Abstract
Physiologic and pathologic stressors promote changes in metabolism that are associated with cardiac remodeling. Metabolic alterations in the heart are a summation of responses of several organs and organ systems, which transform the milieu of circulating substrates and stimuli and prompt cardiac adaptation or remodeling. Nevertheless, the mechanisms by which metabolism causes cardiac remodeling remain unclear. Difficulties in delineating metabolic mechanisms of tissue remodeling are in part due to technical issues as well as to the lack of conceptual clarity with regard to causal entailment of metabolic processes. This review discusses some metabolic mechanisms by which stressors such as exercise, pregnancy, and pressure overload promote metabolism-mediated cardiac remodeling. Adopting conceptual frameworks based in relational biology and delineating hierarchies of metabolic causation could lend new insight into how metabolism coordinates cardiac remodeling.
Keywords: glycolysis, pregnancy, heart failure, exercise, hypertrophy, mitochondria
The mechanisms by which exercise as well as other physiological or pathological stressors regulate cardiac structural and functional remodeling have been difficult to uncover. Such mechanisms, and their causal relationships with each other, are important to identify because they could provide a gateway for developing novel and actionable strategies to deter or delay heart disease. This article tackles a particularly difficult subject—metabolic changes as a cause of cardiac remodeling.
Difficulties in measuring cardiac metabolism in vivo
For several reasons, it has been difficult to delineate how changes in metabolism contribute to cardiac remodeling. This issue is particularly difficult to address in the context of exercise, which promotes dynamic and systemic changes in metabolism. For example, some results suggest that adaptation to exercise increases the basal rate of glycolysis (1, 2**), whereas others suggest diminished (3) or unchanged (4) glycolytic activity. The reasons for such discrepancies could be due to factors specific to each model system (e.g., exercise intensity, type of exercise, rodent strain), differences in cardiac perfusion protocols (e.g., substrate levels, addition of hormones), or the time of day at which heart metabolism is measured (i.e., circadian influences; (5-7)). Uncertainty in interpretation is compounded by the fact that most experimental approaches to address metabolic changes in the heart require us to extrapolate results from in vitro or ex vivo studies to the in vivo system. Although removal of cardiac myocytes from the heart, or the heart from the body, allows for strict control of experimental variables, these approaches irretrievably destroy the complex interactions and relationships of the original system (8, 9). This issue is particularly germane to the heart, which is an opportunistic omnivore with high metabolic demand that responds to numerous humoral factors and neural inputs, many of which change dynamically during or after pathological or physiological stimuli (10, 11).
Although measuring metabolism in vivo would overcome many of these obstacles, it is no easy task. Inferences regarding radiolabeled glucose uptake (e.g., 2-[18F]fluoro-2-deoxy-D-glucose) are limited by the fact that measuring glucose uptake does not provide information of its utilization by metabolic pathways. In this regard, nuclear magnetic resonance and radioactive or stable isotope tracing approaches are useful for understanding changes in cardiac metabolism in vivo (12). Particularly attractive are approaches that strive to minimize obfuscation caused by tracer incorporation and that provide information on the activity of numerous metabolic pathways. For example, deep network tracing, which introduces 13C6-glucose or other stable tracers via a stress-free, ad libitum diet, is capable of revealing the relative activities of numerous anabolic and catabolic pathways simultaneously (13**). This approach could be used to understand how pathways with lower flux, e.g., biosynthetic pathways of glucose metabolism (10), change with exercise adaptation or other stimuli. Importantly, deep network tracing provides information necessary for understanding how metabolic pathway relationships change over time or under conditions of stress, which is more useful than knowing how the activity of only one or a few pathways change.
Although deep network tracing may enable deeper understanding of cardiac metabolism in vivo, its widespread use is currently limited by access to the necessary instrumentation and by the expertise required to analyze and interpret the data (14). In lieu of these measurements, an approach dependent on operationalization can provide understanding of in vivo metabolism. Using operationally defined parameters, phenomena that are not directly measureable are inferred by examining strictly defined variables. We used this approach to find that myocardial glycolytic rate decreases during or near the end of an intense bout of exercise (2**). Required for this analysis was knowledge that: 1) phosphofructokinase 1 (PFK1) is the rate-limiting and committed step of glycolysis (15-17); 2) 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK2) phosphorylation (at S483) can promote PFK1 activation (18-20); 3) glycogenesis occurs when PFK1 is inhibited (21); and 4) elevated levels of circulating lactate and fatty acids diminish cardiac glucose utilization (22). In our analysis, exercise acutely altered all of these operationally defined parameters: PFK2S483 phosphorylation was lower, glycogen levels increased by 5-fold, and circulating levels of competing substrates were higher (2**). These findings provide logically consistent, convergent evidence that a relatively intense bout of exercise acutely decreases PFK1 activity and glycolytic rate.
Difficulties extrapolating ex vivo metabolic measurements to the in vivo system
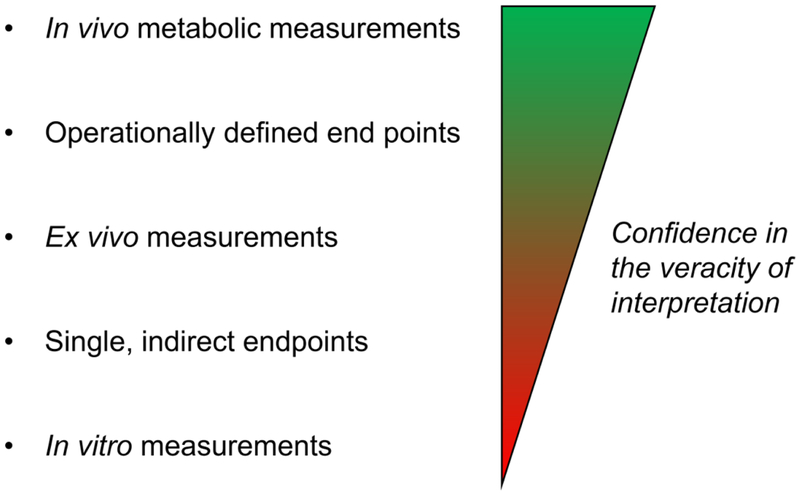
Ex vivo cardiac approaches are useful for assessing metabolism in a tightly controlled system. Data are commonly interpreted assuming that the conditions in the perfusion system are similar to that in vivo. However, recent studies indicate that ex vivo perfusion systems promote regions of anoxia in the heart by disrupting the vasoregulatory network (23*). Moreover, saline-based perfusion solutions have lower oxygen carrying capacity compared with red blood cell perfusion solutions (24) or solutions containing artificial oxygen carriers (25). In addition, perfusion solutions often fail to recapitulate the substrate spectrum normally available to the heart in vivo, which could bias results. Thus, ex vivo analysis of metabolism may not be capable of recapitulating the metabolic state in vivo. Nevertheless, the perfused heart approach has been remarkably successful for quantifying the activity of particular pathways, especially glucose oxidation, fatty acid oxidation, and glycolysis. It is useful for understanding the metabolic inclinations of the isolated organ, especially in the context of gene deletion or overexpression. Although slight differences in perfusion protocols can confer disparate results, combining in vivo endpoints from tissue samples or from operationally defined parameters with ex vivo metabolic measurements increases confidence in interpretation. For example, recent studies of cardiac growth in the context of pregnancy show diminished glucose oxidation in the maternal heart ex vivo, which was complemented by findings of upregulated pyruvate dehydrogenase kinase 4 and higher levels of circulating competing substrates (e.g., fatty acids) (26**). Together, the complementary measurements, along with harmonious reports in the literature (10), provide strong evidence that the maternal heart diminishes its reliance on glucose oxidation. In contrast, single endpoint measurements (e.g., immunoblots of metabolic enzymes) or inductive reasoning from the in vitro setting (e.g., isolated cardiomyocytes) provides the least confidence to interpretations of the in vivo scenario (Fig. 1), at least in the context of intermediary metabolism.
Fig. 1: Relative confidence in the veracity of metabolic measurements.
Generalized schematic of different types of metabolic measurements and the veracity of conclusions derived therefrom.
Evidence that metabolism is a cause of cardiac remodeling
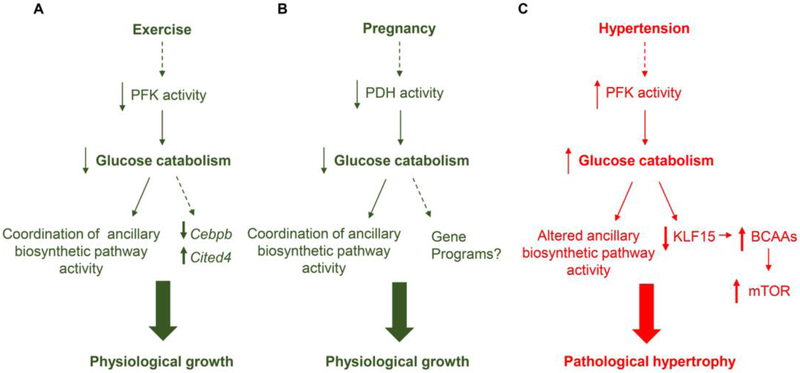
Metabolic pathways provide the cell useable energy (ATP) as well as biosynthetic precursors for nucleotides, phospholipids, and amino acids. Moreover, metabolism coordinates cell signaling, redox state, and transcriptional responses (10, 12). Given the importance of metabolism to each of these factors, it is not surprising that changes in glucose utilization are commonly found to be sufficient causes of cardiac remodeling (10, 11). As reviewed previously (10, 11), exercise-induced metabolic periodicity appears important for physiological cardiac growth. Based on the ability of PFK to regulate ancillary biosynthetic pathway activity (17*, 27*), the decrease in PFK activity and glycolysis in the heart during exercise (2**) would likely change biosynthetic pathway activity, leading to changes in the synthesis of cellular building blocks. Moreover, low PFK activity is sufficient to regulate the cardiac growth program [e.g., Cebpb, Cited4 (28, 29)] (2**), suggesting that decreases in glycolytic rate occurring during exercise may be a formal cause of remodeling (Fig. 2A). Pregnancy-induced cardiac growth follows a similar paradigm: decreases in glucose oxidation could augment ancillary biosynthetic pathway activity to initiate cardiac growth programs (26**) (Fig. 2B). Nevertheless, the fine mechanistic details remain unclear – How do metabolic changes during exercise and pregnancy regulate the transcriptional programs that promote cardiac growth?
Fig. 2: Working models of the role of glucose metabolism in physiological and pathological cardiac remodeling.
Exercise and pregnancy decrease glucose catabolism, which appears critical for physiologic growth. Exercise decreases phosphofructokinase (PFK) activity and glucose catabolism acutely. This coordinates ancillary biosynthetic pathway activity and activates the exercise gene program, i.e., it decreases expression of Cebpb and increases expression of Cited4. Pregnancy-induced changes in progesterone increase Pdk4, which decreases PDH activity and glucose catabolism to promote cardiac growth. The gene programs triggered by this metabolic change remain unclear. Under conditions of pressure overload, as occurs in hypertension, PFK activity is augmented, which would alter ancillary biosynthetic pathway activity. High glucose utilization also appears to diminish Kruppel-like factor (KLF) 15, which suppresses the expression of BCAA catabolic enzymes. This increases BCAA levels in the heart, which activates mTOR and promotes cell hypertrophy.
Changes in metabolism also appear to cause pathological remodeling. For example, that high levels of PFK activity are sufficient to cause a deleterious form of remodeling are supported by the facts that nearly all PFK allosteric activators increase in the pressure-overloaded, hypertrophic heart (30) and that constitutively high PFK activity is sufficient to promote mild dilated cardiomyopathy (2*). The formal mechanism was recently found to be due to the capacity of glucose metabolism to regulate KLF15 transcription, branched chain amino acid (BCAA) abundance, and mTOR activity (31**) (Fig. 2C).
Changes in fatty acid and ketone body utilization also have marked effects on pathological remodeling [reviewed in (10)]. For example, deletion of acetyl CoA carboxylase 2 augments fatty acid oxidation and lowers glucose oxidation in the heart and prevents pressure overload-induced pathological remodeling (32), while deletion of fatty acid oxidation enzymes appear to cause cardiomyopathy (33). Moreover, overexpression of the fatty acid oxidation enzyme, medium-chain acyl coenzyme A dehydrogenase, promotes physiological cardiac growth and prevents pathological remodeling (34). Similarly, enhancing ketone body utilization by overexpressing D-β-hydroxybutyrate dehydrogenase (35) or by delivering ketone bodies in vivo (36) ameliorates pathological remodeling and cardiac dysfunction, and decreasing ketone body oxidation by deleting enzymes important for their utilization worsens cardiac function and remodeling (37). It remains unclear how concomitant metabolic changes that occur during altered fatty acid or ketone utilization, e.g., changes in glucose utilization and anabolic pathway activity, affect cardiac remodeling.
Metabolism may also regulate cardiac health and responses to stress via metabolite signaling. For example, endogenous metabolites of glycolysis (e.g., glucose-6-phosphate) and of the pentose phosphate pathway (5-aminoimidazole-4-carboxamide ribonucleotide; AICAR) as well as changes in adenine nucleotide pools can activate prohypertrophic kinases such as mTOR and AMPK [reviewed in (11)]. Circulating fatty acids also cause cardiac growth, presumably by activating signaling pathways (38, 39). Given that numerous metabolites have cognate GPCRs (40), it is likely that circulating metabolites change the signaling and transcriptional landscapes of the heart.
How might we obtain deeper understanding?
There are several conceptual hurdles to overcome to understand metabolic causation clearly. One philosophical issue is that causation comes in several flavors and layers. For example, there are ultimate causes, proximate causes, sufficient causes, necessary causes, and the construct of classical (Aristotelean) causation, which harbors four additional types of cause (i.e., efficient, material, formal, and final causes). Moreover, that biological organisms are not machines and that the relationships between biological processes have their own emergent properties add additional wrinkles of causal complexity. The relational nature of the metabolic network involves mutually dependent components and interdependent pathways (8, 41), which give rise to properties that emerge from the web of metabolic interactions. The composite effect appears to depend not upon the individual components of metabolism, but on the structural and flux configurations of the web, contemporaneous with signaling events and functional states. Overall, these multifaceted dependencies present a paradox for understanding the extent to which changes in metabolism trigger healthy adaptations or sanction structural and functional decline.
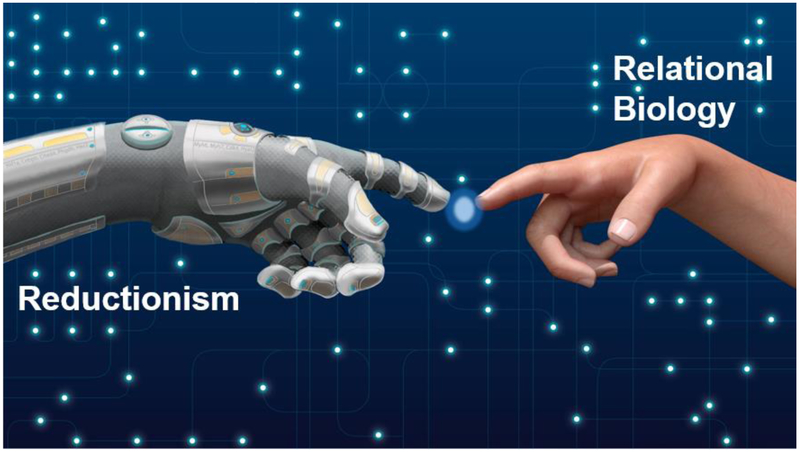
Perhaps one way to think through the problem would be first to acknowledge our (over)reliance on reductionism and our poor understanding of the relational nature of metabolic pathways. We are steeped in reductionist thought patterns and approaches, which have been invaluable for understanding the fundamentals of biological constituents. Yet, they fall short for understanding the relational biology implicit in and controlled by metabolism. In particular, reductionism fails to provide useful information about the emergent properties ascribed to particular metabolic flux configurations. Such properties arise when interactions of the metabolic ensemble yield properties beyond that explained by the sum of the parts. A clearer picture of pathway relationships, perhaps via application of deep network tracing (13**), appears important for understanding how the metabolic system works as a whole and changes upon conditions of stress. Once we have that information, we may be able to address more fully how metabolism causes cardiac remodeling. Constructs that integrate network biology with reductionism and causal states should prove useful for obtaining deeper insights into how metabolic changes cause tissue remodeling (Fig. 3), and could help lay the foundation for future research agendas.
Fig. 3: Conceptual imagery of the creation of new metabolic understanding.
Reductionism views organisms as machines, which has an upper limit for understanding biology. Relational biology attempts to capture the relationships between processes and can reveal emergent properties. The integration of these approaches holds promise for developing new understanding of how changes in metabolism regulate tissue structure and function. Illustration by Ben Smith.
SOURCES OF FUNDING AND ACKNOWLEDGEMENTS
The author would like to acknowledge grants from the National Institutes of Health (HL130174, HL78825, ES028268, GM127607) and the American Diabetes Association Pathway to Stop Diabetes Grant (1-16-JDF-041). The author acknowledges artwork by Ben Smith (https://www.bensmithillustration.com/).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors declare no conflicts of interest.
The author (Bradford Hill) declares no conflict of interest.
REFERENCES
- 1.Riehle C, Wende AR, Zhu Y, Oliveira KJ, Pereira RO, Jaishy BP, Bevins J, Valdez S, Noh J, Kim BJ, Moreira AB, Weatherford ET, Manivel R, Rawlings TA, Rech M, White MF, and Abel ED (2014) Insulin receptor substrates are essential for the bioenergetic and hypertrophic response of the heart to exercise training. Mol Cell Biol 34, 3450–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibb AA, Epstein PN, Uchida S, Zheng Y, McNally LA, Obal D, Katragadda K, Trainor P, Conklin DJ, Brittian KR, Tseng MT, Wang J, Jones SP, Bhatnagar A, and Hill BG (2017) Exercise-Induced Changes in Glucose Metabolism Promote Physiological Cardiac Growth. Circulation 136, 2144–2157**This results of this study suggest that exercise-mediated decreases in phosphofructokinase activity are sufficient to activate the exercise gene program and to promote physiologic cardiac growth.
- 3.Burelle Y, Wambolt RB, Grist M, Parsons HL, Chow JC, Antler C, Bonen A, Keller A, Dunaway GA, Popov KM, Hochachka PW, and Allard MF (2004) Regular exercise is associated with a protective metabolic phenotype in the rat heart. Am J Physiol Heart Circ Physiol 287, H1055–1063 [DOI] [PubMed] [Google Scholar]
- 4.Broderick TL, Poirier P, and Gillis M (2005) Exercise training restores abnormal myocardial glucose utilization and cardiac function in diabetes. Diabetes/metabolism research and reviews 21, 44–50 [DOI] [PubMed] [Google Scholar]
- 5.Seo DY, Lee S, Kim N, Ko KS, Rhee BD, Park BJ, and Han J (2013) Morning and evening exercise. Integr Med Res 2, 139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahara Y, Aoyama S, and Shibata S (2017) The mammalian circadian clock and its entrainment by stress and exercise. J Physiol Sci 67, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatham JC, and Young ME (2013) Regulation of myocardial metabolism by the cardiomyocyte circadian clock. J Mol Cell Cardiol 55, 139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen R (1991) Life itself: a comprehensive inquiry into the nature, origin, and fabrication of life, Columbia University Press, New York [Google Scholar]
- 9.Capra F, and Luisi PL (2014) The systems view of life : a unifying vision, Cambridge University Press, Cambridge [Google Scholar]
- 10.Gibb AA, and Hill BG (2018) Metabolic Coordination of Physiological and Pathological Cardiac Remodeling. Circ Res 123, 107–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulghum K, and Hill BG (2018) Metabolic Mechanisms of Exercise-Induced Cardiac Remodeling. Front Cardiovasc Med 5, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, Des Rosiers C, Gerszten R, Glatz JF, Griffin JL, Gropler RJ, Holzhuetter HG, Kizer JR, Lewandowski ED, Malloy CR, Neubauer S, Peterson LR, Portman MA, Recchia FA, Van Eyk JE, Wang TJ, and American Heart Association Council on Basic Cardiovascular, S. (2016) Assessing Cardiac Metabolism: A Scientific Statement From the American Heart Association. Circ Res 118, 1659–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun RC, Fan TW, Deng P, Higashi RM, Lane AN, Le AT, Scott TL, Sun Q, Warmoes MO, and Yang Y (2017) Noninvasive liquid diet delivery of stable isotopes into mouse models for deep metabolic network tracing. Nat Commun 8, 1646.**This paper describes a novel and stress-free method for tracing metabolic pathway activity in mice. The method incorporates stably labeled metabolic tracers in a liquid diet and utilizes NMR and chromatography-mass spectrometry to trace numerous metabolic networks in vivo.
- 14.Jang C, Chen L, and Rabinowitz JD (2018) Metabolomics and Isotope Tracing. Cell 173, 822–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mor I, Cheung EC, and Vousden KH (2011) Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb Symp Quant Biol 76, 211–216 [DOI] [PubMed] [Google Scholar]
- 16.Yalcin A, Telang S, Clem B, and Chesney J (2009) Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Experimental and molecular pathology 86, 174–179 [DOI] [PubMed] [Google Scholar]
- 17.Cortassa S, Caceres V, Bell LN, O'Rourke B, Paolocci N, and Aon MA (2015) From metabolomics to fluxomics: a computational procedure to translate metabolite profiles into metabolic fluxes. Biophys J 108, 163–172*This study describes a novel procedure to derive flux information from metabolite abundance profiles. The findings of this study demonstrate the importance of phosphofructokinase on ancillary biosynthetic pathway activity.
- 18.Deprez J, Vertommen D, Alessi DR, Hue L, and Rider MH (1997) Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J Biol Chem 272, 17269–17275 [DOI] [PubMed] [Google Scholar]
- 19.Pozuelo Rubio M, Peggie M, Wong BH, Morrice N, and MacKintosh C (2003) 14-3-3s regulate fructose-2,6-bisphosphate levels by binding to PKB-phosphorylated cardiac fructose-2,6-bisphosphate kinase/phosphatase. EMBO J 22, 3514–3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mouton V, Toussaint L, Vertommen D, Gueuning MA, Maisin L, Havaux X, Sanchez-Canedo C, Bertrand L, Dequiedt F, Hemmings BA, Hue L, and Rider MH (2010) Heart 6-phosphofructo-2-kinase activation by insulin requires PKB (protein kinase B), but not SGK3 (serum- and glucocorticoid-induced protein kinase 3). Biochem J 431, 267–275 [DOI] [PubMed] [Google Scholar]
- 21.Hue L, and Taegtmeyer H (2009) The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 297, E578–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schonekess BO (1997) Competition between lactate and fatty acids as sources of ATP in the isolated working rat heart. J Mol Cell Cardiol 29, 2725–2733 [DOI] [PubMed] [Google Scholar]
- 23.Giles AV, Sun J, Femnou AN, Kuzmiak-Glancy S, Taylor JL, Covian R, Murphy E, and Balaban RS (2018) Paradoxical arteriole constriction compromises cytosolic and mitochondrial oxygen delivery in the isolated saline-perfused heart. Am J Physiol Heart Circ Physiol 315, H1791–H1804*Data from this study suggest that isolated perfused hearts partially lack homeostatic balance between cardiac work and oxygen delivery, which results in small regions of total anoxia. The findings imply that the vasoregulatory network of the perfused heart is disrupted by washout of interstitial, vasoactive metabolites.
- 24.Schenkman KA, Beard DA, Ciesielski WA, and Feigl EO (2003) Comparison of buffer and red blood cell perfusion of guinea pig heart oxygenation. Am J Physiol Heart Circ Physiol 285, H1819–1825 [DOI] [PubMed] [Google Scholar]
- 25.Kuzmiak-Glancy S, Covian R, Femnou AN, Glancy B, Jaimes R 3rd, Wengrowski AM, Garrott K, French SA, Balaban RS, and Kay MW (2018) Cardiac performance is limited by oxygen delivery to the mitochondria in the crystalloid-perfused working heart. Am J Physiol Heart Circ Physiol 314, H704–H715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu LX, Rowe GC, Yang S, Li J, Damilano F, Chan MC, Lu W, Jang C, Wada S, Morley M, Hesse M, Fleischmann BK, Rabinowitz JD, Das S, Rosenzweig A, and Arany Z (2017) PDK4 Inhibits Cardiac Pyruvate Oxidation in Late Pregnancy. Circ Res 121, 1370–1378**Findings from this study suggest that progesterone-mediated increases in PDK4 expression diminish glucose oxidation in the maternal heart. Furthermore, the data show that increasing glucose oxidation in the maternal heart prevents pregancy-induced cardiac growth.
- 27.Gibb AA, Lorkiewicz PK, Zheng YT, Zhang X, Bhatnagar A, Jones SP, and Hill BG (2017) Integration of flux measurements to resolve changes in anabolic and catabolic metabolism in cardiac myocytes. Biochem J 474, 2785–2801*Results of this study complement those of Cortassa et al (ref 17) and suggest that phosphofructokinase activity regulates the activity of the pentose phosphate pathway, the hexosamine biosynthetic pathway, and the glycerolipid synthesis pathway in isolated cardiomyocytes.
- 28.Bezzerides VJ, Platt C, Lerchenmuller C, Paruchuri K, Oh NL, Xiao C, Cao Y, Mann N, Spiegelman BM, and Rosenzweig A (2016) CITED4 induces physiologic hypertrophy and promotes functional recovery after ischemic injury. JCI Insight 1, e85904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, Panakova D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, and Spiegelman BM (2010) C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 143, 1072–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, Tornheim K, and Tian R (2004) Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension 44, 662–667 [DOI] [PubMed] [Google Scholar]
- 31.Shao D, Villet O, Zhang Z, Choi SW, Yan J, Ritterhoff J, Gu H, Djukovic D, Christodoulou D, Kolwicz SC Jr., Raftery D, and Tian R (2018) Glucose promotes cell growth by suppressing branched-chain amino acid degradation. Nat Commun 9, 2935.**The findings of this study show that high levels of glycolysis in the heart, such as occurs in pressure overload-induced heart failure (see ref. 28), promotes cardiac hypertrophy by downregulating enzymes responsible for BCAA catabolism. This leads to higher levels of BCAAs, which activate mTOR and promote the hypertrophic growth of cardiomyocytes.
- 32.Kolwicz SC, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, and Tian R (2012) Cardiac-Specific Deletion of Acetyl CoA Carboxylase 2 Prevents Metabolic Remodeling During Pressure-Overload Hypertrophy. Circulation Research 111, 728–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tolwani RJ, Hamm DA, Tian L, Sharer JD, Vockley J, Rinaldo P, Matern D, Schoeb TR, and Wood PA (2005) Medium-chain acyl-CoA dehydrogenase deficiency in gene-targeted mice. PLoS Genet 1, e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernardo BC, Weeks KL, Pongsukwechkul T, Gao X, Kiriazis H, Cemerlang N, Boey EJ, Tham YK, Johnson CJ, Qian H, Du XJ, Gregorevic P, and McMullen JR (2018) Gene delivery of medium chain acylcoenzyme A dehydrogenase (MCAD) induces physiological cardiac hypertrophy and protects against pathological remodelling. Clin Sci 132, 381–397 [DOI] [PubMed] [Google Scholar]
- 35.Uchihashi M, Hoshino A, Okawa Y, Ariyoshi M, Kaimoto S, Tateishi S, Ono K, Yamanaka R, Hato D, Fushimura Y, Honda S, Fukai K, Higuchi Y, Ogata T, Iwai-Kanai E, and Matoba S (2017) Cardiac-Specific Bdh1 Overexpression Ameliorates Oxidative Stress and Cardiac Remodeling in Pressure Overload-Induced Heart Failure. Circ Heart Fail 10, e004417. [DOI] [PubMed] [Google Scholar]
- 36.Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM, Recchia FA, and Kelly DP (2019) The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 4, 124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schugar RC, Moll AR, Andre d'Avignon D, Weinheimer CJ, Kovacs A, and Crawford PA (2014) Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab 3, 754–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foryst-Ludwig A, Kreissl MC, Benz V, Brix S, Smeir E, Ban Z, Januszewicz E, Salatzki J, Grune J, Schwanstecher AK, Blumrich A, Schirbel A, Klopfleisch R, Rothe M, Blume K, Halle M, Wolfarth B, Kershaw EE, and Kintscher U (2015) Adipose Tissue Lipolysis Promotes Exercise-induced Cardiac Hypertrophy Involving the Lipokine C16:1n7-Palmitoleate. J Biol Chem 290, 23603–23615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riquelme CA, Magida JA, Harrison BC, Wall CE, Marr TG, Secor SM, and Leinwand LA (2011) Fatty acids identified in the Burmese python promote beneficial cardiac growth. Science 334, 528–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Husted AS, Trauelsen M, Rudenko O, Hjorth SA, and Schwartz TW (2017) GPCR-Mediated Signaling of Metabolites. Cell Metab 25, 777–796 [DOI] [PubMed] [Google Scholar]
- 41.Gatherer D, and Galpin V (2013) Rosen's (M,R) system in process algebra. BMC Syst Biol 7, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]