. Immunol Rev 2010, 236: 265–275. [DOI] [PubMed] [Google Scholar]
- 5.Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. Journal of clinical immunology 2010, 30 Suppl 1: S9–14. [DOI] [PubMed] [Google Scholar]
- 6.Bournazos S, Woof JM, Hart SP, Dransfield I. Functional and clinical consequences of Fc receptor polymorphic and copy number variants. Clinical and experimental immunology 2009, 157(2): 244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV. General mechanism for modulating immunoglobulin effector function. Proceedings of the National Academy of Sciences of the United States of America 2013, 110(24): 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol 2007, 96: 179–204. [DOI] [PubMed] [Google Scholar]
- 9.Nimmerjahn F, Ravetch JV. FcgammaRs in health and disease. Curr Top Microbiol Immunol 2011, 350: 105–125. [DOI] [PubMed] [Google Scholar]
- 10.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol 2001, 19: 275–290. [DOI] [PubMed] [Google Scholar]
- 11.Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nature reviews Immunology 2010, 10(5): 328–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimberly RP, Wu J, Gibson AW, Su K, Qin H, Li X, et al. Diversity and duplicity: human FCgamma receptors in host defense and autoimmunity. Immunologic research 2002, 26(1–3): 177–189. [DOI] [PubMed] [Google Scholar]
- 13.Takai T Fc receptors and their role in immune regulation and autoimmunity. Journal of clinical immunology 2005, 25(1): 1–18. [DOI] [PubMed] [Google Scholar]
- 14.Jefferis R Isotype and glycoform selection for antibody therapeutics. Archives of biochemistry and biophysics 2012, 526(2): 159–166. [DOI] [PubMed] [Google Scholar]
- 15.Narciso JE, Uy ID, Cabang AB, Chavez JF, Pablo JL, Padilla-Concepcion GP, et al. Analysis of the antibody structure based on high-resolution crystallographic studies. New biotechnology 2011, 28(5): 435–447. [DOI] [PubMed] [Google Scholar]
- 16.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005, 310(5753): 1510–1512. [DOI] [PubMed] [Google Scholar]
- 17.Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. Journal of molecular biology 2003, 325(5): 979–989. [DOI] [PubMed] [Google Scholar]
- 18.Teplyakov A, Zhao Y, Malia TJ, Obmolova G, Gilliland GL. IgG2 Fc structure and the dynamic features of the IgG CH2-CH3 interface. Molecular immunology 2013, 56(1–2): 131–139. [DOI] [PubMed] [Google Scholar]
- 19.Garman SC, Wurzburg BA, Tarchevskaya SS, Kinet JP, Jardetzky TS. Structure of the Fc fragment of human IgE bound to its high-affinity receptor Fc epsilonRI alpha. Nature 2000, 406(6793): 259–266. [DOI] [PubMed] [Google Scholar]
- 20.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature 2000, 406(6793): 267–273. [DOI] [PubMed] [Google Scholar]
- 21.Davies AM, Rispens T, Ooijevaar-de Heer P, Gould HJ, Jefferis R, Aalberse RC, et al. Structural determinants of unique properties of human IgG4-Fc. Journal of molecular biology 2014, 426(3): 630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009, 113(16): 3716–3725. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313(5787): 670–673. [DOI] [PubMed] [Google Scholar]
- 24.Lux A, Nimmerjahn F. Impact of differential glycosylation on IgG activity. Advances in experimental medicine and biology 2011, 780: 113–124. [DOI] [PubMed] [Google Scholar]
- 25.Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. The Journal of biological chemistry 2002, 277(30): 26733–26740. [DOI] [PubMed] [Google Scholar]
- 26.Scherer HU, van der Woude D, Ioan-Facsinay A, el Bannoudi H, Trouw LA, Wang J, et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis and rheumatism 2010, 62(6): 1620–1629. [DOI] [PubMed] [Google Scholar]
- 27.van de Geijn FE, Wuhrer M, Selman MH, Willemsen SP, de Man YA, Deelder AM, et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis research & therapy 2009, 11(6): R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinzaki S, Iijima H, Nakagawa T, Egawa S, Nakajima S, Ishii S, et al. IgG oligosaccharide alterations are a novel diagnostic marker for disease activity and the clinical course of inflammatory bowel disease. The American journal of gastroenterology 2008, 103(5): 1173–1181. [DOI] [PubMed] [Google Scholar]
- 29.Tomana M, Schrohenloher RE, Koopman WJ, Alarcon GS, Paul WA. Abnormal glycosylation of serum IgG from patients with chronic inflammatory diseases. Arthritis and rheumatism 1988, 31(3): 333–338. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proceedings of the National Academy of Sciences of the United States of America 2011, 108(31): 12669–12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borrok MJ, Jung ST, Kang TH, Monzingo AF, Georgiou G. Revisiting the role of glycosylation in the structure of human IgG Fc. ACS chemical biology 2012, 7(9): 1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albert H, Collin M, Dudziak D, Ravetch JV, Nimmerjahn F. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proceedings of the National Academy of Sciences of the United States of America 2008, 105(39): 15005–15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barb AW, Prestegard JH. NMR analysis demonstrates immunoglobulin G N-glycans are accessible and dynamic. Nature chemical biology 2011, 7(3): 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wormald MR, Rudd PM, Harvey DJ, Chang SC, Scragg IG, Dwek RA. Variations in oligosaccharide-protein interactions in immunoglobulin G determine the site-specific glycosylation profiles and modulate the dynamic motion of the Fc oligosaccharides. Biochemistry 1997, 36(6): 1370–1380. [DOI] [PubMed] [Google Scholar]
- 35.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 2008, 320(5874): 373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proceedings of the National Academy of Sciences of the United States of America 2008, 105(50): 19571–19578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hess C, Winkler A, Lorenz AK, Holecska V, Blanchard V, Eiglmeier S, et al. T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. The Journal of clinical investigation 2013, 123(9): 3788–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borthakur S, Andrejeva G, McDonnell JM. Basis of the intrinsic flexibility of the Cepsilon3 domain of IgE. Biochemistry 2011, 50(21): 4608–4614. [DOI] [PubMed] [Google Scholar]
- 39.Dhaliwal B, Yuan D, Pang MO, Henry AJ, Cain K, Oxbrow A, et al. Crystal structure of IgE bound to its B-cell receptor CD23 reveals a mechanism of reciprocal allosteric inhibition with high affinity receptor FcepsilonRI. Proceedings of the National Academy of Sciences of the United States of America 2012, 109(31): 12686–12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selman MH, de Jong SE, Soonawala D, Kroon FP, Adegnika AA, Deelder AM, et al. Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Molecular & cellular proteomics : MCP 2012, 11(4): M111 014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Espy C, Morelle W, Kavian N, Grange P, Goulvestre C, Viallon V, et al. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener’s). Arthritis and rheumatism 2011, 63(7): 2105–2115. [DOI] [PubMed] [Google Scholar]
- 42.Oefner CM, Winkler A, Hess C, Lorenz AK, Holecska V, Huxdorf M, et al. Tolerance induction with T cell-dependent protein antigens induces regulatory sialylated IgGs. The Journal of allergy and clinical immunology 2012, 129(6): 1647–1655 e1613. [DOI] [PubMed] [Google Scholar]
- 43.Diaz de Stahl T, Heyman B. IgG2a-mediated enhancement of antibody responses is dependent on FcRgamma+ bone marrow-derived cells. Scandinavian journal of immunology 2001, 54(5): 495–500. [DOI] [PubMed] [Google Scholar]
- 44.Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. The Journal of experimental medicine 2002, 195(12): 1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. The Journal of experimental medicine 1997, 185(11): 1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. The Journal of clinical investigation 2005, 115(10): 2914–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dhodapkar KM, Kaufman JL, Ehlers M, Banerjee DK, Bonvini E, Koenig S, et al. Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proceedings of the National Academy of Sciences of the United States of America 2005, 102(8): 2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blank U, Launay P, Benhamou M, Monteiro RC. Inhibitory ITAMs as novel regulators of immunity. Immunological reviews 2009, 232(1): 59–71. [DOI] [PubMed] [Google Scholar]
- 49.Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity 2005, 22(5): 539–550. [DOI] [PubMed] [Google Scholar]
- 50.Swanson JA, Hoppe AD. The coordination of signaling during Fc receptor-mediated phagocytosis. Journal of leukocyte biology 2004, 76(6): 1093–1103. [DOI] [PubMed] [Google Scholar]
- 51.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. The Journal of experimental medicine 1999, 189(2): 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dhodapkar KM, Krasovsky J, Williamson B, Dhodapkar MV. Antitumor monoclonal antibodies enhance cross-presentation ofcCellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. The Journal of experimental medicine 2002, 195(1): 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuurhuis DH, van Montfoort N, Ioan-Facsinay A, Jiawan R, Camps M, Nouta J, et al. Immune complex-loaded dendritic cells are superior to soluble immune complexes as antitumor vaccine. Journal of immunology 2006, 176(8): 4573–4580. [DOI] [PubMed] [Google Scholar]
- 54.Desai DD, Harbers SO, Flores M, Colonna L, Downie MP, Bergtold A, et al. Fc gamma receptor IIB on dendritic cells enforces peripheral tolerance by inhibiting effector T cell responses. Journal of immunology 2007, 178(10): 6217–6226. [DOI] [PubMed] [Google Scholar]
- 55.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology 2004, 25(12): 677–686. [DOI] [PubMed] [Google Scholar]
- 56.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nature reviews Immunology 2014, 14(2): 94–108. [DOI] [PubMed] [Google Scholar]
- 57.Jackson SM, Wilson PC, James JA, Capra JD. Human B cell subsets. Advances in immunology 2008, 98: 151–224. [DOI] [PubMed] [Google Scholar]
- 58.Fujiwara H, Kikutani H, Suematsu S, Naka T, Yoshida K, Yoshida K, et al. The absence of IgE antibody-mediated augmentation of immune responses in CD23-deficient mice. Proceedings of the National Academy of Sciences of the United States of America 1994, 91(15): 6835–6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pearse RN, Kawabe T, Bolland S, Guinamard R, Kurosaki T, Ravetch JV. SHIP recruitment attenuates Fc gamma RIIB-induced B cell apoptosis. Immunity 1999, 10(6): 753–760. [DOI] [PubMed] [Google Scholar]
- 60.Tew JG, Wu J, Fakher M, Szakal AK, Qin D. Follicular dendritic cells: beyond the necessity of T-cell help. Trends in immunology 2001, 22(7): 361–367. [DOI] [PubMed] [Google Scholar]
- 61.Barrington RA, Pozdnyakova O, Zafari MR, Benjamin CD, Carroll MC. B lymphocyte memory: role of stromal cell complement and FcgammaRIIB receptors. The Journal of experimental medicine 2002, 196(9): 1189–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Honjo T, Alt FW, Neuberger MS. Molecular biology of B cells. Elsevier: Amsterdam; Boston, 2004. [Google Scholar]
- 63.Ravetch JV, Nussenzweig M. Killing some to make way for others. Nat Immunol 2007, 8(4): 337–339. [DOI] [PubMed] [Google Scholar]
- 64.Xiang Z, Cutler AJ, Brownlie RJ, Fairfax K, Lawlor KE, Severinson E, et al. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat Immunol 2007, 8(4): 419–429. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez D, Castro OE, Kouri G, Perez J, Martinez E, Vazquez S, et al. Classical dengue hemorrhagic fever resulting from two dengue infections spaced 20 years or more apart: Havana, Dengue 3 epidemic, 2001–2002. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases 2005, 9(5): 280–285. [DOI] [PubMed] [Google Scholar]
- 66.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. The American journal of tropical medicine and hygiene 1988, 38(2): 411–419. [DOI] [PubMed] [Google Scholar]
- 67.Kliks S Antibody-enhanced infection of monocytes as the pathogenetic mechanism for severe dengue illness. AIDS research and human retroviruses 1990, 6(8): 993–998. [DOI] [PubMed] [Google Scholar]
- 68.Duerr A, Huang Y, Buchbinder S, Coombs RW, Sanchez J, del Rio C, et al. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study). The Journal of infectious diseases 2012, 206(2): 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monsalvo AC, Batalle JP, Lopez MF, Krause JC, Klemenc J, Hernandez JZ, et al. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat Med 2011, 17(2): 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guihot A, Luyt CE, Parrot A, Rousset D, Cavaillon JM, Boutolleau D, et al. Low titers of serum antibodies inhibiting hemagglutination predict fatal fulminant influenza A(H1N1) 2009 infection. American journal of respiratory and critical care medicine 2014. [DOI] [PubMed] [Google Scholar]
- 71.Ubol S, Halstead SB. How innate immune mechanisms contribute to antibody-enhanced viral infections. Clin Vaccine Immunol 2010, 17(12): 1829–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El Bakkouri K, Descamps F, De Filette M, Smet A, Festjens E, Birkett A, et al. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. Journal of immunology 2011, 186(2): 1022–1031. [DOI] [PubMed] [Google Scholar]
- 73.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 2011, 333(6044): 850–856. [DOI] [PubMed] [Google Scholar]
- 74.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 2007, 449(7158): 101–104. [DOI] [PubMed] [Google Scholar]
- 75.Abboud N, Chow SK, Saylor C, Janda A, Ravetch JV, Scharff MD, et al. A requirement for FcgammaR in antibody-mediated bacterial toxin neutralization. The Journal of experimental medicine 2010, 207(11): 2395–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tedder TF, Baras A, Xiu Y. Fcgamma receptor-dependent effector mechanisms regulate CD19 and CD20 antibody immunotherapies for B lymphocyte malignancies and autoimmunity. Springer Semin Immunopathol 2006, 28(4): 351–364. [DOI] [PubMed] [Google Scholar]
- 77.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002, 99(3): 754–758. [DOI] [PubMed] [Google Scholar]
- 78.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2003, 21(21): 3940–3947. [DOI] [PubMed] [Google Scholar]
- 79.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nature medicine 2000, 6(4): 443–446. [DOI] [PubMed] [Google Scholar]
- 80.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. The Journal of experimental medicine 2004, 199(12): 1659–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008, 26(11): 1789–1796. [DOI] [PubMed] [Google Scholar]
- 82.Varchetta S, Gibelli N, Oliviero B, Nardini E, Gennari R, Gatti G, et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer research 2007, 67(24): 11991–11999. [DOI] [PubMed] [Google Scholar]
- 83.Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009, 27(7): 1122–1129. [DOI] [PubMed] [Google Scholar]
- 84.Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proceedings of the National Academy of Sciences of the United States of America 2012, 109(16): 6181–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. The New England journal of medicine 2014, 370(12): 1101–1110. [DOI] [PubMed] [Google Scholar]
- 86.Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science 2011, 333(6045): 1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.White AL, Chan HTC, Roghanian A, French RR, Mockridge CI, Tutt AL, et al. Interaction with FcγRIIB Is Critical for the Agonistic Activity of Anti-CD40 Monoclonal Antibody. The Journal of Immunology 2011, 187(4): 1754–1763. [DOI] [PubMed] [Google Scholar]
- 88.Xu Y, Szalai AJ, Zhou T, Zinn KR, Chaudhuri TR, Li X, et al. FcγRs Modulate Cytotoxicity of Anti-Fas Antibodies: Implications for Agonistic Antibody-Based Therapeutics. The Journal of Immunology 2003, 171(2): 562–568. [DOI] [PubMed] [Google Scholar]
- 89.Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, et al. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer cell 2011, 19(1): 101–113. [DOI] [PubMed] [Google Scholar]
- 90.Li F, Ravetch JV. A general requirement for FcgammaRIIB co-engagement of agonistic anti-TNFR antibodies. Cell Cycle 2012, 11(18): 3343–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chuntharapai A, Dodge K, Grimmer K, Schroeder K, Marsters SA, Koeppen H, et al. Isotype-dependent inhibition of tumor growth in vivo by monoclonal antibodies to death receptor 4. Journal of immunology 2001, 166(8): 4891–4898. [DOI] [PubMed] [Google Scholar]
- 92.Li F, Ravetch JV. Antitumor activities of agonistic anti-TNFR antibodies require differential FcgammaRIIB coengagement in vivo. Proceedings of the National Academy of Sciences of the United States of America 2013, 110(48): 19501–19506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, et al. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. The Journal of experimental medicine 2013, 210(9): 1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. The Journal of experimental medicine 2013, 210(9): 1695–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Imbach P, Barandun S, d’Apuzzo V, Baumgartner C, Hirt A, Morell A, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet 1981, 1(8232): 1228–1231. [DOI] [PubMed] [Google Scholar]
- 96.Anthony RM, Wermeling F, Ravetch JV. Novel roles for the IgG Fc glycan. Annals of the New York Academy of Sciences 2012, 1253: 170–180. [DOI] [PubMed] [Google Scholar]
- 97.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol 2008, 26: 513–533. [DOI] [PubMed] [Google Scholar]
- 98.Debre M, Bonnet MC, Fridman WH, Carosella E, Philippe N, Reinert P, et al. Infusion of Fc gamma fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet 1993, 342(8877): 945–949. [DOI] [PubMed] [Google Scholar]
- 99.Schwab I, Mihai S, Seeling M, Kasperkiewicz M, Ludwig RJ, Nimmerjahn F. Broad requirement for terminal sialic acid residues and FcgammaRIIB for the preventive and therapeutic activity of intravenous immunoglobulins in vivo. European journal of immunology 2014. [DOI] [PubMed] [Google Scholar]
- 100.Crow AR, Song S, Freedman J, Helgason CD, Humphries RK, Siminovitch KA, et al. IVIg-mediated amelioration of murine ITP via FcgammaRIIB is independent of SHIP1, SHP-1, and Btk activity. Blood 2003, 102(2): 558–560. [DOI] [PubMed] [Google Scholar]
- 101.Crow AR, Song S, Semple JW, Freedman J, Lazarus AH. IVIg inhibits reticuloendothelial system function and ameliorates murine passive-immune thrombocytopenia independent of anti-idiotype reactivity. British journal of haematology 2001, 115(3): 679–686. [DOI] [PubMed] [Google Scholar]
- 102.Tackenberg B, Jelcic I, Baerenwaldt A, Oertel WH, Sommer N, Nimmerjahn F, et al. Impaired inhibitory Fcgamma receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proceedings of the National Academy of Sciences of the United States of America 2009, 106(12): 4788–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Portman MA, Wiener HW, Silva M, Shendre A, Shrestha S. DC-SIGN gene promoter variants and IVIG treatment response in Kawasaki disease. Pediatric rheumatology online journal 2013, 11(1): 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Crow AR, Song S, Semple JW, Freedman J, Lazarus AH. A role for IL-1 receptor antagonist or other cytokines in the acute therapeutic effects of IVIg? Blood 2007, 109(1): 155–158. [DOI] [PubMed] [Google Scholar]
- 105.Schwab I, Biburger M, Kronke G, Schett G, Nimmerjahn F. IVIg-mediated amelioration of ITP in mice is dependent on sialic acid and SIGNR1. European journal of immunology 2012, 42(4): 826–830. [DOI] [PubMed] [Google Scholar]