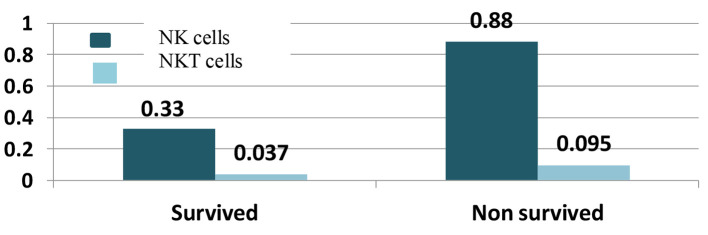Abstract
Background
Natural killer (NK) function defects have been seen in many hematological malignancies, including acute myeloid leukemia (AML). AML is associated with deficient human leukocyte antigen (HLA) expression on leukemia blasts which become targets for killing by NK and natural killer-like T (NKT) cells. However, NK and NKT cells are not effective in killing autologous leukemia blasts, maybe due to number or functional abnormalities. The aim of the work was to detect the number and percentage of NK and NKT cells in patients with AML and the impact of their percentage on the prognosis, response to treatment and survival.
Methods
Bone marrow and peripheral blood samples were collected from 50 adult patients diagnosed as de novo AML who presented to the Hematology Unit in the Oncology Center Mansoura University (OCMU) at time of diagnosis. NK and NKT cells were detected by using immunophenotyping by expression of cell surface and cytoplasmic markers (anti-CD3 fluorescein isothiocyanate (FITC), anti-CD16/56 phycoerythrin (PE)).
Results
We observed significant reduction in the median values of NK and NKT cells in AML patients in comparison to normal values. There was an insignificant correlation to response to induction treatment. While a significant correlation to overall survival (OS) (P = 0.03) was observed. The correlation to risk stratification was significant with NK cells (P < 0.001), but not with NKT cells (P = 0.23).
Conclusion
We concluded that the number and percentage of NK and NKT cells decreased significantly in AML patients and the frequency of NK and NKT cells is inversely proportionate with prognosis and OS in studied AML patients. We recommend correlating both number and function of NK and NKT cells in future studies to help provide a wide field of interest for possibility of demonstrating novel therapies using NK cells for curing AML.
Keywords: AML, NK cells, NKT cells
Introduction
Clinical usage of natural killer (NK) and natural killer-like T (NKT) cells in treatment of leukemia is considered a field of interest and under intense investigations based on the evidence that both the innate and adaptive immune systems have the ability to identify acute myeloid leukemia (AML) cells, killing leukemic cells or keeping a balance in inhibiting tumor proliferation and spread. AML can escape from NK cell immune surveillance by multiple mechanisms, which all share in an abnormal anti-leukemic immune response including abnormalities of NK cell, immunosuppressive, devious characters of leukemic cells and interactions with other immune cells for immune escape [1].
The development of new immunotherapeutic therapies is allowed by improved understanding of the biology of NK cells because for NK and NKT cells unlike T lymphocytes, their anti-tumor activity does not depend on recognition of tumor-specific antigens, which remain poorly defined for most of malignancies [2].
Many limitations are detected during use of the autologous or allogenic transfer of NK cells, including poor survival of NK cells, reduced activity by inhibitory receptors or decreased signaling via activating receptors (e.g. shedding of ligands or downregulation of activating receptors) [2].
Some functional studies have been interested in explaining weaken function of NK cell and impaired cytokine production in myeloid neoplasms, mainly AML, that may return to normal after complete remission but these studies did not handle the absolute/relative numbers or percentage of NK cells or their subsets and their correlation with survival. But in our case-control study, prospective data detecting the number and percentage but not the function of NK cells depend on available surface markers evaluated at the time of diagnosis in bone marrow (BM) samples from AML patients.
Patients and Methods
Patients and treatment strategy
This study was an observational prospective study that was approved by the Institutional Review Board of the Faculty of Medicine Mansoura University. It was conducted on 50 adult patients of AML who presented to the Hematology Unit in the Oncology Center Mansoura University (OCMU) from March 2015 to April 2016. All patients met the inclusion and exclusion criteria and informed consent was provided according to the Declaration of Helsinki.
For fit patients (age < 65 years, low performance status, no or controlled comorbidities, high and intermediate risk patients), 3 + 7 protocol was used (3 days doxorubicin by dose 30 mg/m2 + 7 days cytarabine by dose 200 mg divided every 12 h), but for unfit patients (elderly patients > 65 years, high performance status, uncontrolled comorbidities), subcutaneous Ara C (cytarabine given SC by dose 20 mg/12 h for 10 days) was used. In cases of acute promyelocytic leukemia (APL), all trans-retinoic acid (ATRA) with induction chemotherapy (3 days doxorubicin by dose 30 mg/m2) or 7 + 3 with ATRA was used.
In consolidation regimens for patients in remission, either 2 + 5 protocol (2 days doxorubicin by dose 30 mg/m2 + 5 days cytarabine by dose 200 mg divided every 12 h) one cycle, then high-dose Ara C (HDA) for three cycles, or HDA (cytarabine given by dose of 2 g/m2/12 h for 3 days day after day) for 3 - 4 cycles, was used.
Re-induction treatment was used for patients who achieved partial hematological response (reduction of blast cells > 50% of their number at diagnosis) after first cycle in the form of 7 + 3 and 5 + 2 protocols. Salvage protocols were used for refractory and relapsed cases: FLAG protocol (fludarabine 25 mg/m2 + cytarabine 2 g/m2 for 5 days) and HAM protocol (cytarabine 2 g/m2/12 h for 3 days + mitoxantrone 20 mg/m2 for 3 days). Patients with human leukocyte antigen (HLA)-matched sibling were referred for allogenic BM transplantation.
Diagnosis of AML and detection of NK cells
AML patients are diagnosed by ≥ 20% of blast count in marrow or blood samples, except for AML with t(8;21), t(15;17), inv(16) and other cytogenetics. Myeloblast, monoblasts and megakaryoblasts are included in the blast count. In AML with monocytes or myelomonocytic differentiation, monoblasts and promonocytes, but not abnormal monocytes, are included as blast cells according to World Health Organization (WHO) diagnostic criteria. This diagnosis was confirmed by using immunophenotyping through the detection of expression of cell surface and cytoplasmic markers.
Cell counting, preparation, staining and fluorescence-activated cell sorting (FACS) analysis
All samples were processed within 24 h after blood withdrawal through the following steps. 1) The whole Di-K-EDTA (ethylene diamine tetra acetic acid) blood samples were counted by an automated hematology analyzer (Cell-Dyn 1800 Abbot Diagnostics, USA), and then the leukocyte count was recorded. 2) Leucocytes were prepared by incubation of whole blood with a fourfold excess of ammonium chloride (NH4Cl 0.86% in distilled H2O) in Falcon tubes at room temperature for 15 min. 3) The tubes were centrifuged at 2,000 rpm for 5 min, and then the excess of ammonium chloride was discarded leaving the pellet of cells at the bottom of the tube. 4) Three milliliter of phosphate-buffered saline (PBS) was added to the tubes, well mixed by vortex, and then the tubes were centrifuged for 5 min at 2,000 rpm. 5) Excess PBS was removed, and then the pellet of the cells was mixed with 1 mL PBS, well mixed by vortex, and then counted using an automated hematology analyzer. 6) The count was adjusted to be 1 × 106 cells suspended in 1 mL PBS and well mixed by vortex. 7) Two hundred micrometers of the cells suspension were aliquoted into three tubes, and then antibodies were added to each tube. 8) Cells were incubated with 5 µL of the following antibody mixes: a) Anti-CD3 fluorescein isothiocyanate (FITC), anti-CD16/56 phycoerythrin (PE). b) Unstained tube represents the auto fluorescence of the cells to each filter. c) Anti-IgG1kappa FITC and PE (isotopic control for CD3 FITC, CD16/56 PE). 9) Cells were incubated for 20 - 30 min in the dark at 4 °C, then washed twice with PBS, suspended in 1 mL PBS and acquired immediately using an FACS Canto II flow cytometer.
For each sample at least 100,000 events were acquired on an FACS Canto II which is equipped with blue (488-nm, air-cooled, 20-mW solid state, red 633-nm, 17-mW HeNe), and violet (405-nm, 30-mW solid state) (Becton Dickinson, USA) and analyzed with the FACS DIVA software system (Becton Dickinson, USA).
Statistical analysis
The statistical analysis of data was done by using excel (Office program 2010) and SPSS program (SPSS, Inc., Chicago, IL) version 20. Kolmogorov-Smirnov test was done to evaluate the normality of distribution of data. Qualitative data were detected as number and percentage. Chi-square or Fisher’s exact tests were used in group’s comparison. Quantitative data were detected as mean, standard deviation, median and range. Student’s t-test and Mann-Whitney test (for non-parametric data) were used for comparison between two groups. To compare between more than two groups, analysis of variance (ANOVA) (for non-parametric data) was used. Also, Pearson correlation was used to correlate between different parameters. Kaplan-Meier test was used for survival analysis and the statistical significance of differences among curves was determined by log-rank test. P-value less than 0.05 was considered statistically significant. Receiver operating characteristic (ROC) curves were used to demonstrate the association between achieving MR4.5 and white blood cells counts at presentation. The optimal thresholds along the ROC curves were calculated using the Youden index (sensitivity + specificity - 1).
Results
In this study, the mean age of studied AML cases was 44.7 ± 15.36 years. Twenty-five patients (50%) were males and the same percent was females in the studied AML cases as shown in Table 1.
Table 1. Demographic Data of Studied AML Cases in Relation to Control.
| Demographic data | Cases (N = 50) |
|---|---|
| Age | |
| Mean | 44.7 |
| Standard deviation | 15.36 |
| Gender | |
| Male, n (%) | 25 (50) |
| Female, n (%) | 25 (50) |
AML: acute myeloid leukemia.
The patients were diagnosed according to the WHO classification (2016), and percentages are shown in Table 2.
Table 2. Laboratory Data of the Studied AML Cases at Presentation.
| Parameters | Number of cases (N = 50) |
|
|---|---|---|
| N | % | |
| WHO classification of AML cases | ||
| AML with recurrent cytogenetics | ||
| AML with t(8;21) | 4 | 8.0 |
| AML with inv16 | 3 | 6.0 |
| Acute promyelocytic leukemia with PML/RARA | 7 | 14.0 |
| AML with t(6;9) | 1 | 2.0 |
| AML with bi-allelic mutation of CEPBA | 1 | 2.0 |
| AML without maturation | 7 | 14.0 |
| AML with maturation | 12 | 24.0 |
| Acute myelomonocytic leukemia | 10 | 20.0 |
| Acute monoblastic/monocytic leukemia | 3 | 6.0 |
| Pure erythroid leukemia | 2 | 4.0 |
| Conventional karyotyping | ||
| Normal | 43 | 86.0 |
| 47,xy,+8 | 4 | 8.0 |
| 47,xy,+9 | 1 | 2.0 |
| 46,xy,-9 | 1 | 2.0 |
| 46,xy,t(6:9) | 1 | 2.0 |
| Cancer cytogenetics (FISH) | 34 | |
| Positive | ||
| inv16 | 3 | 6.0 |
| t(15;17) | 7 | 14.0 |
| t(8;21) | 4 | 8.0 |
| Negative | 20 | 40 |
| Not available | 16 | 32 |
AML: acute myeloid leukemia; FISH: fluorescent in situ hybridization.
In this study, the patients were classified into three risk categories according to available data. Five patients (10%) were categorized as low risk group, 31 patients (62%) were considered intermediate risk and 14 patients (28%) were classified as high risk group.
Almost AML patients included in this study received treatment according to the induction protocols, 37 (74%) patients received 7 + 3 protocol and three patients (6%) received SC Ara C as they refused ordinary regimens. From the seven patients who were diagnosed as APL, five patients (10%) received 3 days doxorubicin with ATRA and the other two patients (4%) received 7 + 3 with ATRA. The remaining three patients (6%) died before receiving induction treatment.
Regarding the assessment of response to treatment after induction, complete hematological remission was achieved in 17 patients (34%), partial response was achieved in eight patients (26%) and treatment failure occurred in 22 patients (44%) as five patients (10%) were refractory to treatment and 14 patients (28%) died in aplasia as shown in Table 3.
Table 3. Response to Induction Treatment in Studies AML Patients.
| Response to induction treatment | Number of patients (N = 44) |
|
|---|---|---|
| N | % | |
| CR | 17 | 34.0 |
| PR | 8 | 26.0 |
| Refractory | 5 | 10.0 |
| Died in aplasia | 14 | 28.0 |
AML: acute myeloid leukemia; CR: complete remission; PR: partial response.
NK cells studied in AML cases ranged from 0 to 3.70 × 109/µL (0-11.66%) with median 0.595 × 109/µL (4.24%). Mature NK (mNK) cells were classified according to IPT into two types: NK CD56bright and NK CD56dim, and most of cells detected in our study were NK CD56dim by about 65%. As regards NKT cells, they ranged from 0 to 2.01 × 109/µL (0-11.56%) in AML cases with median 0.070 × 109/µL (1.375%).
NK cells showed weak positive correlation with different parameters with insignificant P values including blast cell percentage in peripheral blood (PB) and in BM. While NKT cells showed weak negative correlation with the rest of parameters with insignificant P value except for blast cells in PB in which r is 0.292 with P value of 0.040.
As regards response to induction treatment, in patients with complete hematological response, NK cells ranged from 0 to 1.90 × 109/µL (0-10.47%) with median 0.435 × 109/µL (4.06%), while in patients with partial response, they ranged from 0 to 1.02 × 109/µL (0-11.66%) with median 0.5352 × 109/µL (3.85%). While in refractory patients, they ranged from 0 to 2.56 × 109/µL (0-11%) with median 0.88 × 109/µL (5.50%) and they ranged from 0.08 to 3.70 × 109/µL (0.56-11.30%) with median 0.77 × 109/µL (4.50%) in patients who died after induction with insignificant P value of 0.720 as shown in Table 4. Also, median values of NKT cells in relation to induction treatment are shown in Table 4 and comparison of P value between groups is shown in Table 5.
Table 4. NK Cells and NKT Cells With Response to Induction Treatment.
| Response to induction | NK cells |
NKT cells |
||
|---|---|---|---|---|
| Median | Range | Median | Range | |
| CR | 0.4350 | 0.00 - 1.90 | 0.026 | 0.01 - 0.390 |
| PR | 0.5325 | 0.00 - 1.02 | 0.095 | 0.004 - 0.270 |
| Refractory | 0.88 | 0.00 - 2.56 | 0.050 | 0.020 - 0.210 |
| Died after induction | 0.77 | 0.08 - 3.70 | 0.090 | 0.00 - 2.01 |
NK: natural killer; NKT: natural killer-like T; CR: complete remission; PR: partial response.
Table 5. Comparison of P Values Between Groups.
| Response to induction groups | NK cells | NKT cells |
|---|---|---|
| CR vs. PR | 0.862 | 0.63 |
| CR vs. refractory cases | 0.29 | 0.33 |
| CR vs. died after induction | 0.43 | 0.56 |
NK: natural killer; NKT: natural killer-like T; CR: complete remission; PR: partial response.
Based on the results shown in Figure 1, NK cells ranged from 0 to 2.60 × 109/µL (0-11.66%) with median 0.33 × 109/µL (3.58%) in survived cases, while they ranged from 0 to 3.70 × 109/µL (0-11.47%) with median 0.88 × 109/µL (4.31%) in non-survived patients with significant P value of 0.035.
Figure 1.
Natural killer (NK) and natural killer-like T (NKT) cells with survival.
NKT cells were also correlated with survival of AML patients, the median of NKT cells was 0.037 × 109/µL (1.205%) in survived patients with a range from 0.004 to 0.330 × 109/µL (0.06-23.25%) and the median was 0.095 × 109/µL (1.825%) with a range from 0 to 2.01 × 109/µL (0-9.88%) in non-survived with significant P value of 0.036 as shown in Figure 1.
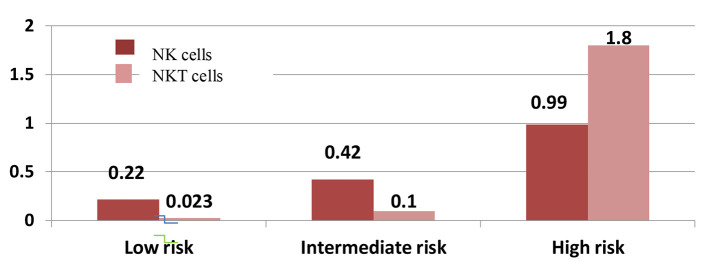
As regards NK cells and risk stratification, patients with low risk had NK cells in a range from 0 to 0.76 × 109/µL (0-9.50%) with median 0.22 × 109/µL (3.54%), patients with intermediate risk had NK cell range from 0 to 2.60 × 109/µL (0-11.66%) with median 0.42 × 109/µL (4.10%) and patients with high risk had range from 0.22 to 3.7 × 109/µL (0.56-11.30%) with median 0.99 × 109/µL (4.84%) with significant P value of 0.001 as shown in Figure 2 (P values comparison between groups is shown in Table 6).
Figure 2.
Natural killer (NK) and natural killer-like T (NKT) cells with risk stratification.
Table 6. Comparison of P Values According to Risk Stratification.
| Risk groups | NK cells | NKT cells |
|---|---|---|
| Low vs. intermediate risk | 0.29 | 0.39 |
| Low vs. high risk | 0.009 | 0.05 |
| Intermediate vs. high risk | 0.06 | 0.12 |
NK: natural killer; NKT: natural killer-like T.
For correlation of NKT cells with risk stratification of studied AML cases, their median in low risk patients was 0.023 × 109/µL (1.115%) with a range from 0.01 to 0.088 × 109/µL (0.68-7.34%), in intermediate risk patients, the median was 0.100 × 109/µL (1.87%) with a range from 0 to 1.46 × 109/µL (0-23.25%) and the median was 0.040 × 109/µL (1.09%) in high risk patients with a range from 0.008 to 2.01 × 109/µL (0.03-3.66%) with insignificant P value of 0.328 as shown in Figure 2 (P values comparison between groups is shown in Table 6).
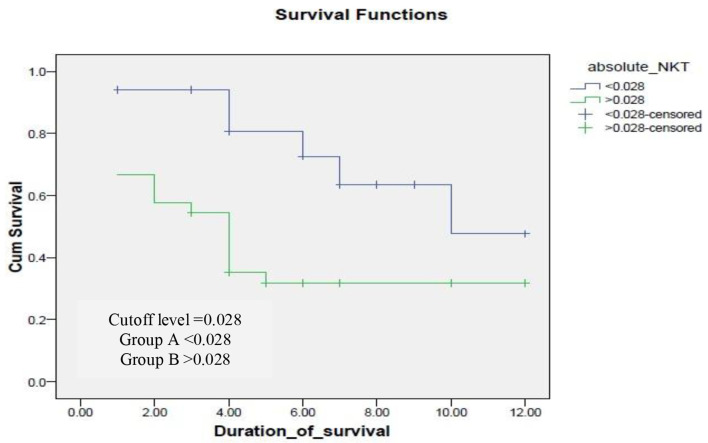
ROC curve was done for both absolute number and percentage of NK and NKT cells to evaluate presence of significant cutoff level. It revealed no significant cutoff except for an absolute number of NKT cells of 0.028 with P value of 0.020 with sensitivity 81.5% and specificity 48% with area under the curve (AUC) of 69%. The cases were divided into two groups: group A < 0.028 (number of cases = 17) and group B > 0.028 (number of cases = 33).
Kaplan-Mayer survival analysis was conducted to compare OS between both groups. In group A < 0.028, the median survival was 10 months, while in group B, the median survival was 4 months as shown in Figure 3. A log-rank test was conducted and revealed that the survival distributions for both groups were statistically significant (P = 0.02).
Figure 3.
Survival of cases in according to cutoff level of natural killer-like T (NKT) cells.
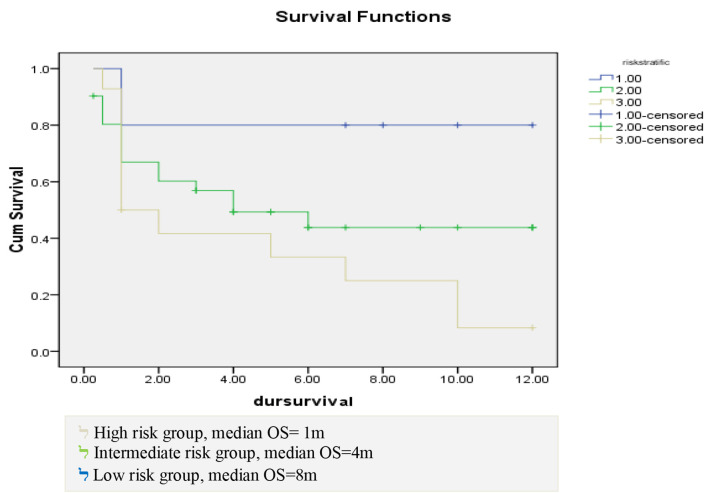
OS differed according to risk stratification in this study. In low risk patients, the median of OS was 8 months with a range from 1 to 12 months, in intermediate risk patients, the median duration was 4 months with a range from 0 to 8.18 months, while in high risk patients, the median duration of OS was 1 month with a range from 0 to 1.76 months. Estimated OS was 80%, 50% and 35% at 6 months interval in low, intermediate and high risk patients, respectively, while estimated OS was 80%, 50% and 10% at 12 months interval in low, intermediate and high risk patients, respectively with significant P value of 0.036 as shown in Figure 4.
Figure 4.
Correlation between risk stratification and duration of survival.
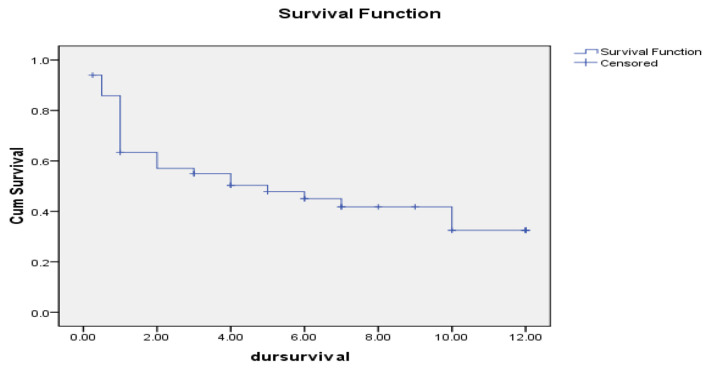
At the end of follow-up period (12 months), the median OS of studied AML cases was 5 months with a range from 0.234 to 9.766 months. OS estimated 45% at 6 months interval and 30% at 12 months interval as shown in Figure 5.
Figure 5.
Overall survival of studied acute myeloid leukemia cases.
As regards event-free survival (EFS), it was 77% at 6 and 12 months with median 10.14 months and a range from 8.5 to 11.7 months in studied AML cases. Relapse-free survival (RFS) was also 77% at 6 and 12 months interval with median 10.12 months and a range from 7.02 to 9.22 months.
Discussion
Normally, NK cells present in PB in 3-20% of circulating lymphocytes in males and 1-28% in females with different variation according to many factors such as hormones, ethnic and environmental factors [3]. NKT cells form normally 5-15% of peripheral T lymphocytes [4]. In our study, we found the range of NK cells in females 2-11% and in males 1-14%, and range of NKT cells in 1.5-9.5% from total lymphocytes in PB in both genders.
Some studies were interested in correlation of NK cells to age and gender. Regarding age, no significant differences were found [5, 6]. While as regards gender, some studies suggested that no significant gender-related difference was found in contrast to other studies which found significant fluctuation of NK cells in females in certain situations as menstruation [7, 8]. As regards NKT cells, studies suggested reduction in their number and function with aging [9]. In our study, there is no significant correlation to age for both NK and NKT cells.
In this study, the median age of AML group was 44.7 ± 15.36 years, from the fact that AML affects mostly old age patients and aging may affect NK and NKT cells at many levels as in frequency and capability of NK cells to respond and produce cytokines after stimulation, a reduction in intracellular signaling of NK and NKT cells and its cytotoxicity reduction and this is accompanied with an increased risk of infections and death rate [10, 11].
mNK group differs in the expression of their surface receptor, functional capability and proliferation. There are mainly two functionally mNK subtypes present in humans’ PB (NK CD56dim) and other NK cell subtypes are present in the thymus, liver and uterus (NK CD 56bright) [12].
In this study, most of the detected NK cells were NK CD56dim. According to Spits et al [12], CD56dim NK cells (negative for CD34 and CD117, dimming for CD94 and bright CD16) dominate in PB producing lower levels of monokine-stimulated cytokine, but those cells are more powerful in production of target cell-induced cytokine and cytotoxicity that gives us a clue that increasing this type of cells will be associated with better prognosis in AML cases. But in our study, the higher percentage correlates with poor prognosis. In our opinion, this can be explained that autologous NK cells have been shown to be functionally weak against many neoplastic clones that arise in both solid tumors and other neoplasms including AML, maybe due to inhibition by the physiologic identification of “self” major histocompatibility complex (MHC) molecules on the malignant cells by the NK cell identification [13].
Results in this study revealed significant decreases in number and percentage of mNK cells and NKT cells in patients with AML. As regards NKT cells, we found that their number decreased significantly in studied AML cases, which agreed with Aggarwal et al [13], who studied the percentage of NKT cells in AML and myelodysplastic syndrome (MDS) cases, and reported that the percentage of NKT cells was lower in AML cases than in controls. Our results also agreed with Boeck et al [14], which studied NK and NKT in different types of leukemia including AML, acute lymphoid leukemia (ALL) and chronic lymphocytic leukemia (CLL) and reported that their frequencies decreased in patients in comparison to healthy controls.
Zimmer et al [15] studied both the number and function of NK cells only not NKT cells in 30 non-promyelocytic patients aged 60 - 80 years who achieved first complete remission after chemotherapy and samples of PB were examined before consolidation and every week after treatment for 8 weeks in comparison to 15 controls, NK cell numbers in the patients were decreased before treatment in comparison with the control group and it was suggested that the expression of activating receptors of NK cells much differed in patients in comparison with the control group. So, the study concluded that the expected basis of the modification pattern may depend on an interaction between the direct and indirect effects of treatment, enhancement of immune system, differentiation of NK cells and its interaction with AML blasts which agreed with our results in this study.
Vidriales et al [16] found that NK cells were significantly increased at the diagnosis of AML cases and their level in PB correlated with those of BM and this result was opposite to our results and to the results of the above mentioned studies. Also, Vidriales et al (1993) evaluated NK cells in patients who achieved complete remission and concluded that the NK cells reduced to be within the normal range but we did not assess NK after chemotherapy.
In this study, we correlated NK with laboratory data of AML patients at diagnosis including blast cell count in PB and BM and concluded that increasing blasts in PB and BM was associated with higher NK cells which agreed with results reported by Vidriales et al [16]. In contrast to Boeck et al [14] who reported high blasts count in studied patients including AML, ALL patients directly correlated with lower numbers of NK.
As regards NKT with lab data, our results showed that lower NKT cells were associated with higher blasts in PB and this was agreed with Boeck et al [14], who reported that high blasts count in studied patients including AML, ALL patients directly correlated with lower numbers of NKT.
We correlated in this study the number and percentage of NK cells with risk stratification of the studied patients and reported that there is significant variation of NK cells number in different risk groups, as high risk AML group shows higher NK cells percentage than low and intermediate risk groups. On the other hand, there was no significant correlation between number and percentage of NKT cells with risk stratification of the studied cases.
These findings are agreed with Levy et al [17] and Aggarwal et al [13], who studied the proportionality of NK and NKT cells in AML and MDS patients in a case-control study, and reported that the mNK cells (CD56dim/CD16bright) are lower than controls and relatively increased in high risk AML patients, while it did not significantly vary between MDS BM samples in comparison to the controls or patients with AML.
This study focused on assessing number and percentage of autologous NK cells in AML patients and we concluded that higher numbers of NK and NKT cells were associated with lower survival of those patients. On the other hand, some studies focused on assessing number and percentage of NK cells during their adoptive transfer for leukemia treatment in contrast to manipulated donor lymphocyte infusion (DLI). Suck et al [1] studied the role of NK cells in allogeneic hematopoietic stem cell transplantation (allo-HSCT) in haplo-identical transplants, which included excessive T-cell depletion of three-locus mismatched hematopoietic stem cell grafts, thus making effective transplantation upon the MHC barrier. Very large doses of T-cell-depleted grafts together with no given drugs for prophylaxis against graft-versus-host disease (GVHD), NK cells recovered rapidly and had a critical role in immune recovery and had a strong anti-leukemic activity.
NK cell number is significantly correlated with survival and prognosis of studied AML cases as we concluded that a lower percentage of NK (CD65dim) cells at time of diagnosis came with better survival and this goes with Aggarwal et al [13]. It was also reported that a lower NK (CD65dim) cells proportion came with better survival in AML but did not correlate with survival in MDS cases. While Alcasid et al’s [2] study was opposed to our results as it studied 130 AML patients and reported that NK cells (specifically CD56+/CD16+ NK cells at a cutoff of ≥ 5%) were found to be an independent indicator of improved overall and disease-free survival.
Boeck et al [14] also did not agree with our results and reported that increased frequency of NK and cells correlated with better prognosis and OS of studied cases of different types of leukemia including AML, ALL and CLL.
This study also reported that there is significant correlation between reduction of number and percentage of NKT cells with prognosis and survival of studied AML cases, as cases were divided according to cutoff level of 0.028/µL into group A < 0.028/µL with better survival. This is not agreed with Chuc et al [8], who studied invariant NKT cells in 28 de novo AML cases in comparison with healthy donors and patients with megaloblastic anemia and reported that reduced number of NKT cells came with lower OS with cutoff of 0.2/µL and concluded that invariant NKT < 0.2/µL was defined as an independent variable for poor prognosis of newly diagnosed AML cases.
In conclusion, NK and NKT cells affect prognosis and survival of AML either by their percentage and proportion or by their functions. Therefore, we recommend correlating both number and function of NK and NKT cells in future studies to help provide a wide field of interest for possibility of demonstrating novel therapies using NK cells for curing AML.
Acknowledgments
I am thankful to my colleagues in hematology department including the residents and assistant lecturers, nursing staff, laboratory and radiology technicians in Oncology Center of Mansoura University. I am also thankful to all our patients, wishing them good health and happiness in life. At last, I am thankful for our team for their great help and support my supervisors. The abstract has been published in EHA Hope_ Cairo 2018 as poster presentation and planned to be published in the EHA 25th virtual conference.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
Protocol of the study, study design, methodology, follow-up of the patient, collecting data, data analysis and original draft preparation: Esraa Jamal, Noha Eisa. Laboratory investigation: Salah Aref, Mohamed Ayed. Writing review and editing: Esraa Jamal. Supervision: Emad Azmy.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Suck G, Linn YC, Tonn T. Natural killer cells for therapy of leukemia. Transfus Med Hemother. 2016;43(2):89–95. doi: 10.1159/000445325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcasid M, Ma L, Gotlib JR, Arber DA, Ohgami RS. The clinicopathologic significance of lymphocyte subsets in acute myeloid leukemia. Int J Lab Hematol. 2017;39(2):129–136. doi: 10.1111/ijlh.12594. [DOI] [PubMed] [Google Scholar]
- 3.Lee S, Kim J, Jang B, Hur S, Jung U, Kil K, Na B. et al. Fluctuation of peripheral blood T, B, and NK cells during a menstrual cycle of normal healthy women. J Immunol. 2010;185(1):756–762. doi: 10.4049/jimmunol.0904192. [DOI] [PubMed] [Google Scholar]
- 4.Kokordelis P, Kramer B, Boesecke C, Voigt E, Ingiliz P, Glassner A, Wolter F. et al. CD3(+)CD56(+) natural killer-like T cells display anti-HCV activity but are functionally impaired in HIV(+) patients with acute hepatitis C. J Acquir Immune Defic Syndr. 2015;70(4):338–346. doi: 10.1097/QAI.0000000000000793. [DOI] [PubMed] [Google Scholar]
- 5.Al-Jabri AA, Al Belushi MS, Nsanze H. Frequency and levels of autoantibodies in healthy adult Omanis. Ann Saudi Med. 2003;23(6):372–375. doi: 10.5144/0256-4947.2003.372. [DOI] [PubMed] [Google Scholar]
- 6.Al-Mawali A, Pinto AD, Al Busaidi R, Al-Zakwani I. Lymphocyte subsets: reference ranges in an age- and gender-balanced population of Omani healthy adults. Cytometry A. 2013;83(8):739–744. doi: 10.1002/cyto.a.22322. [DOI] [PubMed] [Google Scholar]
- 7.Chng WJ, Tan GB, Kuperan P. Establishment of adult peripheral blood lymphocyte subset reference range for an Asian population by single-platform flow cytometry: influence of age, sex, and race and comparison with other published studies. Clin Diagn Lab Immunol. 2004;11(1):168–173. doi: 10.1128/CDLI.11.1.168-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Najera Chuc AE, Cervantes LA, Retiguin FP, Ojeda JV, Maldonado ER. Low number of invariant NKT cells is associated with poor survival in acute myeloid leukemia. J Cancer Res Clin Oncol. 2012;138(8):1427–1432. doi: 10.1007/s00432-012-1251-x. [DOI] [PubMed] [Google Scholar]
- 9.Chen YJ, Liao HF. NK/NKT cells and aging. International Journal of Gerontology. 2007;1(2):65–76. doi: 10.1016/S1873-9598(08)70025-5. [DOI] [Google Scholar]
- 10.Le Garff-Tavernier M, Beziat V, Decocq J, Siguret V, Gandjbakhch F, Pautas E, Debre P. et al. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9(4):527–535. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez CJ, Le Treut T, Boehrer A, Knoblauch B, Imbert J, Olive D, Costello RT. Natural killer cells and malignant haemopathies: a model for the interaction of cancer with innate immunity. Cancer Immunol Immunother. 2011;60(1):1–13. doi: 10.1007/s00262-010-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S. et al. Innate lymphoid cells - a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal N, Swerdlow SH, TenEyck SP, Boyiadzis M, Felgar RE. Natural killer cell (NK) subsets and NK-like T-cell populations in acute myeloid leukemias and myelodysplastic syndromes. Cytometry B Clin Cytom. 2016;90(4):349–357. doi: 10.1002/cyto.b.21349. [DOI] [PubMed] [Google Scholar]
- 14.Boeck CL, Amberger DC, Doraneh-Gard F, Sutanto W, Guenther T, Schmohl J, Schuster F. et al. Significance of frequencies, compositions, and/or antileukemic activity of (DC-stimulated) invariant NKT, NK and CIK cells on the outcome of patients with AML, ALL and CLL. J Immunother. 2017;40(6):224–248. doi: 10.1097/CJI.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 15.Zimmer J, Michel T, Andres E, Hentges F. Up-regulation of NKG2D ligands by AML cells to increase sensitivity to NK cells: the tumour might strike back. Comment on "differentiation-promoting drugs up-regulate NKG2D ligand expression and enhance the susceptibility of acute myeloid leukemia cells to natural killer cell-mediated lysis" by Rohner et al. [Leuk Res 2007;31:1393-402] Leuk Res. 2008;32(4):676–677. doi: 10.1016/j.leukres.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Vidriales MB, Orfao A, Lopez-Berges MC, Gonzalez M, Hernandez JM, Ciudad J, Lopez A. et al. Lymphoid subsets in acute myeloid leukemias: increased number of cells with NK phenotype and normal T-cell distribution. Ann Hematol. 1993;67(5):217–222. doi: 10.1007/BF01715050. [DOI] [PubMed] [Google Scholar]
- 17.Levy EM, Roberti MP, Mordoh J. Natural killer cells in human cancer: from biological functions to clinical applications. J Biomed Biotechnol. 2011;2011:676198. doi: 10.1155/2011/676198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.