Abstract
Background
Ribosome profiling has been widely used for studies of translation under a large variety of cellular and physiological contexts. Many of these studies have greatly benefitted from a series of data-mining tools designed for dissection of the translatome from different aspects. However, as the studies of translation advance quickly, the current toolbox still falls in short, and more specialized tools are in urgent need for deeper and more efficient mining of the important and new features of the translation landscapes.
Results
Here, we present RiboMiner, a bioinformatics toolset for mining of multi-dimensional features of the translatome with ribosome profiling data. RiboMiner performs extensive quality assessment of the data and integrates a spectrum of tools for various metagene analyses of the ribosome footprints and for detailed analyses of multiple features related to translation regulation. Visualizations of all the results are available. Many of these analyses have not been provided by previous methods. RiboMiner is highly flexible, as the pipeline could be easily adapted and customized for different scopes and targets of the studies.
Conclusions
Applications of RiboMiner on two published datasets did not only reproduced the main results reported before, but also generated novel insights into the translation regulation processes. Therefore, being complementary to the current tools, RiboMiner could be a valuable resource for dissections of the translation landscapes and the translation regulations by mining the ribosome profiling data more comprehensively and with higher resolution. RiboMiner is freely available at https://github.com/xryanglab/RiboMiner and https://pypi.org/project/RiboMiner.
Background
Based on deep sequencing of the ribosome-protected mRNA fragments, ribosome profiling enables genome-wide investigations of translation with sub-codon resolution [1]. In the past decade, ribosome profiling has been widely used for studies of translation under various contexts in human and almost all the major model organisms such as bacteria, C. elegans, yeast, and mouse, et al. [2, 3]. In these studies, the most popular applications of ribosome profiling data include quantifications of translation efficiency (TE) [4, 5], annotations of open reading frames (ORFs) [4, 6], meta-gene analysis of the ribosome distribution patterns [7, 8], and identification of translation initiation sites and pausing regions [3, 7].
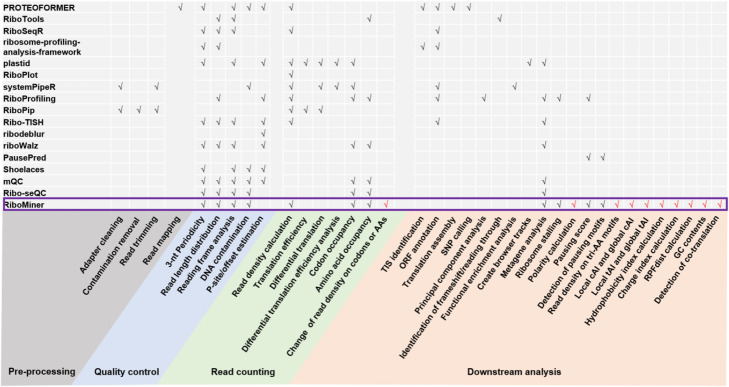
A large variety of algorithms, software, and online resources have been developed to accommodate the ever-growing needs for data processing and analysis of ribosome profiling as well as presentation and interpretation of the results [9–11]. For example, RiboCode [12], ORF-RATER [13], Ribowave [14], ORFscore [15], RiboTaper [16], and Ribo-TISH [17] were designed for annotation of active ORFs. Xtail [18], anota [19], Riborex [20], Babel [21], and RiboDiff [22] were developed for quantification of TE changes. In addition to these highly focused analyses, more customized down-stream analyses of ribosome profiling data are of great value as well for better understanding of the translatome and the translation regulation. As summarized in Fig. 1, these pipelines implemented a broad range of data analyses, from pre-processing to down-stream information mining [17, 23–36]. For example, RiboPip is focused on the pre-processing of ribosome profiling and RNA-seq data; mQC [35] and Ribo-seQC [36] were mainly designed for quality control of the data; RiboPlot [28] and Shoelaces [34] can be used for quality control and visualization of the data; PausePred [33] was specifically designed to identify pausing motifs; RiboTools [24] is a Galaxy toolbox with functions including detection of translational ambiguities and identification of readthrough events; PROTEOFORMER [23] is a python package for automatic processing of ribosome profiling data, which includes SNP calling, ORF assembly and TIS identification; Plastid [27] is used for differential translation analysis and metagene analysis; RiboProfiling [30], riboSeqR [25], and systemPipeR [29] are R packages for tasks such as data pre-processing, quality control, TE calculation and ORF annotation, et al. These tools together cover many key procedures for ribosome profiling data analysis and presentation. However, as shown in Fig. 1, new types of analyses, many of which are related to the complicated machinery of translation regulation and only emerged in recent studies, have not been implemented by the current methods.
Fig. 1.
Major functions of the data analysis tools for ribosome profiling. Partly adapted from a review article by Wang, et al. [9]. RiboMiner is highlighted at the bottom, and the red ticks represent the unique functions of RiboMiner
Here, we present RiboMiner, a python toolset for ribosome profiling data analysis, in response to the emerging needs for deeper mining of the hidden information about translation and its regulation, such as co-translation events, metagene analyses of the ribosome footprint density, and the potential regulatory factors of ribosome distribution, e.g., codon usage, tRNA gene copy numbers, and properties of nascent amino acids (Fig. 1). Characterization of these multi-dimensional features, under specific experimental or physiological conditions, provides closer views on the shift of translation landscapes with more details, which could be informative for pursuing the underlying machinery of translation regulation.
Implementation
Overview of RiboMiner
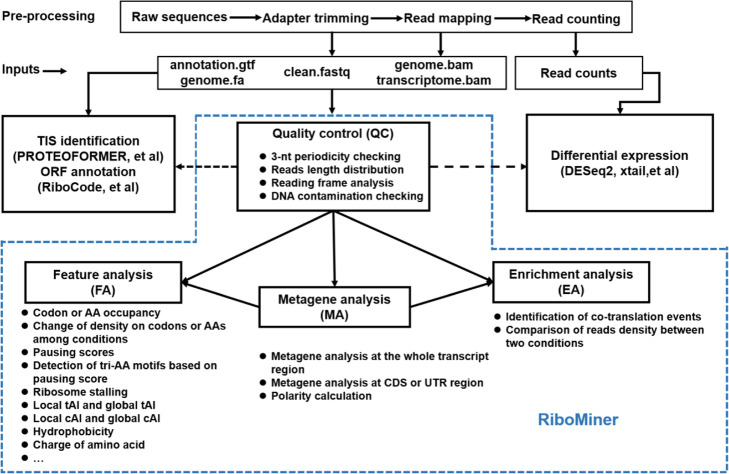
The pipeline of RiboMiner is composed of 4 major function modules (Fig. 2): 1) Quality Control (QC), designed for assessment of ribosome profiling data quality with a multitude of benchmarks including 3-nt periodicity, distribution of read lengths, reads distribution in non-coding genome/transcriptome, etc. 2) Metagene Analysis (MA) for global distribution patterns of ribosome footprints on pooled transcripts of the full or any given subset of the transcriptome. This analysis helps identifying potential ribosome stalling events that take place at the global scale or just for subsets of the transcripts. Via direct comparisons between different conditions, metagene analysis of the footprints shed lights on how and at which stage these conditions perturb translation. 3) Feature Analysis (FA) for mining of various features that are enriched in predefined gene sets, e.g., the subset of genes with ribosome stalling events detected by MA as introduced above. The features being tested here include ribosome footprint densities on different amino acid (AA) and tri-amino acid (tri-AA) motifs, local and global codon adaptation index, local and global tRNA adaptation index, AA hydrophobicity and charge, etc. By identifying such features that are enriched in selected genes, this module of functions could provide insights into the machinery of translation landscape shift, from a multitude of different perspectives. 4) Enrichment Analysis (EA), designed for direct comparison of the ribosome footprint distributions across two experiments. For instance, this function can be used for data analysis of selective ribosome profiling to help the identification of co-translation regulation.
Fig. 2.
Overview of RiboMiner. There are four function modules in RiboMiner. QC: quality control of ribosome profiling data. MA: metagene analysis for different samples with the full genome or pre-defined gene sets. FA: feature analysis for given sets of genes, which covers a series of translation-related indices. EA: enrichment analysis for the data of selective ribosome profiling to identify the potential co-translation regulation events
The python package of RiboMiner is freely available at https://github.com/xryanglab/RiboMiner and https://pypi.org/project/RiboMiner. We also offer a Docker image for RiboMiner at https://hub.docker.com/r/yanglab/ribocode_ribominer. The RiboMiner pipeline, including the testing data used in the present study, is also available as a Gene Container Service (GCS) on the Huawei Cloud.
Inputs of RiboMiner
As previously described, the common practice of raw data pre-processing before downstream analysis of ribosome profiling data includes quality control of sequencing, adapter trimming, read mapping and read counting (Fig. 2). The counts files could be used for differential expression analysis or quantification of differential translation efficiency with specialized tools such as DESeq2 [37] or Xtail [18]. The BAM files generated by read mapping can be used for TIS identification [23, 26] or ORF annotation [12, 13, 15] with specialized tools such as RiboCode [12] and PROTEOFORMER [23]. There are three categories of input files for RiboMiner (Fig. 2): 1) genome sequences in FASTA format and an annotation file in GTF format, which would be used for annotations of the RNA transcripts and the protein coding sequences; 2) RPF sequences in FASTA format, after pre-processing such as adapter trimming and quality filtering; 3) Two BAM files generated by mapping of the RPF reads to the genome and the transcriptome. These BAM files can be supplied by the users or generated by the script we have offered, which can be found on the GitHub page of RiboMiner. See supplementary file for a detailed tutorial of RiboMiner, including preparation of input files and all the down-stream analyses.
Data preparation
Alternative splicing of the eukaryotic genes generates multiple transcript isoforms for each gene [38]. To avoid ambiguous alignments of the sequencing reads, the longest transcript of each protein coding gene would be used for the following analyses. The function OutputTranscriptInfo performs this task and generates annotation files containing all the selected transcripts of the protein coding genes. GetProteinCodingSequence returns the sequences of these transcripts, the protein coding sequences, and the amino acid sequences, whereas GetUTRSequences extracts the UTR sequences of these transcripts specifically. It should be noted that all the functions above are dependent on the transcript annotation file generated by prepare_transcripts of RiboCode [12] that our group developed before.
Quality control
Four functions were designed for quality control of the ribosome footprints. Periodicity, which is adapted from metaplots of RiboCode [12], is used for assessment of the 3-nt periodicity and identification of P-sites of the ribosome footprints. It reports the distributions of RPFs aligned by their 5′ end in relative to the start and stop codons, which is done for the reads of each specific length or for all the reads combined. RiboDensityOfDiffFrames returns the read densities of each reading frame. LengthDistribution or ReadsLengthOfSpecificRegions provides the length distributions of all the ribosome footprints or the ones from specific regions such as CDS, 5’UTR, and 3’UTR. StatisticReadsOnDNAsContam counts the RPF reads mapped to introns and intergenic regions of the genome, which are potentially DNA contaminations or other non-ribosome-footprint fragments and could be indicative to the data quality in general.
Metagene analysis
Metagene analysis aligns the transcripts of all the genes or a pre-defined gene set by their start codons and quantifies the relative read densities at each nucleotide or codon. Such analysis is particularly useful for identifying the potential global ribosome stalling sites under certain experimental or physiological conditions. MetageneAnalysisForTheWholeRegions can be used for calculating the read densities along the transcripts for an overall view, which would be helpful for testing whether the ribosome distributions are biasedly enriched. The function PolarityCalculation then evaluates the ribosome distribution bias for each gene and returns an overall distribution of such bias for all the genes. Finally, MetageneAnalysis was developed to zoom in and study the footprint densities in any particular region of the transcripts, including the UTR regions. This is particularly useful for allocating the ribosome stalling regions. All the results from the functions above can be readily presented as figures (see Supplementary file).
Feature analysis
A number of factors have been shown to be involved in the regulation of translation initiation and elongation, such as poly-proline motifs [8, 39, 40], codon usage [41], tRNA gene copy numbers [42], amino acids with positive charges and high isoelectric points (pI) [43], etc. RiboMiner provides a series of functions for mining of such hidden features that are related to ribosome occupancy, with a goal of providing valuable insights into the molecular machinery of translation regulation. RiboDensityAtEachKindAAOrCodon calculates the ribosome footprint density at each amino acid (AA) or codon to show the differences under the experimental or physiological conditions. Furthermore, PausingScore was developed to quantify the ribosome density at each tri-AA motif and identify the motifs with enriched ribosome occupancy. Next, RiboDensityAroundTripleteAAMotifs can be used for computing the ribosome occupancy around the P/E site of the tri-AA motifs identified by the functions above.
tRNA adaptation index (tAI) and codon adaptation index (cAI) have been found to be potentially influential to translation, especially during elongation [41–43]. RiboMiner thereby provides two functions tAI and cAI to calculate the global tAI and cAI values as well as the local tAI and cAI values at each position along the transcripts for a specified organism. Note that although the weights of tAI are not exactly the same in all species, most of them are highly correlated to the weights fitted in yeast [44]. Finally, hydropathyCharge calculates the hydrophobicity and charge indexes of amino acids encoded by each codon along the transcripts.
Previously, the ratio of RPF read counts in 5’UTR to CDS was reported to be negatively correlated with the translation efficiency (TE) [45]. RiboMiner includes a function, RPFdist, to calculate these ratios, which would be potentially informative for evaluating the translation efficiencies in cases when the RNA-seq data in parallel with the ribosome profiling data is not available. See Supplementary file for more details about usages of these functions above.
Enrichment analysis
Direct comparison between different ribosome profiling data could reveal the translation landscape shifts in details. For example, selective ribosome profiling (SeRP) is a powerful tool for studying the interaction of molecular chaperones and their potential targeting factors in the process of translation elongation [46]. It also reveals the co-translation events among different subunits of protein complexes [47]. Detailed analysis of the data from SeRP has been carefully done [46, 48], but these pipelines are not available in any of the current data analysis programs. Thus, RiboMiner incorporated a specially designed pipeline for mining of ribosome footprint enrichments with selective ribosome profiling data, which is quite different from the normal ribosome profiling data. This pipeline is composed of at least three steps: First, RiboDensityAtEachPosition calculates the ribosome density at each position for each transcript; Second, EnrichmentAnalysis performs an enrichment analysis by calculating the ribosome density ratio at each position in one ribosome profiling data over another one, e.g., ribosome profiling with IP of a specific protein and the normal ribosome profiling data. Finally, PlotEnrichmentRatio or EnrichmentAnalysisForSingleTrans generates plots of the results. In cases of multiple replicates for each condition, enrichmentMeanDensity can be used to calculate the mean ribosome density at each position ahead of the function EnrichmentAnalysis. It is worth noting that although this module was designed for SeRP data analysis, it could also be used for read density comparison between two normal ribosome profiling data under different conditions.
Results
To showcase the application of RiboMiner for mining of insights into the translation regulation machineries, we used two published datasets, one from the study of eIF5A in translation regulation in yeast (GSE89704) [8] and the other from the study of co-translation of the aminoacyl-tRNA-synthetase complex (GSE116570) [47], which is specifically used for the Enrichment Analysis.
Quality control
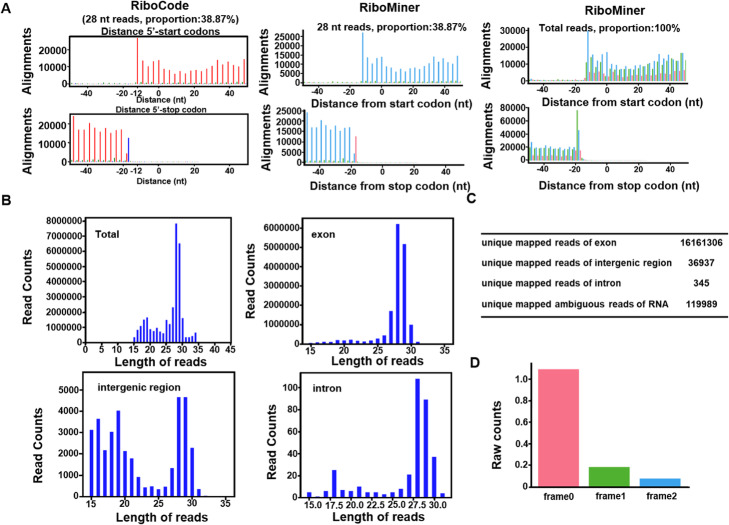
Benchmarks for the quality of a ribosome profiling dataset include 3-nt periodicity, appropriate distribution of the footprint lengths (usually ~ 28–30 nt), and enrichment in coding regions. As for GSE89704 (SRR5008135 for example), RiboMiner exhibited strong 3-nt periodicity for the reads with a specific length or for all the reads combined and aligned by their P-site positions (Fig. 3a). The lengths of ribosome footprints are usually 28 ~ 30 nt [1]. However, the footprint length distribution of SRR5008135, generated by RiboMiner, showed that although the main peak was indeed around 28–30 nt, there is a small peak at 19 nt (Fig. 3b), which seems quite abnormal. This was further addressed by genome mapping of the RPF reads by RiboMiner, which showed that a small proportion of the reads were mapped to the intergenic regions and introns, suggesting some level of potential DNA contamination (Fig. 3b, c). After removal of these reads, the footprints strongly unified around 28–30 nt. Finally, frame analysis of the footprints showed that most reads were enriched in the first reading frame (Fig. 3d). Taken together, the results above indicate generally good quality of the data and identified the source of potential contamination, which could be easily eliminated by size selection of the raw reads.
Fig. 3.
Results of the Quality Control (QC) module of RiboMiner. a 3-nt periodicity plots generated by RiboCode and RiboMiner, using the reads of 28 nt as an example. b Length distributions of all the RPF reads and the reads mapped to different genomic regions including exons, intergenic regions, and introns. c Numbers of reads mapped to different regions of the genome. d Reads mapped to different reading frames. The sample SRR5008135 in the dataset GSE89704 was used as an example here
Metagene analysis
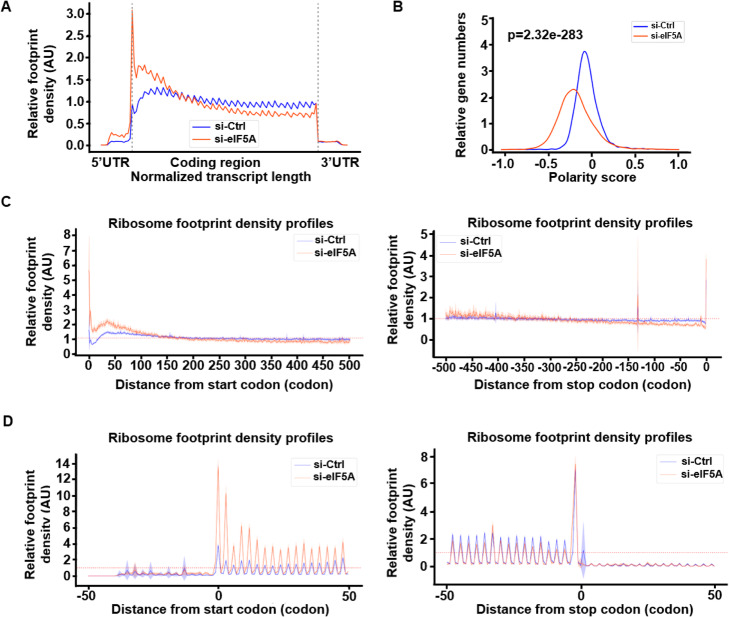
RiboMiner was used for metagene analysis of the ribosome footprint distributions with a similar procedure as previously described [8]. As shown in Fig. 4a, RiboMiner reproduced the dramatically changed pattern of ribosome occupancy upon knock-down of eIF5A, suggesting strong stalling at the early stage of elongation in the first 100 ~ 150 codons (Fig. 4a, c). Distributions of the polarity scores, generated by RiboMiner, confirmed the significant shift of the ribosome footprints towards the 5′ ends (Fig. 4b). In addition to the metagene analysis along the whole transcript or the CDS region, RiboMiner could also be used for metagene analysis for the UTR regions (Fig. 4d). Finally, it is worth noting that the metagene analysis with RiboMiner can be done at the global scale or just for a subset of the genes.
Fig. 4.
Results of Metagene Analysis (MA) by RiboMiner. a Overall read density distributions along all the transcripts merged. b Distributions of polarity scores. c Ribosome densities along the CDS regions. Left: read density after the start codon. Right: read density before the stop codon. d Ribosome densities along the UTR regions. Left: read density around the start codon. Right: read density around the stop codon. Shading area represents 95% confidence interval
Mining of features related to translation regulation
Although the global metagene analysis as shown above indicates strong ribosome stalling upon eIF5A knock-down (Fig. 4a, c), it is possible that such pattern was mainly contributed by a subset of the genes. To specifically identify the genes with ribosome footprints enriched in the first 100 codons upon eIF5A knock-down, we compared the read densities on the first 100 codons for each transcript between the control and si-eIF5A samples. Two thousand nine hundred fifty-four genes showed up-regulated ribosome densities (“up-regulated genes”) with the ratio of si-eIF5A/si-Ctrl > = 1.5. This list of genes could then be used for mining of common features that are potentially related to the ribosome stalling during early elongation.
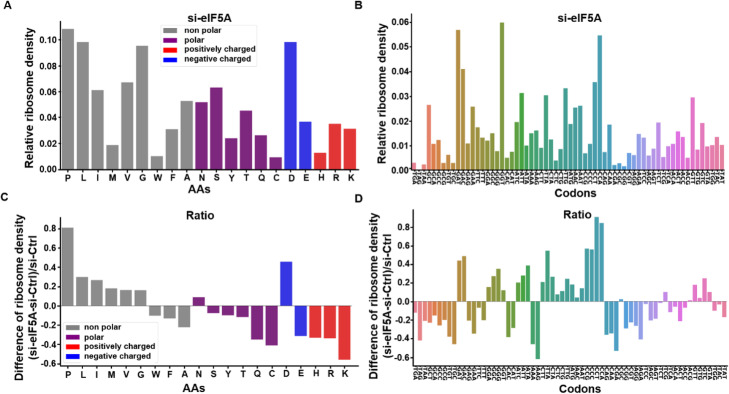
For example, RiboMiner can calculate ribosome density at each codon or amino acid (AA) for a set of genes (Fig. 5a, b) and further evaluate the change of ribosome density under different conditions (Fig. 5c, d). The results showed that read densities on proline (P) and aspartic acid (D) were largely increased after eIF5A-KD (si-eIF5A), suggesting that these amino acids may have contributed to the ribosome stalling observed above.
Fig. 5.
Comparison of the ribosome densities among different amino acids and codons. a Relative ribosome density on each amino acid for the sample of si-eIF5A. b Relative ribosome densities on different codons for the si-eIF5A sample. c The ratio (log2) of ribosome densities for each amino acid in the sample of si-eIF5A over si-Ctrl. d The ratio (log2) of ribosome densities for each codon in the sample of si-eIF5A over si-Ctrl. All the analyses above were based on the 2954 up-regulated transcripts. Ribosome densities were calculated with reads aligned by their P-sites
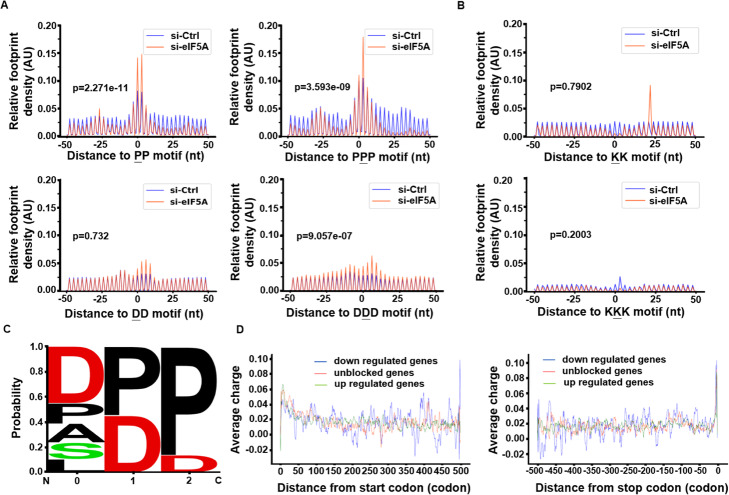
Next, RiboMiner was also used for calculating the ribosome densities on tri-AA motifs. Results showed that the motifs of poly-proline (PPP) and poly-aspartic acid (DDD) were significantly enriched by ribosome upon eIF5A knock-down (Fig. 6a), whereas the ribosome density on poly-lysine was decreased, but with no statistical significance (Fig. 6b). These results are consistent to the original reports in [8] (Fig. 6c). Besides, although the charges of amino acids have been reported to be negatively correlated to the speed of translation elongation [43], we found no difference in the charge of amino acids among the different gene sets (Fig. 6d).
Fig. 6.
Ribosome densities among tri-AA motifs. a Relative ribosome densities on poly-proline and poly-Aspartic acid. b Relative ribosome densities on poly-Lysine. c The tri-AA motifs with enriched ribosome, reported by RiboMiner. 0,1,2 represents E, P, A site of a tri-AA motif, respectively. d Average charge of the amino acids encoded by each codon along the transcripts. All the analyses above were based on the 2954 up-regulated transcripts
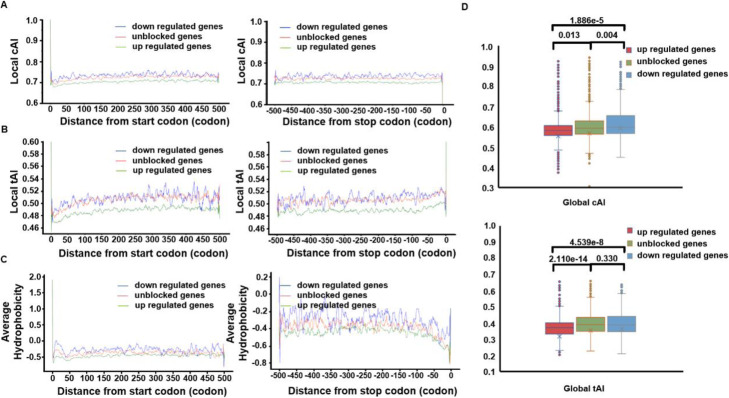
Interestingly, analysis with RiboMiner revealed some more novel features that have not been reported in the original study. For example, we found that both the local and global cAI and tAI values of the genes with ribosome enrichment during early elongation (“up-regulated gene”) tend to be much smaller than those of the other transcripts used as control (“unblocked genes” and “down-regulated genes”) and so did the local cAI and tAI values (Fig. 7a, b, d). This suggests that the “up-regulated genes” have more sub-optimal codons, which then potentially led to slower moving of the ribosomes along the transcripts. In addition, the hydrophobicity of the amino acids encoded by these “up-regulated genes” are also much smaller, which we suspect may be related to ribosome stalling as well (Fig. 7c).
Fig. 7.
Results of Feature Analysis (FA) by RiboMiner. a Distributions of local cAI along different gene sets. b Distributions of local tAI along different gene sets. c Average hydrophobicity of each amino acid encoded along the transcripts of different gene sets. d Distributions of global cAI and global tAI for different gene sets. The P-values with T-tests are provided on the plots
In summary, based on the results of RiboMiner, we reproduced the ribosome stalling event upon eIF5A knock-down as reported by the original study. Such stalling was mainly contributed by a subset of genes, and further mining of multiple features of these genes indicated that tri-AA motifs, codon usage, tRNA copy numbers, and amino acid hydrophobicity may be related to the translation dysregulations due to eIF5A knock-down. Many of these new insights have not been reported by the original study and therefore could worth further investigations.
Enrichment analysis with selective ribosome profiling data
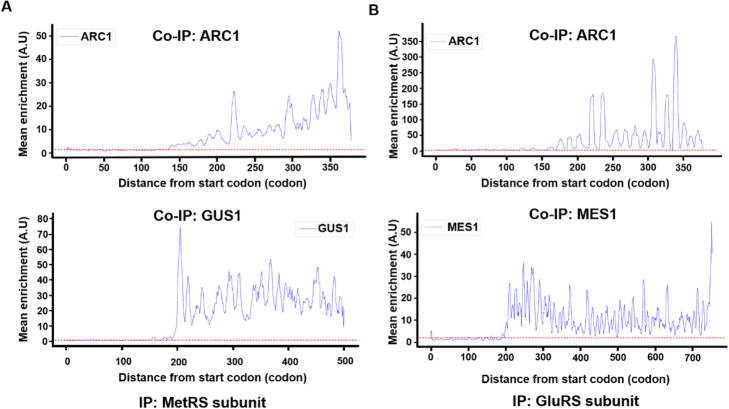
We used RiboMiner to revisit the selective ribosome profiling data in the study of the assembly of a hetero-trimeric complex, the multi-aminoacyl-tRNA synthetase [47]. This complex is composed of three major subunits, i.e., the essential methionyl- and glutamyl-tRNA synthetases MetRS and GluRS (encoded by MES1 and GUS1, respectively) and the Arc1p cofactor (encoded by ARC1) regulating the catalytic activities and subcellular distributions of the complex [47]. RiboMiner regenerated the distributions of ribosome footprints that are enriched in the selective ribosome profiling (Fig. 8). This confirms the main conclusion of the original study, i.e., proteins MetRS and GluRS could co-translate with each other, and both participate in the translation of Arc1p starting at a specific position [47].
Fig. 8.
Results of Enrichment Analysis by RiboMiner. a Engagement of nascent ARC1 (top), GUS1 (bottom) by C-terminally tagged MetRS. b Engagement of nascent ARC1 (top), MES1 (bottom) by C-terminally tagged GluRS. The red dotted lines represent the two-fold threshold
Running time of different modules of RiboMiner
Last, the running time of each module of RiboMiner with the data of 4 different model organisms are provided in Table 1. We did not consider the pre-filtering and data preparation steps. In general, the time mainly depends on the total reads, i.e., the sequencing depth. All jobs were run on a Linux server with Intel R Xeon R CPUs at 2.40GHz, 64 G memory. Note that the table reports the total running time of all the functions in each module. The scripts and results for evaluation of the running time are available at https://github.com/xryanglab/RiboMiner/tree/master/RuningTimeTest.
Table 1.
Computation time of each module in RiboMiner for different organisms
| Modules | Yeast | Fly | Mouse | Human |
|---|---|---|---|---|
| Data source | GSE89704 | GSE79626 | GSE32060 | GSE59821 |
| Test samples | SRR5008135/ SRR5008137 | SRR3297802/ SRR3297804 | SRR649755/ SRR649757 | SRR2873530/ SRR2873532 |
| Unique mapped reads | 16,318,577/ 18,441,750 | 7,627,569/ 12,099,927 | 16,457,639/ 7,282,998 | 11,557,500/ 12,896,290 |
| QC | 32.2 min | 16.3 min | 35.1 min | 29.2 min |
| MA | 24.6 min | 20.0 min | 14.2 min | 22.7 min |
| FA | 21.6 min | 17.7 min | 14.6 min | 20.0 min |
| EA | 2.4 min | 2.4 min | 2.4 min | 3.4 min |
| Total | 80.8 min | 56.4 min | 66.3 min | 75.3 min |
Conclusions
RiboMiner is a python toolset for deep mining of multi-dimensional features of the translatomes with ribosome profiling data. A multitude of functions incorporated in RiboMiner are useful for quality control of ribosome profiling data, metagene analysis for detection of translation dysregulations such as ribosome stalling, mining of various features related to the translation dysregulations, and exploration of selective ribosome profiling for fine maps of translation regulation such as co-translation. Applications of RiboMiner on two published datasets did not only reproduce the main results reported before, but also generated novel insights into the translation regulation processes. RiboMiner provides quantitative data for visualization as well as statistical analyses. In summary, here we present RiboMiner as a complementary toolset to the current methods, to facilitate the comprehensive and thorough dissections of the translation landscapes as well as the translation regulations with the technique of ribosome profiling.
Availability and requirements
Project name: RiboMiner
Project home page:https://github.com/xryanglab/RiboMiner
Operating system(s): Platform independent
Programming language: Python
Other requirements: Python (version > = 3.6)
License: GNU GPL
Any restrictions to use by non-academics: Licence needed
Supplementary information
Acknowledgements
The authors wish to acknowledge the supports from the core facilities of Genome Sequencing & Analysis and High-Performance Computing of the National Protein Science Facility (Beijing).
Abbreviations
- TE
Translation efficiency
- ORFs
Open reading frames
- TIS
Translation initiation site
- QC
Quality control
- MA
Metagene analysis
- FA
Feature analysis
- EA
Enrichment analysis
- AA
Amino acid
- RPF
Ribosome profiling footprint
- UTR
Un-translated region
- CDS
Coding sequence
- tAI
tRNA adaptation index
- cAI
Codon adaptation index
- SeRP
Selective ribosome profiling
Authors’ contributions
FL, XX and XY conceived the idea for RiboMiner. FL, XX and ZX developed the methods and python package. GX developed the docker image for RiboMiner. FL and XY wrote the article. All the authors read and approved the final manuscript.
Funding
This work was supported by the National key research and development program, Precision Medicine Project (2016YFC0906001), the Tsinghua University Spring Breeze Fund, the Tsinghua University Initiative Scientific Research Program (2019Z06QCX01), and the National Natural Science Foundation of China (81972912 and 31671381). The funding bodies did not play any roles in the design of the study, in the collection, analysis, interpretation of data, or in writing of the manuscript.
Availability of data and materials
RiboMiner is freely available at https://github.com/xryanglab/RiboMiner and https://pypi.org/project/RiboMiner. Detailed instructions and the information of all the sequencing data used in the current manuscript are available at https://github.com/xryanglab/RiboMiner/blob/master/Implementation.md. We also offer a Docker image for RiboMiner at https://hub.docker.com/r/yanglab/ribocode_ribominer. The RiboMiner pipeline, including the testing data used in the present study, is also available as a Gene Container Service (GCS) on the Huawei Cloud.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12859-020-03670-8.
References
- 1.Ingolia NT, Ghaemmaghami S, Newman JRS, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324(5924):218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingolia NT. Ribosome profiling: new views of translation, from single codons to genome scale. Nat Rev Genet. 2014;15(3):205–213. doi: 10.1038/nrg3645. [DOI] [PubMed] [Google Scholar]
- 3.Ingolia NT, Hussmann JA, Weissman JS. Ribosome Profiling: Global Views of Translation. CSH PERSPECT BIOL. 2019;11(5):a032698. 10.1101/cshperspect.a032698. [DOI] [PMC free article] [PubMed]
- 4.Zou Q, Xiao Z, Huang R, Wang X, Wang X, Zhao H, Yang X. Survey of the translation shifts in hepatocellular carcinoma with ribosome profiling. Theranostics. 2019;9(14):4141–4155. doi: 10.7150/thno.35033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Z, Hsu PJ, Xing X, Fang J, Lu Z, Zou Q, Zhang KJ, Zhang X, Zhou Y, Zhang T, et al. Mettl3−/Mettl14-mediated mRNA N (6)-methyladenosine modulates murine spermatogenesis. Cell Res. 2017;27(10):1216–1230. doi: 10.1038/cr.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson R, Kroehling L, Khitun A, Bailis W, Jarret A, York AG, Khan OM, Brewer JR, Skadow MH, Duizer C, et al. The translation of non-canonical open reading frames controls mucosal immunity. Nature. 2018;564(7736):434–438. doi: 10.1038/s41586-018-0794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin Y, Li F, Huang L, Polte C, Duan H, Fang J, Sun L, Xing X, Tian G, Cheng Y, et al. eIF3 associates with 80S ribosomes to promote translation elongation, mitochondrial homeostasis, and muscle health. Mol Cell. 2020. 10.1016/j.molcel.2020.06.003. [DOI] [PubMed]
- 8.Schuller AP, Wu CC, Dever TE, Buskirk AR, Green R. eIF5A functions globally in translation elongation and termination. Mol Cell. 2017;66(2):194–205.e195. doi: 10.1016/j.molcel.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Wang Y, Xie Z. Computational resources for ribosome profiling: from database to web server and software. Brief Bioinform. 2019;20(1):144–155. doi: 10.1093/bib/bbx093. [DOI] [PubMed] [Google Scholar]
- 10.Andreev DE, O'Connor PBF, Loughran G, Dmitriev SE, Baranov PV, Shatsky IN. Insights into the mechanisms of eukaryotic translation gained with ribosome profiling. Nucleic Acids Res. 2017;45(2):513–526. doi: 10.1093/nar/gkw1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Zhang H, Lu J. Recent advances in ribosome profiling for deciphering translational regulation. Meth. 2020;176:46–54. 10.1016/j.ymeth.2019.05.011. [DOI] [PubMed]
- 12.Xiao Z, Huang R, Xing X, Chen Y, Deng H, Yang X. De novo annotation and characterization of the translatome with ribosome profiling data. Nucleic Acids Res. 2018;46(10):e61. doi: 10.1093/nar/gky179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields AP, Rodriguez EH, Jovanovic M. A regression-based analysis of ribosome-profiling data reveals a conserved complexity to mammalian translation. Mol Cell. 2015;60(5):11. doi: 10.1016/j.molcel.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu ZY, Hu L, Shi BB, Geng SS, Xu LC, Wang D, Lu ZJ. Ribosome elongating footprints denoised by wavelet transform comprehensively characterize dynamic cellular translation events. Nucleic Acids Res. 2018;46(18):e109. doi: 10.1093/nar/gky533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bazzini AA, Johnstone TG, Christiano R, Mackowiak SD, Obermayer B, Fleming ES, Vejnar CE, Lee MT, Rajewsky N, Walther TC, et al. Identification of small ORFs in vertebrates using ribosome footprinting and evolutionary conservation. EMBO J. 2014;33(9):981–993. doi: 10.1002/embj.201488411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calviello L, Mukherjee N, Wyler E, Zauber H, Hirsekorn A, Selbach M, Landthaler M, Obermayer B, Ohler U. Detecting actively translated open reading frames in ribosome profiling data. Nat Methods. 2016;13(2):165–170. doi: 10.1038/nmeth.3688. [DOI] [PubMed] [Google Scholar]
- 17.Zhang P, He DD, Xu Y, Hou JK, Pan BF, Wang YF, Liu T, Davis CM, Ehli EA, Tan L, et al. Genome-wide identification and differential analysis of translational initiation. Nat Commun. 2017;8(1):1749. doi: 10.1038/s41467-017-01981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao Z, Zou Q, Liu Y, Yang X. Genome-wide assessment of differential translations with ribosome profiling data. Nat Commun. 2016;7:11194. doi: 10.1038/ncomms11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsson O, Sonenberg N, Nadon R. anota: analysis of differential translation in genome-wide studies. Bioinformatics. 2011;27(10):1440–1441. doi: 10.1093/bioinformatics/btr146. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Wang W, Uren PJ, Penalva LOF, Smith AD. Riborex: fast and flexible identification of differential translation from Ribo-seq data. Bioinformatics. 2017;33(11):1735–1737. doi: 10.1093/bioinformatics/btx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olshen AB, Hsieh AC, Stumpf CR, Olshen RA, Ruggero D, Taylor BS. Assessing gene-level translational control from ribosome profiling. Bioinformatics. 2013;29(23):2995–3002. doi: 10.1093/bioinformatics/btt533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong Y, Karaletsos T, Drewe P, Sreedharan VT, Kuo D, Singh K, Wendel HG, Ratsch G. RiboDiff: detecting changes of mRNA translation efficiency from ribosome footprints. Bioinformatics. 2017;33(1):139–141. doi: 10.1093/bioinformatics/btw585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crappe J, Ndah E, Koch A, Steyaert S, Gawron D, De Keulenaer S, De Meester E, De Meyer T, Van Criekinge W, Van Damme P, et al. PROTEOFORMER: deep proteome coverage through ribosome profiling and MS integration. Nucleic Acids Res. 2015;43(5):e29. doi: 10.1093/nar/gku1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Legendre R, Baudin-Baillieu A, Hatin I, Namy O. RiboTools: a galaxy toolbox for qualitative ribosome profiling analysis. Bioinformatics. 2015;31(15):2586–2588. doi: 10.1093/bioinformatics/btv174. [DOI] [PubMed] [Google Scholar]
- 25.Chung BY, Hardcastle TJ, Jones JD, Irigoyen N, Firth AE, Baulcombe DC, Brierley I. The use of duplex-specific nuclease in ribosome profiling and a user-friendly software package for Ribo-seq data analysis. RNA. 2015;21(10):1731–1745. doi: 10.1261/rna.052548.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Klerk E, Fokkema IF, Thiadens KA, Goeman JJ, Palmblad M, den Dunnen JT, von Lindern M, ‘t Hoen PA. Assessing the translational landscape of myogenic differentiation by ribosome profiling. Nucleic Acids Res. 2015;43(9):4408–4428. doi: 10.1093/nar/gkv281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn JG, Weissman JS. Plastid: nucleotide-resolution analysis of next-generation sequencing and genomics data. BMC Genomics. 2016;17(1):958. doi: 10.1186/s12864-016-3278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel AM, Mullan JPA, Velayudhan V, O'Connor PBF, Donohue CA, Baranov PV. RiboGalaxy: a browser based platform for the alignment, analysis and visualization of ribosome profiling data. RNA Biol. 2016;13(3):316–319. doi: 10.1080/15476286.2016.1141862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Backman TWH, Girke T. systemPipeR: NGS workflow and report generation environment. BMC Bioinformatics. 2016;17:388. doi: 10.1186/s12859-016-1241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popa A, Lebrigand K, Paquet A, Nottet N, Robbe-Sermesant K, Waldmann R, Barbry P. RiboProfiling: a Bioconductor package for standard Ribo-seq pipeline processing. F1000Res. 2016;5:1309. doi: 10.12688/f1000research.8964.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Kingsford C, McManus CJ. Using the Ribodeblur pipeline to recover A-sites from yeast ribosome profiling data. Methods. 2018;137:67–70. doi: 10.1016/j.ymeth.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauria F, Tebaldi T, Bernabo P, Groen EJN, Gillingwater TH, Viero G. riboWaltz: optimization of ribosome P-site positioning in ribosome profiling data. PLoS Comput Biol. 2018;14(8):e1006169. doi: 10.1371/journal.pcbi.1006169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumari R, Michel AM, Baranov PV. PausePred and Rfeet: webtools for inferring ribosome pauses and visualizing footprint density from ribosome profiling data. RNA. 2018;24(10):1297–1304. doi: 10.1261/rna.065235.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birkeland A, Chyzynska K, Valen E. Shoelaces: an interactive tool for ribosome profiling processing and visualization. BMC Genomics. 2018;19(1):543. doi: 10.1186/s12864-018-4912-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verbruggen S, Menschaert G. mQC: A post-mapping data exploration tool for ribosome profiling. Comput Methods Programs Biomed. 2019;181:104806. 10.1016/j.cmpb.2018.10.018. [DOI] [PubMed]
- 36.Calviello L, Sydow D, Harnett D, Ohler U. Ribo-seQC: Comprehensive analysis of cytoplasmic and organellar ribosome profiling data. Cold Spring Harbor Laboratory Press; 2019. 10.1101/601468.
- 37.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291–336. doi: 10.1146/annurev.biochem.72.121801.161720. [DOI] [PubMed] [Google Scholar]
- 39.Doerfel LK, Wohlgemuth I, Kothe C, Peske F, Urlaub H, Rodnina MV. EF-P is essential for rapid synthesis of proteins containing consecutive Proline residues. Science. 2013;339(6115):85–88. doi: 10.1126/science.1229017. [DOI] [PubMed] [Google Scholar]
- 40.Woolstenhulme CJ, Guydosh NR, Green R, Buskirk AR. High-precision analysis of translational pausing by ribosome profiling in Bacteria lacking EFP. Cell Rep. 2015;11(1):13–21. doi: 10.1016/j.celrep.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S, Hu H, Zhou J, He X, Jiang T, Zeng J. Analysis of ribosome stalling and translation elongation dynamics by deep learning. Cell Syst. 2017;5(3):212–220. doi: 10.1016/j.cels.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Tuller T, Carmi A, Vestsigian K, Navon S, Dorfan Y, Zaborske J, Pan T, Dahan O, Furman I, Pilpel Y. An evolutionarily conserved mechanism for controlling the efficiency of protein translation. Cell. 2010;141(2):344–354. doi: 10.1016/j.cell.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 43.Riba A, Di Nanni N, Mittal N, Arhne E, Schmidt A, Zavolan M. Protein synthesis rates and ribosome occupancies reveal determinants of translation elongation rates. Proc Natl Acad Sci U S A. 2019;116(30):15023–15032. doi: 10.1073/pnas.1817299116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabi R, Daniel RV, Tuller T. stAI (calc): tRNA adaptation index calculator based on species-specific weights. Bioinformatics. 2017;33(4):589–591. doi: 10.1093/bioinformatics/btw647. [DOI] [PubMed] [Google Scholar]
- 45.Murat P, Marsico G, Herdy B, Ghanbarian AT, Portella G, Balasubramanian S. RNA G-quadruplexes at upstream open reading frames cause DHX36- and DHX9-dependent translation of human mRNAs. Genome Biol. 2018;19(1):229. doi: 10.1186/s13059-018-1602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becker AH, Oh E, Weissman JS, Kramer G, Bukau B. Selective ribosome profiling as a tool for studying the interaction of chaperones and targeting factors with nascent polypeptide chains and ribosomes. Nat Protoc. 2013;8(11):2212–2239. doi: 10.1038/nprot.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shiber A, Doring K, Friedrich U, Klann K, Merker D, Zedan M, Tippmann F, Kramer G, Bukau B. Cotranslational assembly of protein complexes in eukaryotes revealed by ribosome profiling. Nature. 2018;561(7722):268–272. doi: 10.1038/s41586-018-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galmozzi CV, Merker D, Friedrich UA, Döring K, Kramer G. Selective ribosome profiling to study interactions of translating ribosomes in yeast. Nat Protoc. 2019;14(8):2279–2317. doi: 10.1038/s41596-019-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RiboMiner is freely available at https://github.com/xryanglab/RiboMiner and https://pypi.org/project/RiboMiner. Detailed instructions and the information of all the sequencing data used in the current manuscript are available at https://github.com/xryanglab/RiboMiner/blob/master/Implementation.md. We also offer a Docker image for RiboMiner at https://hub.docker.com/r/yanglab/ribocode_ribominer. The RiboMiner pipeline, including the testing data used in the present study, is also available as a Gene Container Service (GCS) on the Huawei Cloud.