Abstract
Opioid agents play a unique role in pain and symptom management for cancer patients. Research shows that opiate use, especially when associated with underlying cancer, has significant effects on hematological parameters. These changes may lead to greater risk for immunosuppression, tumor growth and progression of metastatic processes. The aim of this review is to explore the effects of opiates on various metabolic and biological processes, as well as the hematopoietic system, especially in cancer patients. Our findings demonstrate that the tumor-promoting effects of opiates remain contradictory, as both growth-promoting and anti-tumor effects have been observed. However, available data suggest that opiates can facilitate the proliferation and migration of tumor cells, and understanding of this process on cancer treatment is tremendously important.
Keywords: Opioids, Morphine, Cancer, Metastasis, Leukemia, Lymphoma, Oncology, Immune system
Introduction
According to the European Association for Palliative Care’s (EAPC) guidelines on tumor-related pain, oral morphine is the gold standard for treating moderate-intensity pain [1]. The most commonly used opioids are buprenorphine, codeine, fentanyl, hydrocodone, hydromorphone, methadone, morphine, oxycodone, tramadol and tapentadol [2, 3].
The main physiological mechanisms of opioid analgesics have been well documented. However, current data indicate that the spectrum of opioid side effects may be much wider than originally thought. It has been confirmed that the immunosuppressive effect of opioid agents is mediated by mu opioid receptors (MORs), increasing the risk of opportunistic infections in drug users and cancer patients. Furthermore, it has been suggested that opiates may significantly influence the proliferation of hematopoetic cells and stimulate cancerous processes [4]. Exploring the effects of opioids on various hematological parameters may be helpful in understanding the progression of these devastating conditions.
The primary aim of this review is to explore the effects of opioid agents on the development and progression of hematologic conditions. To this end, we attempted to 1) analyze the mechanisms of the immunomodulatory effects of opioid medications, 2) present the most up-to-date information on the possible effects of opioid drugs on the manifestation and progression of major hematologic diseases, and 3) discuss how changes in these parameters may influence the prognosis or the severity of pathological processes.
Immunomodulatory Effects of Opioid Agents
To date, much information has been accumulated on the basic physiological effects of opioids. However, the lesser-studied effects that these drugs have on immune system function have never before been summarized.
The inhibitory effects that opioid compounds have on the pattern of immunosecretion are well known [4]. Opiates have been shown to alter the intracellular signaling pathways responsible for the development, differentiation and function of immune cells. Moreover, endogenous opioids can affect the innate and acquired immune systems by interacting with the activation of different opioid receptors and altering their expression. Interestingly, chronic morphine exposure may be associated with receptor down-regulation and tolerance in neural cells, causing mu-receptor overexpression in immune cells and increased Th2 T-helper cell differentiation [5].
Other authors suggest that centrally acting opioid agents increase catecholamines and steroid plasma concentrations, reflecting a central activation of sympathetic outflow, thereby inhibiting the immune response even further [6, 7]. Long-term opioid use increases activity in the hypothalamus-pituitary-adrenal axis, resulting in an increased production of glucocorticoids that reduces the cytotoxicity of natural killer (NK) cells. Opioid agents may also activate the sympathetic nervous system and provoke an immunosuppressive effect, and morphine can act through type I dopamine receptors, increasing the release of neuropeptide Y and decreasing NK cytotoxicity [8].
At the level of the cell nucleus, the immunosuppressive effects of opioids are mediated by epigenetic mechanisms. Temporary connections formed between the MOR gene promoter and the following are shown in Jurkat T cells upon activation of MOR: histone H3 trimethylated at lysine at position 4, phosphorylated at serine at position 10 and acetylated at lysine at position 14. They are also formed between the MOR gene promoter and histone H4 acetylated at lysine at the 16th position and with the chromatin-remodeling protein Brg-1 [9].
Opiates alter the cancerous process by either modulating proliferation or interfering with differentiation and maturation of cell progenitors. It has been suggested that mitogen-activated protein kinases (MAPKs) can be activated by opiates. Members of the MAPK family such as the ribosomal S6 kinases 1 and 2 (RSKs) may facilitate G1-phase progression through the phosphorylation of cyclin-dependent kinase (CDK) inhibitor p27kip1 [10]. Moreover, the extracellular signal-regulated kinases (ERKs) and the RSKs can also mediate progression through the G2 phase [11]. Opiates may induce apoptosis via overexpression of p53 and Bax mRNA and reduced expression level of Bcl-2 [12]. It has been demonstrated that opiates induce phosphorylation of Bad, a pro-apoptotic member of the Bcl-2 family proteins via activation of AKT [13].
It has been shown that morphine may activate vascular endothelial growth factor (VEGF) receptors and other signaling molecules such as AKT and ERK1/2. Stimulation of opioid receptors which belong to the family of G protein-coupled receptors (GPCRs) triggers a number of intracellular signaling processes which leads to the inhibition of adenylyl cyclase activity, activation of tyrosine kinases, phospholipase C, PI3K/AKT and the RAS/RAF/ERK1/2 signaling pathways [14, 15]. These findings confirm the pro-angiogenic effects of morphine and other opioid agents. However, high doses of morphine have also been shown to inhibit the pro-angiogenic activity of endothelial cells through the increased production of nitrogen oxide (NO) [16].
Figure 1 summarizes the potential immunomodulatory effects of opioids.
Figure 1.
Potential immunomodulatory effects of opioids.
Opioid agents may also affect a number of characteristics in immune cells, such as chemotaxis, white blood cell (WBC) count, lymphocyte proliferation and NK cell activity [17-19]. According to Roy et al (2001), long-term use of morphine has been associated with significant suppression of interleukin (IL)-1α, tumor necrosis factor (TNF)-α-induced chemotaxis, decreased levels of IL-2 and interferon (IFN)-γ and elevated production of IL-4 and IL-5 [20]. This is in contrast to Peng et al (2000), who stated that mice peritoneal macrophages exhibited elevated expression of IL-12 and TNF-α following administration of morphine [21].
Haghpanah et al noted that opium-dependent patients had a significant increase in WBC count, while heroin users and withdrawal groups had not experienced any significant changes [19]. Moreover, neutrophil and lymphocyte counts in heroin- and opium-addicted groups were significantly lower than expected. By contrast, the red blood cell (RBC) count remained normal, while the hematocrit (HCT) percentage and the mean corpuscular hemoglobin concentration (MCHC) level were significantly higher in all study groups [19].
Opiates can directly affect immune system cells through opioid receptors, leading to a decrease in the proliferation of T cells in vitro and the production of cytokines [22]. It is also believed that the suppressor effect of morphine on NK activity is not a direct result of MOR stimulation but is mediated by additional factors. Mice and rats taking morphine intravenously experience a significant decrease in the activity of NK cells as soon as 3 h after injection, and this effect persists for at least 24 h [23]. However, in an in vitro study of morphine effects (with doses from 3 pg/mL to 32 µg/mL), there was no decrease in NK activity, but there was an increase in the activity of human cytotoxic T lymphocytes [24]. Morphine also suppresses the formation of peripheral blood T cells, an effect that can be blocked by pre-treatment with the opioid receptor antagonist naloxone [23].
Opiate-induced immunosuppression appears to be largely mediated by microRNA (miRNA). Studies at the molecular level prove that morphine inhibits the production of several transcription factors and induces miRNAs that inhibit inflammatory reactions. The enhancing effects of heroin on HIV replication processes are based on the heroin inhibition processes of certain miRNAs (specifically, miRNA-28, miRNA-125b, miRNA-150 and miRNA-382) that normally limit the replication of viral particles [25]. Clinical studies confirm lower levels of several biologically significant miRNAs (in particular, miRNA-582-5p and miRNA-590-5p) in the blood of long-term heroin users [26].
MiRNAs have been found to mediate the apoptosis of immune system cells in opiate users. In experiments in mice, morphine has been linked to reduced miRNA-873 activity in spleen cells and in macrophages of the abdominal cavity. Such apoptosis can be suppressed with miRNA-873 mimetics [23]. We can assume that morphine and other opiates also inhibit the expression of factor nuclear factor (NF)-κB, which correlates with a decrease in pro-inflammatory cytokine production. In some studies, the peritoneal macrophages of mice treated with micromolar doses of morphine displayed reduced NF-κB levels [27].
Morphine use has been linked to atrophy of the spleen and thymus cells [23, 28] and to the induction of apoptosis in monocyte cultures. Changes in the spleens and thymi of mice and monkeys receiving daily morphine injections for 2 years were detected at a ratio of CD4 and CD8 T cells [23, 29]. Another study showed that 7 days after mice were implanted with slowly dissolving morphine granules, the numbers of B cells and CD4 and CD8 T cells in their spleens and lymph nodes were reduced [23].
It has been concluded that morphine may increase chemokine receptor expression but decrease chemokine levels [23]. Opiate use is linked to macrophage infection of one of the HIV R5 strains since opiates suppress the production of β-chemokines and increase the expression level of the CCR5 receptor [30]. Suzuki et al showed similar overexpression in CCR5 in a human lymphocytic cell line caused by methadone [31].
However, it is still unclear whether opiates induce gene expression of other molecules, such as GPCRs. Recent evidence has shown that many GPCRs, such as endothelin receptors, chemokine receptors and lysophosphatidic acid receptors, have been involved in the progression of several forms of cancer [32]. It appears that opiates may increase the expression of CXCR4 receptors [33, 34], which may play a crucial role in tumor invasion and metastatic process [32].
Such effects could have an extremely negative impact on cancer patients. However, research data obtained from relatively healthy volunteers and animal models cannot be automatically transferred to cancer patients. The results of a number of studies are summarized in Table 1 [35-47].
Table 1. The Effects of Opioids on the Immune System in Various Forms of Cancer.
| Authors, year | Study goal | Study description | Findings |
|---|---|---|---|
| Gaspani et al, 2002 [35] | Analysis of tramadol’s ability to stimulate NK cell activity and prevent metastasis progression in rodents | Experimental surgery was conducted with subsequent analysis of: 1) tramadol’s ability to prevent the effects of laparotomy on NK activity, and 2) the progression of the metastatic process of the NK-sensitive tumor model MADB106 in rodents. | Compared to morphine, the administration of tramadol (20 and 40 mg/kg) before and after surgery was associated with decreased progression of lung metastasis and preserved NK activity in post-operative animals. |
| Shavit et al, 2004 [36] | Analysis of the effects of different doses of fentanyl, administered at different time points relative to tumor inoculation, on natural killer cell cytotoxicity (NKCC) and on tumor metastasis in rats | Three hundred forty-four (344) rats were injected with low or high doses of fentanyl, 6 or 2 h before, simultaneously with or 1 h after being inoculated intravenously with MADB106 experimental tumor cells. Following the surgery, lung tumor retention, the severity of the metastatic process and NK cell activity were assessed at different time points. | Fentanyl administration was associated with impaired NKCC and increased risk of tumor metastasis. |
| Provinicali et al, 1991 [37] | The effect of morphine on the activity of NK and lymphokine-activated killer (LAK) cells in cancer patients | Twenty (20) patients with various forms of cancer were studied. At the end of a month of morphine administration, test results were compared with the results of healthy volunteers. | A significant decrease in NK activity and an increase in LAK activity were observed. |
| Provinicali et al, 1996 [38] | To study the effects of short-term and long-term administration of morphine on the activity of NK and LAK cells | Eighteen (18) patients with cancer of various natures were studied. Ten (10) patients received morphine, but eight patients did not. Changes were evaluated 30 min after a single injection of 10 mg of morphine and 1 month after daily administration of 90 mg of morphine. | Short-term effect: decrease in NK activity, increase in LAK activity, no change in the number of lymphocytes in peripheral blood. Long-term effect: an even greater decrease in NK activity and an increase in LAK activity, an increase in the content of CD3+ and CD4+ lymphocytes, a decrease in CD16+ lymphocytes. |
| Sacerdote et al,1997 [39] | Analysis of the potential immunosuppressive effects of morphine and morphine-derived drugs (codeine, hydromorphone, oxycodone) | Effects of listed agents on immune parameters (splenocyte proliferation, NK cell activity and interleukin-2 (IL-2) production) were evaluated in the mouse model. | Morphine had a potent immunosuppressive effect; codeine possessed limited immunosuppressive activity; hydromorphone and oxycodone were devoid of immunosuppressive effects. The pure antagonists naloxone and naltrexone potentiated immune responses. |
| Makimura et al, 2011 [40] | Analysis of cytokine levels as a possible predictor of morphine resistance | Forty-four (44) cancer patients received morphine according to the standard protocol for 8 days. | The study revealed no correlation between the administration of morphine and immune system functions. |
| Hashiguchi et al, 2005 [41] | Analysis of possible morphine effects on immune system function in cancer patients | Fourteen (14) patients were split into two groups. Group 1 contained patients who had not received morphine previously. The final dose of morphine was 20 - 30 mg by oral or intravenous administration. Group 2 contained patients who received morphine previously for a month. The initial dose of morphine was 40 - 120 mg. The final dose of morphine was 20 - 240 mg, by oral, intravenous, subcutaneous, rectal administration. | In group 1, there was a negative correlation with the level of immunoglobulins and proliferation of lymphocytes induced by phytohemagglutinin, but not with NK activity or the ratio of CD4/CD8 cells. In group 2, no correlations were found. |
| Palm et al, 1998 [42] | Analysis of the possible effects of morphine on immune function when administered orally | Ten (10) patients received morphine at a dose of 30 - 240 mg per day. Tests were taken before the start of morphine administration, as well as 1, 4 and 12 weeks after the start of morphine administration. | There was no change in the total number of lymphocytes or their populations, the rate of lymphocyte proliferation induced by phytohemagglutinin or the levels of IgM and IgG. Elevated levels of IL-2 were secreted by lymphocytes. |
| Sacerdote et al, 2000 [43] | Authors explored the impact of morphine and tramadol on pain and the immune system in 30 patients undergoing surgery for uterine carcinoma. | T lymphocyte proliferation and NK activity were assessed at different time points: before and after surgery, and 2 h after the IM administration of 10 mg of morphine (group 1) or 100 mg tramadol (group 2) for pain. | Tramadol and morphine showed comparable analgesic activity. However, in group 1, proliferative values remained lower than basal levels for 2 h after treatment, whereas in tramadol-treated patients proliferative values returned to basal levels. Tramadol, in contrast to morphine, enhanced the activity of NK cells. |
| Franchi et al, 2007 [44] | Analyzed the ability of buprenorphine to prevent the effects of surgery on HPA activation, NK activity and lung diffusion of the NK-sensitive tumor MADB106 | Buprenorphine (0.1 mg/kg) was compared with equianalgesic doses of 1) fentanyl (0.1 mg/kg) and 2) morphine (10 mg/kg) in an animal model. | In normal animals, morphine and fentanyl stimulate the HPA axis, decrease NK activity and trigger tumor metastasis, while buprenorphine is devoid of these effects. Buprenorphine, in contrast to other agents, was able to prevent the HPA and immune system alterations and ameliorate the increase of tumor metastasis induced by surgery-induced stress reaction. |
| Desmond et al, 2015 [45] | To verify whether anesthetic technique influences the distribution of NK cells, T lymphocytes and macrophages in intra-tumoral tissue and predict prognosis and response to therapy | Breast cancer patients were randomized to receive either a propofol-paravertebral anesthetic with continuing analgesia (PPA) or a standardized general anesthesia with opioid analgesia (GA) for 24 h post-operatively. | PPA induces increased levels of NK and T helper cell infiltration into breast cancer tissue compared with GA but not T suppressor cells or macrophages. Lack of immune cell infiltration could negatively affect the protective anti-tumor immunity. |
| Shen et al, 2014 [46] | To analyze the effects of morphine +/- flurbiprofen as post-operative analgesics on the immune systems of patients undergoing gastric cancer surgery | Sixty (60) patients undergoing gastric cancer surgery were randomized into two groups based on post-operative intravenous (IV) analgesia using morphine either with or without flurbiprofen. | Analgesia combined with morphine and flurbiprofen ameliorates the immune depression in T-lymphocyte subtypes and NK cells, while providing a similar analgesic effect to morphine alone. |
| Gong et al, 2014 [47] | Evaluation of effects of fentanyl anesthesia and sufentanil anesthesia on regulatory T-cell frequencies | Authors compared the immunosuppressive effects of sufentanil and fentanyl on CD4+CD25+Foxp3+ regulatory T-cell (Tregs) frequencies both in vitro and in breast cancer (BC) patients undergoing eradicative operation. | Treg cells can inhibit anti-tumor immune responses. Sufentanil is more powerful than fentanyl in increasing the quantity of Tregs in vitro. Both agents have very similar analgesic potential; no significant differences in Treg frequencies between sufentanil and fentanyl noted. |
The effect of opioids on immune function may correlate with the duration of their administration. In some of the studies included in Table 1, there was a negative correlation between serum levels of morphine metabolites and immunoglobulins in patients who had just started taking morphine. In such patients, there was also a negative correlation between morphine metabolites and indicators of lymphocyte proliferation induced by phytohemagglutinin (a non-specific T-cell activator). However, these effects were not observed in patients who had been receiving morphine for longer than a month.
Some evidence indicates that the method of administration may influence how morphine affects the immune system. For example, when it is administered intrathoracically, the effects tend to be more pronounced than when it is administered orally. This suggests that the effects of morphine on the immune system are mediated by central mechanisms involving the central nervous system [8].
Based on the evidence provided above, it should be noted that not all immune cells are beneficial to the anti-tumor response. Moreover, while NK cell activity correlates with cancer progression in animal models, these outcomes have not been properly assessed in clinical settings with humans. Furthermore, the degrees of change in immune response to opioid dosages necessary to produce a clinically significant effect are unknown, and it could be difficult to connect opiate-induced immunosuppression to poorer outcomes in patients with cancer.
Other relevant effects of opiates
Jankovic and Maric (1987) suggested that small doses of enkephalins increased humoral immune responses in rats [48]. Evidence has also shown that some opiate agents can function as cytokines, alternating the function of various cells involved in host immunity. DAMGO ([D-Ala2, N-MePhe4, Gly-ol]-enkephalin), a synthetic opioid agent with high affinity for MOR, can provoke the release of monocyte chemoattractant protein-1 (MCP-1), RANTES and IFN-γ from human mononuclear cells [49].
Adham et al detected a significant increase in the level of serum alpha-2-macroglobulin (a-2-M), the main factor in the acute phase of inflammation, in drug addicts undergoing methadone treatment at a rehabilitation center. The patients’ average level of a-2-M was found to be 341 ± 14 mg/dL, compared with 231 ± 8 mg/dL in a control group (P < 0.01). The authors attribute this increase to the increased synthesis of macroglobulin by lymphoid tissues due to chronic antigenic stimulation of the lymphoid system [50].
Heroin users tend to have increased serum IgY levels in combination with relatively normal levels of IgG and IgA [51]. Piepenbrink et al found a significant (twofold) increase in the total number of B cells in heroin users compared with healthy individuals. Although in this study the total IgG in plasma was similar in both groups, drug users had significantly higher levels of IgG3 and IgG4, indicating chronic B-cell activation. Several clinical parameters (i.e., an increase in the levels of plasma metabolites CD40L, TNF-α, transforming growth factor (TGF)-α, IL-8 and ceramide) may indicate the presence of systemic inflammation in heroin drug users. Interestingly, rising levels of ceramide, which have been observed in this population, can facilitate the penetration of viral particles into cells and lead to viral infections [52].
These potential side effects should be considered when prescribing opioid as analgesics. However, it should be noted that the data obtained from monitoring patients receiving morphine cannot be extrapolated to all opioids because of the heterogeneous nature of this class of drugs. Other opioids are significantly different in their physicochemical and pharmacological characteristics.
The Role of the Opioid System in Hemato-Oncological Conditions
Association of opiate abuse with the manifestation and progression of various hematologic disorders
Some experts consider intravenous opiate use a significant risk factor for the development of devastating hematologic conditions [53, 54]. Polyclonal hyper macroglobulinemia (increased IgM levels) have been detected in drug-dependent individuals using heroin intravenously [55].
Ahmed et al studied five African-American patients diagnosed with Waldenstrom macroglobulinemia at a relatively young age, all of whom used heroin intravenously. The authors point out that Waldenstrom macroglobulinemia is a disease that mainly affects elderly patients, indicating a possible connection between intravenous heroin use and the development of the disease at a young age [56].
The literature also suggests a connection between the use of opioid drugs and the development of secondary amyloidosis, a pathological condition in which fibrils composed of fragments of amyloid A are deposited across the body. Current epidemiological studies show that diagnostic signs of chronic inflammation often occur in heroin users. In some regions and groups, the dynamics of heroin consumption coincide with the etiological indicators of amyloidosis [57].
Opiate agents and autoimmunity
Only a few studies have attempted to determine factors that connect opiate use with the development of autoimmune processes. Several authors have suggested that administration of an opiate agent was associated with elevated levels of autoantibodies to mu delta-opiate receptors (MDORs) in non-drug users as well as in heroin addicts [58, 59].
Experimental autoimmune encephalitis (EAE) in rats has always been considered a model for human multiple sclerosis. Panerai et al suggest the possibility of opioid system involvement in the pathogenesis of this autoimmune process. The development of EAE has been linked to Th1 responses, while remission of symptoms is associated with protective Th2 immune response. Research has confirmed the role of opioids in the modulation of Th1/Th2 responses, with the administration of naloxone worsening the clinical presentation of EAE and increasing mortality rates by increasing Th1 responses and suppressing Th2 cytokines [60].
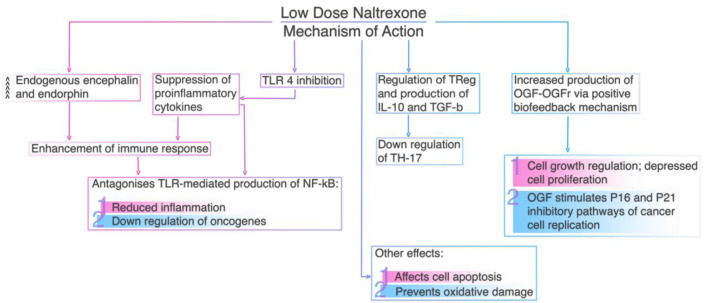
Low-dose naltrexone (LDN) can be administered within the therapeutic range (when its plasma concentration would be between the minimum effective concentration for obtaining the desired immunomodulatory action and the minimum toxic concentration) as an immunoregulator in many autoimmune conditions and malignant neoplasms [61]. LDN’s ability to suppress opioid growth factor (OGF) activity, the inhibitory growth factor that stimulates p16 and/or p21 cyclin-dependent inhibitory kinases to slow cell replication, has been proven to be effective in a number of malignant tumors, including non-small cell lung cancer (NSCLC) [62], adenoid cystic carcinoma [63], ovarian cancer [64] and B-cell lymphoma [65]. LDN affects inter-cell signaling and cell cycle regulation and reduces tumor growth by blocking opioid growth factor receptors (OGFrs) and upregulating serum enkephalin levels [66]. Additionally, cancer cells previously exposed to LDN tend to be more sensitive to chemotherapeutic agents [61]. Figure 2 summarizes LDN’s mechanism of action.
Figure 2.
Low dose naltrexone’s mechanism of action.
Association of opiates with hemolytic and thrombotic consequences
Opiates have been implicated as a risk factor for hemolytic anemia [67], RBC alloimmunization and significant hemolytic disease of the fetus and newborn [68] in previous case reports; however, the mechanisms that lead to these changes remain unclear. Evidence shows that microangiopathic hemolytic anemia (MAHA) may be triggered by using a non-sterile syringe to draw up and inject the drug and tablet filler in a non-sterile environment [67]. Moreover, regular use of opiates can be associated with increased risk of developing thrombotic microangiopathy (TMA). In 2012, the development of hemolytic anemia and thrombocytopenia, similar to thrombotic thrombocytopenic purpura (TTP), was reported in several patients with a history of oxymorphone hydrochloride abuse. However, the mechanism underlying thrombotic complications from the administration of dissolved tablets of oral oxymorphone remains unclear [69-71].
Other authors have confirmed the presence of MOR in human RBC, finding that the density of these receptors is significantly higher in chronic opiate abusers. They note that erythrocytes from opiate-dependent individuals have a high degree of deformability (r = 0.74, P < 0.005). These data may lead to a better understanding of hemolytic and prothrombotic changes associated with illicit drug use [72].
Anti-tumor properties of opiates in hematological neoplasms
Some clinical studies report that opioids may have anti-tumor effects in hematologic conditions. It was already known at the end of the last century that opioid receptors play a role in the regulation of blood cell differentiation processes [73]. Opioid receptors are present in the cells and interstitial fluid of bone marrow [74]. They may affect hematopoietic cells in bone marrow in two ways: 1) by working indirectly through ILs secreted from stromal cells during stimulation of opioid receptors, and 2) directly on hematopoietic precursors [75].
In the 1980s, endogenous opioids and receptors were found in benign and malignant tumors of ectodermal, mesodermal and endodermal origin. A specific, high-affinity, saturable binding of multiple opioid ligands with neural and non-neural human and animal tumors was shown. Radio-immunological analysis confirmed the presence of leucine-endorphin and methionine-enkephalin in the tumors, both of which were found in tumor tissue in connection with the cortical cytoplasm of tumor cells but not with their cell nuclei [76]. Wick et al showed that delta opioid receptors (DORs) and kappa opioid receptors (KORs) are expressed in human peripheral blood lymphocytes as well as in several tumor lines of human lymphoid cells [77]. Receptors with high affinity and specificity binding to met-enkephalin have been found in cells of the NALM 6 line (B-cell acute lymphoblastic leukemia) and Jurkat cells (T-cell lymphoma) [78].
Yin el al conclude that morphine may facilitate apoptosis in Jurkat cells (leukemia) via activation of FADD/p53, anti-apoptotic PI3K/Akt and NF-κB pathways [79]. Moreover, it appears that the effect of opioids on atypical blood cells can be mediated by different types of opioid receptors. An in vitro analysis of the viability and proliferation of NALM-1 leukemia cells showed that the DOR DSLET agonist and the MOR DAMGO agonist both reduced cell viability according to the MTT test 6 h after treatment, while the j-opioid receptor agonist U-69593 suppressed NALM-1 cell proliferation up to 48 h after treatment [80].
It should be taken into consideration that the medications used to treat hematological conditions can affect the expression of opioid receptors, meaning that the effects of opioid therapy can vary. For example, Beltran et al show that treating HL-60 promyelocytic leukemia cells with 12-o-tetradecanoyl phorbol-13-acetate (TPA) leads to a significant increase in the number of MOR in differentiated HL-60 cells [81].
There is no doubt that when used to treat hematologic conditions, opioids can directly affect tumor cells, but the nature of such effects varies depending on the type of opioid used. It is known that methadone inhibits the proliferation of leukemia cells, enhancing their apoptosis, while endogenous and synthetic opioid peptides can stimulate the cell migration processes of early acute lymphocytic leukemia. Differing opioids and differing target cells lead to very different outcomes [82].
Noscapine, a benzylisoquinoline alkaloid derived from opium, has been shown to inhibit the development of various types of cancer, including leukemia and myeloma. While the mechanism of this process has not yet been fully elucidated, it has been suggested that the effect is mediated through the activation of the transcription factor NF-κB, which is associated with inflammation regulation as well as progression, invasion and angiogenesis in cancer cells. Noscapine has been proven to enhance apoptosis of tumor cells under the action of cytokines and chemotherapeutic agents, suppressing activation of NF-κB by inhibiting IκB kinase (IKK). It has also been found to suppress phosphorylation of the p65 protein, leading to inhibition of the reporter activity of NF-κB that is induced by various components of the NF-κB activating pathway. Noscapine also inhibited activity of the NF-κB-containing COX-2 promoter. Experimental data obtained to date led the authors to conclude that noscapine inhibits the proliferation of leukemic cells, thus increasing sensitivity to TNF and the action of chemotherapeutic agents [83]. It has also been suggested that morphine may inhibit tumor cell growth via inhibition of NK-κB activation and suppression of TNF-α expression in HL-60 promyelocytic leukemia cells and SKNO-1 leukemia cells [84, 85].
Papaverine, another non-narcotic opium alkaloid with well-established antispasmodic activity, has shown some anti-tumor effects, but its potential has been a matter of controversy. As a high-mobility group box 1 protein (HMGB1)/receptor for advanced glycation end products (RAGEs) inhibitor, papaverine was able to suppress cell proliferation and cancer cell migration in human glioblastoma (GBM) [86]. These papaverine properties have been observed in other cell lines, but the mechanism of the effect can vary [87-89]. Alkaloids such as narceine have papaverine-like properties but are also active on µ-opiate and κ-receptors. Based on limited data, it has been concluded that narceine has no proapoptotic or cytotoxic qualities but may cause some DNA damage on HT1080 fibrosarcoma cells [90].
It is well reported in the medical literature that opioids such as D,L-methadone have an anti-tumor effect. D,L-methadone may induce cell death in leukemia cells, but the mechanism is still unclear. Exposure to these agents can occur through the cyclic adenosine monophosphate (cAMP) system, which regulates several cellular processes and modulates the process of induced cell death. cAMP levels change upon stimulation of G protein-specific receptors that inhibit or activate adenylate cyclases. Stimulation of opioid receptors can activate inhibitory Gi proteins, which, in turn, block adenylate cyclase activity. Enhancing cAMP levels by blocking specific opioid receptors strongly reduces D,L-methadone-induced apoptosis, caspase activation and doxorubicin sensitivity. Induction of cell death in leukemia cells by activation of opioid receptors using D,L-methadone depends on the expression level of opioid receptors on the cell surface. In this case, doxorubicin increases the expression of opioid receptors in leukemia cells. Thus, D,L-methadone and doxorubicin mutually enhance cytotoxicity, making activating opioid receptors a promising strategy for increasing the effectiveness of antitumor therapy [91].
Other data show the ways in which opioids affect the activity of apoptosis regulatory proteins in leukemia cells. Two recently described endogenous MOR agonists, endomorphine 1 and endomorphine 2, have been shown to reduce the viability of cultured HL-60 promyelocytic leukemia cells by inducing apoptosis through activation of the Bcl-2, Bax and Fas, FasL signaling pathways [92].
Studies have also shown that methionine-enkephalin may inhibit the metabolic activity of the leukemic cell line (NALM-1) [93]. Several in vitro experiments reported the ability of morphine to decrease signs of blast transformation, especially in samples obtained from patients with laryngeal cancer [94]. Moreover, we know that the anti-leukemic activity of methadone is synergistic with the activity of ABT-737, an antagonist of the Bcl-2 protein. Methadone combined with ABT-737 induces distinct changes in the mitochondria of tumor cells [95], and there is experimental evidence that met-enkephalin increases apoptosis in human erythroid leukemia K562 cells [96].
Perez-Alvarez et al witnessed the death of SH-SY5Y leukemic cells in methadone users. This seemed to be associated with necrotic processes rather than with typical apoptosis, as the authors were unable to identify the involvement of typical apoptosis proteins such as Bcl-X and p53 in these processes. The methadone-induced cell death occurred independently of caspases, and the authors hypothesize that the cytotoxic effect of methadone may be based on its ability to cause a sharp depletion of ATP stores in the cell [97]. However, the effects of opioid drugs on the formation of reactive oxygen species in HL-60 leukemic cells have not been confirmed [98]. In contrast, morphinone, the final product of morphine metabolism, has been shown to cause non-apoptotic cell death in HL-60 cells [99].
There is a possibility that opioids can influence tumor cells of hematological origin through other non-opioid receptors. Specifically, it is thought that the common acute lymphoblastic leukemia antigen CD10 could be involved in the regulation of enkephalin-mediated inflammatory reactions in phylogenetically distant organisms [100].
Counter-arguments
Nevertheless, we need to consider that the effects that opioids have on the hematopoietic cells in a patient with one condition will differ from the effects they have on another individual. The results of earlier experimental studies indicated the possibility of significant differences between the reactions of leukemia cells and the effect of opioid compounds. At first, no difference was seen between control cells and those exposed to methionine enkephalinamide in a level analysis of intracellular-free ionized calcium (Ca2+) in human T-leukemia Jurkat cell clones. However, more detailed data analysis found that methionine enkephalinamide did change the level of intracellular calcium, but only in 20-40% of the cases [101].
It is clear that morphine suppresses the cell DNA synthesis and population growth (by 50% or more compared to a control group) of B-lymphoma cells (Namalva cells), but it also activates (by 1.5 times) the proliferation of K562 myeloid cells and Jurkat T-lymphoma cells [102]. Similarly, Bosshart found that morphine given at concentrations of 10-8 M can significantly reduce the release of H2O2 from the cells of acute monocytic leukemia THP-1. This effect, contrary to those described above, can promote cancerous cell growth [103].
Zhou et al (2019) confirmed that morphine is able to promote proliferation in blast phase-chronic myeloid leukemia (BP-CML) K562 and LAMA84 cell lines, while cell survival remained unchanged. Moreover, morphine impaired the anti-proliferative and pro-apoptotic effects of BCR-ABL tyrosine kinase inhibitor (TKI) and enhanced the self-renewal capacity of human CD34 stem cells. Their experiments revealed that morphine may influence Wnt signaling via increasing β-catenin activity and Wnt target gene transcription in K562 and CD34 cells. In addition, morphine suppresses the inhibitory effects of TKIs in cell survival and proliferation in BP-CML CD34 multipotential progenitor cells [104].
Similarly, in mice infected with leukemia, daily administration of morphine (10 mg/kg) over a period of 10 days resulted in increased tumor growth, with signs of atrophy in the spleen and thymus [105]. Massive lysis of tumor cells during a course of chemotherapy can also lead to renal dysfunction, which makes the patient susceptible to the side effects associated with morphine [106]. Additionally, while morphine given to mice infected with the mouse leukemia virus LP-BM5 (MuLV) led to an increase in the relative content of macrophages, it had the opposite effect on controls groups [107].
In some cases, the resistance of tumor cells to treatment seems to be related to the characteristics of the reception of opioid compounds. One recent study analyzed acute lymphoblastic leukemia cell resistance to L-asparaginase, the most important chemotherapeutic agent in the disease management. A full-scale genomic analysis and screening of RNA cells of 10 L-asparaginase resistant clones of acute lymphoblastic leukemia revealed that six genes and their products could be associated with this resistance. One of the revealed genes was an OPRM1 opioid receptor gene, encoding the mu 1 receptor. OPRM1 was expressed in all tested leukemia cells, and its knockdown made the cells resistant to L-asparaginase. Methadone, an OPRM1 agonist, increases the sensitivity of leukemia cells to L-asparaginase treatment, but it does not have this effect on OPRM1 knockdown cells. Thus, the loss of OPRM1 contributes to the survival of leukemia cells, probably due to the suppression of the OPRM1-mediated apoptosis. The cells of patients with relatively high levels of OPRM1 are more sensitive to treatment with L-asparaginase. OPRM1 can thus be used as a biomarker for predicting the effectiveness of chemotherapy for acute lymphoblastic leukemia [108].
Opioid use and the risk of infections
Many studies confirm that opioid use can suppress immune mechanisms in some patients with hemato-oncological conditions [109]. Other authors note a significant weakening of antiviral immunity linked to chronic exposure to morphine. Furthermore, opiates have been found to inhibit the production of antiviral molecules such as IFN-a, IFN-b and IFN-y [23, 110].
Opiate use is also associated with impaired macrophage phagocytosis and chemotaxis [22]. Injecting morphine into mice for 3 days has been found to dramatically reduce the ability of ex vivo macrophages to kill Candida albicans cells. In the same study, naltrexone blocked the morphine-induced inhibition of Candida albicans phagocytosis [111]. Opioid compounds with MOR, KOR and DOR affinity have shown similar effects, while selective antagonists of different types of opioid receptors inhibit such effects. Evidence suggests that morphine blocks the production of reactive oxygen and the intermediate products of peroxidation, both of which are involved in the bactericidal function of neutrophils [23].
In another study, mice implanted with morphine granules developed sepsis. The mesenteric lymph nodes, peritoneal cavities, spleens and livers of the animals were found to contain microorganisms normally found in intestinal microflora. Eisenstein suggests that morphine compromises the integrity of the intestinal epithelial barrier, causing microorganisms to enter the systemic circulation [23].
Studies have also shown that morphine increases the sensitivity of the body to infections of Acinetobacter baumannii and Listeria monocytogenes. Eisenstein et al (2019) noted that when animals in the control group received a sublethal dose of bacteria, 100% of the animals survived, while 100% of those treated with morphine died. Similar results were obtained in experiments with Toxoplasma gondii: in a group of animals treated with morphine, mortality reached 86%, while none died in the control group.
Although it has not been explored further, it can be expected that these animal experiments do predict human reactions, especially in immunocompromised patients. Interestingly, in this study, in cases where the pathogen was administered at least 9 days before the administration of morphine, no similar increase in mortality was recorded [23].
The impact of opiates on prognosis in cancer patients
In 2015, scientists at the University of Texas MD Anderson Cancer Center carried out one of the largest retrospective studies ever to look at the effects of opioid drugs on the condition of patients with acute lymphoblastic anemia. The study included all patients undergoing treatment according to the main protocol between January 1, 2006 and January 31, 2013. Results showed no statistical difference for risk of death between patients who received opioids and those who did not. These results are in contrast with those of similar studies conducted on patients with prostate cancer and breast cancer, in which opioid use was associated with an increased risk of relapse and decreased survival rate [82].
Although it has been concluded that opiates may increase the risk of cancer relapse, the data from randomized controlled trials remain questionable [112]. According to a recent retrospective analysis of 7,030 patients undergoing elective oncological surgery, combined volatile anesthesia was associated with an increased risk of death, with a hazard ratio of 1.80 [113]. Another study suggested that IV morphine-equivalent doses (IVME) > 20 mg/day were associated with worse prognosis compared with doses ≤ 17 mg/day. Based on the data provided, patients on ≤ 17 mg/day IVME had a mean survival time of 27 days, while patients on 20 - 25 mg/day IVME survived only 12 days [114]. Similarly, systemic opioid administration with increasing dose was associated with shorter survival [115]. However, poor survival in patients with advanced cancer may also be associated with the cancerous process itself rather than with opiate administration.
Conclusion
Based on an analysis of the literature, it can be concluded that opiates can negatively affect immune system cells, decreasing the body’s resistance to pathogenic agents and stimulating cancerous processes through the suppression of apoptosis, triggering angiogenesis and tumor cell migration. However, the literature also contains a significant number of reports on the anti-tumor effects of widely known opioid agents. As such, it is important to remember that the dispute over the anti-tumor activity of opioid series drugs cannot be considered unambiguously resolved. The body of preclinical evidence is certainly growing; however, strong data to support the use of one specific opiate or strategy over others are currently lacking.
All studies included in this review were extremely heterogeneic due to the differing doses of morphine used, and the effects may also vary depending upon whether in vivo or in vitro methods were used. Moreover, some of those studies were limited in that they included heterogeneous populations, did not identify clear starting points for opioid use and lacked information on durations of opioid use. For that reason, their results cannot show causality, but they may provide some associations.
Thus, the available published data indicate that the prescription of opioids to cancer patients should be carried out with extreme caution to prevent possible side effects and progression of the underlying disease. At the same time, the possible anti-tumor activity of some medications in this group requires further research.
Acknowledgments
We are grateful for a number of mentors in encouraging us to start the work, persevere with it and finally to publish it. We thank the anonymous referees for their useful suggestions. Thank you to all of those with whom we have had the pleasure to work during this and other related projects.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
Both authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content. PT was in charge of overall direction and planning and made substantial contribution to conception and design, acquisition of data, analysis and interpretation of data. PT conceived the presented idea, while VZ participated in drafting the article and revising it critically for important intellectual content. Both authors discussed the results and implications, commented on the manuscript at all stages and carried out the review of different sections outlined in this article.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
References
- 1.Hanks GW, Conno F, Cherny N, Hanna M, Kalso E, McQuay HJ, Mercadante S. et al. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer. 2001;84(5):587–593. doi: 10.1054/bjoc.2001.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anghelescu DL, Faughnan LG, Hankins GM, Ward DA, Oakes LL. Methadone use in children and young adults at a cancer center: a retrospective study. J Opioid Manag. 2011;7(5):353–361. doi: 10.5055/jom.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiffen PJ, Wee B, Derry S, Bell RF, Moore RA. Opioids for cancer pain - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;7:CD012592. doi: 10.1002/14651858.CD012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown SM, Stimmel B, Taub RN, Kochwa S, Rosenfield RE. Immunologic dysfunction in heroin addicts. Arch Intern Med. 1974;134(6):1001–1006. doi: 10.1001/archinte.1974.00320240035003. [DOI] [PubMed] [Google Scholar]
- 5.Roy S, Wang J, Kelschenbach J, Koodie L, Martin J. Modulation of immune function by morphine: implications for susceptibility to infection. J Neuroimmune Pharmacol. 2006;1(1):77–89. doi: 10.1007/s11481-005-9009-8. [DOI] [PubMed] [Google Scholar]
- 6.Vallejo R, de Leon-Casasola O, Benyamin R. Opioid therapy and immunosuppression: a review. Am J Ther. 2004;11(5):354–365. doi: 10.1097/01.mjt.0000132250.95650.85. [DOI] [PubMed] [Google Scholar]
- 7.Hoehe M, Duka T. Opiates increase plasma catecholamines in humans. Psychoneuroendocrinology. 1993;18(2):141–148. doi: 10.1016/0306-4530(93)90065-S. [DOI] [PubMed] [Google Scholar]
- 8.Boland JW, McWilliams K, Ahmedzai SH, Pockley AG. Effects of opioids on immunologic parameters that are relevant to anti-tumour immune potential in patients with cancer: a systematic literature review. Br J Cancer. 2014;111(5):866–873. doi: 10.1038/bjc.2014.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraus J, Lehmann L, Borner C, Hollt V. Epigenetic mechanisms involved in the induction of the mu opioid receptor gene in Jurkat T cells in response to interleukin-4. Mol Immunol. 2010;48(1-3):257–263. doi: 10.1016/j.molimm.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Fujita N, Sato S, Tsuruo T. Phosphorylation of p27Kip1 at threonine 198 by p90 ribosomal protein S6 kinases promotes its binding to 14-3-3 and cytoplasmic localization. J Biol Chem. 2003;278(49):49254–49260. doi: 10.1074/jbc.M306614200. [DOI] [PubMed] [Google Scholar]
- 11.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68(2):320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian M, Jin L, Li R, Zhu S, Ji M, Li W. Comparison of oxycodone and morphine on the proliferation, apoptosis and expression of related molecules in the A549 human lung adenocarcinoma cell line. Exp Ther Med. 2016;12(2):559–566. doi: 10.3892/etm.2016.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Shi W, Li H, Sun X, Fan X, Lesage G, Li H. et al. Critical role of toll-like receptor 9 in morphine and Mycobacterium tuberculosis-Induced apoptosis in mice. PLoS One. 2010;5(2):e9205. doi: 10.1371/journal.pone.0009205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115(6):1363–1381. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heiss A, Ammer H, Eisinger DA. delta-Opioid receptor-stimulated Akt signaling in neuroblastoma x glioma (NG108-15) hybrid cells involves receptor tyrosine kinase-mediated PI3K activation. Exp Cell Res. 2009;315(12):2115–2125. doi: 10.1016/j.yexcr.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Hsiao P, Chang M, Cheng W, Chen C, Lin H, Hsieh C, Sun W. Morphine induces apoptosis of human endothelial cells through nitric oxide and reactive oxygen species pathways. Toxicology. 2009;256:83–91. doi: 10.1016/j.tox.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Miyagi T, Chuang LF, Lam KM, Kung H, Wang JM, Osburn BI, Chuang RY. Opioids suppress chemokine-mediated migration of monkey neutrophils and monocytes - an instant response. Immunopharmacology. 2000;47(1):53–62. doi: 10.1016/S0162-3109(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Castrillon JL, Perez-Arellano JL, Garcia-Palomo JD, Jimenez-Lopez A, De Castro S. Opioids depress in vitro human monocyte chemotaxis. Immunopharmacology. 1992;23(1):57–61. doi: 10.1016/0162-3109(92)90009-2. [DOI] [PubMed] [Google Scholar]
- 19.Haghpanah T, Afarinesh M, Divsalar K. A review on hematological factors in opioid-dependent people (opium and heroin) after the withdrawal period. Addict Health. 2010;2(1-2):9–16. [PMC free article] [PubMed] [Google Scholar]
- 20.Roy S, Balasubramanian S, Sumandeep S, Charboneau R, Wang J, Melnyk D, Beilman GJ. et al. Morphine directs T cells toward T(H2) differentiation. Surgery. 2001;130(2):304–309. doi: 10.1067/msy.2001.116033. [DOI] [PubMed] [Google Scholar]
- 21.Peng X, Mosser DM, Adler MW, Rogers TJ, Meissler JJ Jr, Eisenstein TK. Morphine enhances interleukin-12 and the production of other pro-inflammatory cytokines in mouse peritoneal macrophages. J Leukoc Biol. 2000;68(5):723–728. [PubMed] [Google Scholar]
- 22.McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 2001;62(2):111–123. doi: 10.1016/S0376-8716(00)00181-2. [DOI] [PubMed] [Google Scholar]
- 23.Eisenstein TK. The Role of Opioid Receptors in Immune System Function. Front Immunol. 2019;10:2904. doi: 10.3389/fimmu.2019.02904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuggetta MP, Di Francesco P, Falchetti R, Cottarelli A, Rossi L, Tricarico M, Lanzilli G. Effect of morphine on cell-mediated immune responses of human lymphocytes against allogeneic malignant cells. J Exp Clin Cancer Res. 2005;24(2):255–263. [PubMed] [Google Scholar]
- 25.Wang X, Ma TC, Li JL, Zhou Y, Geller EB, Adler MW, Peng JS. et al. Heroin inhibits HIV-restriction miRNAs and enhances HIV infection of macrophages. Front Microbiol. 2015;6:1230. doi: 10.3389/fmicb.2015.01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long X, Li Y, Qiu S, Liu J, He L, Peng Y. MiR-582-5p/miR-590-5p targeted CREB1/CREB5-NF-kappaB signaling and caused opioid-induced immunosuppression in human monocytes. Transl Psychiatry. 2016;6:e757. doi: 10.1038/tp.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy S, Cain KJ, Chapin RB, Charboneau RG, Barke RA. Morphine modulates NF kappa B activation in macrophages. Biochem Biophys Res Commun. 1998;245(2):392–396. doi: 10.1006/bbrc.1998.8415. [DOI] [PubMed] [Google Scholar]
- 28.Bryant HU, Bernton EW, Holaday JW. Morphine pellet-induced immunomodulation in mice: temporal relationships. J Pharmacol Exp Ther. 1988;245(3):913–920. [PubMed] [Google Scholar]
- 29.Carr DJ, France CP. Immune alterations in morphine-treated rhesus monkeys. J Pharmacol Exp Ther. 1993;267(1):9–15. [PubMed] [Google Scholar]
- 30.Guo CJ, Li Y, Tian S, Wang X, Douglas SD, Ho WZ. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor. J Investig Med. 2002;50(6):435–442. doi: 10.1136/jim-50-06-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki S, Carlos MP, Chuang LF, Torres JV, Doi RH, Chuang RY. Methadone induces CCR5 and promotes AIDS virus infection. FEBS Lett. 2002;519(1-3):173–177. doi: 10.1016/S0014-5793(02)02746-1. [DOI] [PubMed] [Google Scholar]
- 32.Li S, Huang S, Peng SB. Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. Int J Oncol. 2005;27(5):1329–1339. doi: 10.3892/ijo.27.5.1329. [DOI] [PubMed] [Google Scholar]
- 33.Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309(1):99–107. doi: 10.1016/S0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 34.Mahajan SD, Schwartz SA, Shanahan TC, Chawda RP, Nair MP. Morphine regulates gene expression of alpha- and beta-chemokines and their receptors on astroglial cells via the opioid mu receptor. J Immunol. 2002;169(7):3589–3599. doi: 10.4049/jimmunol.169.7.3589. [DOI] [PubMed] [Google Scholar]
- 35.Gaspani L, Bianchi M, Limiroli E, Panerai AE, Sacerdote P. The analgesic drug tramadol prevents the effect of surgery on natural killer cell activity and metastatic colonization in rats. J Neuroimmunol. 2002;129(1-2):18–24. doi: 10.1016/S0165-5728(02)00165-0. [DOI] [PubMed] [Google Scholar]
- 36.Shavit Y, Ben-Eliyahu S, Zeidel A, Beilin B. Effects of fentanyl on natural killer cell activity and on resistance to tumor metastasis in rats. Dose and timing study. Neuroimmunomodulation. 2004;11(4):255–260. doi: 10.1159/000078444. [DOI] [PubMed] [Google Scholar]
- 37.Provinciali M, Di Stefano G, Raffaeli W, Pari G, Desiderio F, Fabris N. Evaluation of NK and LAK cell activities in neoplastic patients during treatment with morphine. Int J Neurosci. 1991;59(1-3):127–133. doi: 10.3109/00207459108985455. [DOI] [PubMed] [Google Scholar]
- 38.Provinciali M, Di Stefano G, Stronati S, Raffaeli W, Pari G, Fabris N. Role of prolactin in the modulation of NK and LAK cell activity after short- or long-term morphine administration in neoplastic patients. Int J Immunopharmacol. 1996;18(10):577–586. doi: 10.1016/S0192-0561(96)00059-8. [DOI] [PubMed] [Google Scholar]
- 39.Sacerdote P, Manfredi B, Mantegazza P, Panerai AE. Antinociceptive and immunosuppressive effects of opiate drugs: a structure-related activity study. Br J Pharmacol. 1997;121(4):834–840. doi: 10.1038/sj.bjp.0701138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makimura C, Arao T, Matsuoka H, Takeda M, Kiyota H, Tsurutani J, Fujita Y. et al. Prospective study evaluating the plasma concentrations of twenty-six cytokines and response to morphine treatment in cancer patients. Anticancer Res. 2011;31(12):4561–4568. [PubMed] [Google Scholar]
- 41.Hashiguchi S, Morisaki H, Kotake Y, Takeda J. Effects of morphine and its metabolites on immune function in advanced cancer patients. J Clin Anesth. 2005;17(8):575–580. doi: 10.1016/j.jclinane.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Palm S, Lehzen S, Mignat C, Steinmann J, Leimenstoll G, Maier C. Does prolonged oral treatment with sustained-release morphine tablets influence immune function? Anesth Analg. 1998;86(1):166–172. doi: 10.1213/00000539-199801000-00033. [DOI] [PubMed] [Google Scholar]
- 43.Sacerdote P, Bianchi M, Gaspani L, Manfredi B, Maucione A, Terno G, Ammatuna M. et al. The effects of tramadol and morphine on immune responses and pain after surgery in cancer patients. Anesth Analg. 2000;90(6):1411–1414. doi: 10.1097/00000539-200006000-00028. [DOI] [PubMed] [Google Scholar]
- 44.Franchi S, Panerai AE, Sacerdote P. Buprenorphine ameliorates the effect of surgery on hypothalamus-pituitary-adrenal axis, natural killer cell activity and metastatic colonization in rats in comparison with morphine or fentanyl treatment. Brain Behav Immun. 2007;21(6):767–774. doi: 10.1016/j.bbi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Desmond F, McCormack J, Mulligan N, Stokes M, Buggy DJ. Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. 2015;35(3):1311–1319. [PubMed] [Google Scholar]
- 46.Shen JC, Sun HL, Zhang MQ, Liu XY, Wang Z, Yang JJ. Flurbiprofen improves dysfunction of T-lymphocyte subsets and natural killer cells in cancer patients receiving post-operative morphine analgesia. Int J Clin Pharmacol Ther. 2014;52(8):669–675. doi: 10.5414/CP202027. [DOI] [PubMed] [Google Scholar]
- 47.Gong L, Qin Q, Zhou L, Ouyang W, Li Y, Wu Y, Li Y. Effects of fentanyl anesthesia and sufentanil anesthesia on regulatory T cells frequencies. Int J Clin Exp Pathol. 2014;7(11):7708–7716. [PMC free article] [PubMed] [Google Scholar]
- 48.Jankovic BD, Maric D. Enkephalins and immunity. I: In vivo suppression and potentiation of humoral immune response. Ann N Y Acad Sci. 1987;496:115–125. doi: 10.1111/j.1749-6632.1987.tb35754.x. [DOI] [PubMed] [Google Scholar]
- 49.Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol. 1998;83(1-2):63–69. doi: 10.1016/S0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- 50.Adham NF, Song MK, Eng BF. Hyper-alpha-2-macroglobulinemia in narcotic addicts. Ann Intern Med. 1978;88(6):793–795. doi: 10.7326/0003-4819-88-6-793. [DOI] [PubMed] [Google Scholar]
- 51.Grieco MH, Chuang CY. Hypermacroglobulinemia associated with heroin use in adolescents. J. Allergy Clin. Immunol. 1973;51:156–160. doi: 10.1016/0091-6749(73)90020-1. [DOI] [PubMed] [Google Scholar]
- 52.Piepenbrink MS, Samuel M, Zheng B, Carter B, Fucile C, Bunce C, Kiebala M. et al. Humoral dysregulation associated with increased systemic inflammation among injection heroin users. PLoS One. 2016;11(7):e0158641. doi: 10.1371/journal.pone.0158641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Randall D, Degenhardt L, Vajdic CM, Burns L, Hall WD, Law M, Butler T. Increasing cancer mortality among opioid-dependent persons in Australia: a new public health challenge for a disadvantaged population. Aust N Z J Public Health. 2011;35(3):220–225. doi: 10.1111/j.1753-6405.2011.00682.x. [DOI] [PubMed] [Google Scholar]
- 54.Rashidian H, Zendehdel K, Kamangar F, Malekzadeh R, Haghdoost AA. An Ecological Study of the Association between Opiate Use and Incidence of Cancers. Addict Health. 2016;8(4):252–260. [PMC free article] [PubMed] [Google Scholar]
- 55.Cushman P Jr, Grieco MH. Hyperimmunoglobulinemia associated with narcotic addiction. Effects of methadone maintenance treatment. Am J Med. 1973;54(3):320–326. doi: 10.1016/0002-9343(73)90026-0. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed S, Shurafa MS, Bishop CR, Varterasian M. Waldenstrom's macroglobulinemia in young African-American adults. Am J Hematol. 1999;60(3):229–230. doi: 10.1002/(SICI)1096-8652(199903)60:3<229::AID-AJH11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 57.Mani D. Secondary amyloidosis associated with heroin use and recurrent infections - A case report. Ann Med Surg (Lond) 2019;37:38–41. doi: 10.1016/j.amsu.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Granstrem O, Adriani W, Shumilina M, Izykenova G, Dambinova S, Laviola G. Specific changes in levels of autoantibodies to glutamate and opiate receptors induced by morphine administration in rats. Neurosci Lett. 2006;403(1-2):1–5. doi: 10.1016/j.neulet.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 59.Dambinova SA, Izykenova GA. Recombinant mu-delta receptor as a marker of opiate abuse. Ann N Y Acad Sci. 2002;965:497–514. doi: 10.1111/j.1749-6632.2002.tb04191.x. [DOI] [PubMed] [Google Scholar]
- 60.Panerai AE, Radulovic J, Monastra G, Manfredi B, Locatelli L, Sacerdote P. Beta-endorphin concentrations in brain areas and peritoneal macrophages in rats susceptible and resistant to experimental allergic encephalomyelitis: a possible relationship between tumor necrosis factor alpha and opioids in the disease. J Neuroimmunol. 1994;51(2):169–176. doi: 10.1016/0165-5728(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 61.Li Z, You Y, Griffin N, Feng J, Shan F. Low-dose naltrexone (LDN): A promising treatment in immune-related diseases and cancer therapy. Int Immunopharmacol. 2018;61:178–184. doi: 10.1016/j.intimp.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 62.Miskoff JA, Chaudhri M. Low Dose Naltrexone and Lung Cancer: A Case Report and Discussion. Cureus. 2018;10(7):e2924. doi: 10.7759/cureus.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan A. Long-term remission of adenoid cystic tongue carcinoma with low dose naltrexone and vitamin D3—a case report. Oral Health Dent Manag. 2014;13(3):721–724. [PubMed] [Google Scholar]
- 64.Donahue RN, McLaughlin PJ, Zagon IS. Low-dose naltrexone suppresses ovarian cancer and exhibits enhanced inhibition in combination with cisplatin. Exp Biol Med (Maywood) 2011;236(7):883–895. doi: 10.1258/ebm.2011.011096. [DOI] [PubMed] [Google Scholar]
- 65.Berkson BM, Rubin DM, Berkson AJ. Reversal of signs and symptoms of a B-cell lymphoma in a patient using only low-dose naltrexone. Integr Cancer Ther. 2007;6(3):293–296. doi: 10.1177/1534735407306358. [DOI] [PubMed] [Google Scholar]
- 66.Liu WM, Scott KA, Dennis JL, Kaminska E, Levett AJ, Dalgleish AG. Naltrexone at low doses upregulates a unique gene expression not seen with normal doses: Implications for its use in cancer therapy. Int J Oncol. 2016;49(2):793–802. doi: 10.3892/ijo.2016.3567. [DOI] [PubMed] [Google Scholar]
- 67.Schofferman J, Billesdon J, Hall R. Microangiopathic hemolytic anemia. Another complication of drug abuse. JAMA. 1974;230(5):721. doi: 10.1001/jama.1974.03240050049026. [DOI] [PubMed] [Google Scholar]
- 68.Markham KB, Scrape SR, Prasad M, Rossi KQ, O'Shaughnessy RW. Hemolytic Disease of the Fetus and Newborn due to Intravenous Drug Use. AJP Rep. 2016;6(1):e129–132. doi: 10.1055/s-0036-1579646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kapila A, Chhabra L, Chaubey VK, Summers J. Opana ER abuse and thrombotic thrombocytopenic purpura (TTP)-like illness: a rising risk factor in illicit drug users. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2013-203122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ambruzs JM, Serrell PB, Rahim N, Larsen CP. Thrombotic microangiopathy and acute kidney injury associated with intravenous abuse of an oral extended-release formulation of oxymorphone hydrochloride: kidney biopsy findings and report of 3 cases. Am J Kidney Dis. 2014;63(6):1022–1026. doi: 10.1053/j.ajkd.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 71.Amjad AI, Parikh RA. Opana-ER used the wrong way: intravenous abuse leading to microangiopathic hemolysis and a TTP-like syndrome. Blood. 2013;122(20):3403. doi: 10.1182/blood-2013-05-503904. [DOI] [PubMed] [Google Scholar]
- 72.Zeiger AR, Patkar AA, Fitzgerald R, Lundy A, Ballas SK, Weinstein SP. Changes in mu opioid receptors and rheological properties of erythrocytes among opioid abusers. Addict Biol. 2002;7(2):207–217. doi: 10.1080/135562102200120433. [DOI] [PubMed] [Google Scholar]
- 73.Golub ES, Diaz De' Pagan T, Sun I, Hall AK, Crane FL, Isom G. Can opioids regulate hemopoietic differentiation? Ann N Y Acad Sci. 1988;521:123–128. doi: 10.1111/j.1749-6632.1988.tb35270.x. [DOI] [PubMed] [Google Scholar]
- 74.Sardelli S, Petraglia F, Massolo F, Messori A, Santoro V, Facchinetti F, Genazzani AR. Changes in immunoreactive beta-endorphin, methionine-enkephalin and ACTH in bone marrow cells and fluid from leukemic children. Clin Immunol Immunopathol. 1986;41(2):247–253. doi: 10.1016/0090-1229(86)90108-X. [DOI] [PubMed] [Google Scholar]
- 75.Janda SS, Boranic M, Skodlar J, Petrovecki M, Nemet D, Labar B. Effect of opioid peptide methionine-enkephalin in long-term cultures of human bone marrow. Acta Med Croatica. 2000;54(3):99–105. [PubMed] [Google Scholar]
- 76.Zagon IS, McLaughlin PJ, Goodman SR, Rhodes RE. Opioid receptors and endogenous opioids in diverse human and animal cancers. J Natl Cancer Inst. 1987;79(5):1059–1065. [PubMed] [Google Scholar]
- 77.Wick MJ, Minnerath SR, Roy S, Ramakrishnan S, Loh HH. Differential expression of opioid receptor genes in human lymphoid cell lines and peripheral blood lymphocytes. J Neuroimmunol. 1996;64(1):29–36. doi: 10.1016/0165-5728(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 78.Heagy W, Teng E, Lopez P, Finberg RW. Enkephalin receptors and receptor-mediated signal transduction in cultured human lymphocytes. Cell Immunol. 1999;191(1):34–48. doi: 10.1006/cimm.1998.1409. [DOI] [PubMed] [Google Scholar]
- 79.Yin D, Woodruff M, Zhang Y, Whaley S, Miao J, Ferslew K, Zhao J. et al. Morphine promotes Jurkat cell apoptosis through pro-apoptotic FADD/P53 and anti-apoptotic PI3K/Akt/NF-kappaB pathways. J Neuroimmunol. 2006;174(1-2):101–107. doi: 10.1016/j.jneuroim.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 80.Martin-Kleiner I. The effect of opioid agonists of delta-class DSLET, mu-class DAMGO, kappa-class U-69593 and an opioid antagonist, naloxone, on MTT activity of NALM-1 leukemic cells. Biomed Pharmacother. 2002;56(9):458–462. doi: 10.1016/S0753-3322(02)00288-3. [DOI] [PubMed] [Google Scholar]
- 81.Beltran JA, Peek J, Chang SL. Expression and regulation of the mu opioid peptide receptor in TPA-differentiated HL-60 promyelocytic leukemia cells. Int Immunopharmacol. 2006;6(8):1331–1340. doi: 10.1016/j.intimp.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 82.Owusu-Agyemang P, Borthakur G, Rebello E, Arunkumar R, Wang SA, Rytting M, Jorgensen JL. et al. The association between opioid administration and response to therapy in patients with acute lymphoblastic leukemia. Leuk Lymphoma. 2015;56(6):1903–1905. doi: 10.3109/10428194.2014.981176. [DOI] [PubMed] [Google Scholar]
- 83.Sung B, Ahn KS, Aggarwal BB. Noscapine, a benzylisoquinoline alkaloid, sensitizes leukemic cells to chemotherapeutic agents and cytokines by modulating the NF-kappaB signaling pathway. Cancer Res. 2010;70(8):3259–3268. doi: 10.1158/0008-5472.CAN-09-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sueoka E, Sueoka N, Kai Y, Okabe S, Suganuma M, Kanematsu K, Yamamoto T. et al. Anticancer activity of morphine and its synthetic derivative, KT-90, mediated through apoptosis and inhibition of NF-kappaB activation. Biochem Biophys Res Commun. 1998;252(3):566–570. doi: 10.1006/bbrc.1998.9695. [DOI] [PubMed] [Google Scholar]
- 85.Sueoka N, Sueoka E, Okabe S, Fujiki H. Anti-cancer effects of morphine through inhibition of tumour necrosis factor-alpha release and mRNA expression. Carcinogenesis. 1996;17(11):2337–2341. doi: 10.1093/carcin/17.11.2337. [DOI] [PubMed] [Google Scholar]
- 86.Inada M, Shindo M, Kobayashi K, Sato A, Yamamoto Y, Akasaki Y, Ichimura K. et al. Anticancer effects of a non-narcotic opium alkaloid medicine, papaverine, in human glioblastoma cells. PLoS One. 2019;14(5):e0216358. doi: 10.1371/journal.pone.0216358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang H, Li LJ, Zhang HB, Wei AY. Papaverine selectively inhibits human prostate cancer cell (PC-3) growth by inducing mitochondrial mediated apoptosis, cell cycle arrest and downregulation of NF-kappaB/PI3K/Akt signalling pathway. J BUON. 2017;22(1):112–118. [PubMed] [Google Scholar]
- 88.Sajadian S, Vatankhah M, Majdzadeh M, Kouhsari SM, Ghahremani MH, Ostad SN. Cell cycle arrest and apoptogenic properties of opium alkaloids noscapine and papaverine on breast cancer stem cells. Toxicol Mech Methods. 2015;25(5):388–395. doi: 10.3109/15376516.2015.1045656. [DOI] [PubMed] [Google Scholar]
- 89.Noureini SK, Wink M. Antiproliferative effect of the isoquinoline alkaloid papaverine in hepatocarcinoma HepG-2 cells - inhibition of telomerase and induction of senescence. Molecules. 2014;19(8):11846–11859. doi: 10.3390/molecules190811846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Afzali M, Ghaeli P, Khanavi M, Parsa M, Montazeri H, Ghahremani MH, Ostad SN. Non-addictive opium alkaloids selectively induce apoptosis in cancer cells compared to normal cells. Daru. 2015;23:16. doi: 10.1186/s40199-015-0101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Friesen C, Roscher M, Hormann I, Fichtner I, Alt A, Hilger RA, Debatin KM. et al. Cell death sensitization of leukemia cells by opioid receptor activation. Oncotarget. 2013;4(5):677–690. doi: 10.18632/oncotarget.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin X, Chen Q, Xue LY, Ma XJ, Wang R. Endomorphins, endogenous opioid peptides, induce apoptosis in human leukemia HL-60 cells. Can J Physiol Pharmacol. 2004;82(11):1018–1025. doi: 10.1139/y04-087. [DOI] [PubMed] [Google Scholar]
- 93.Martin-Kleiner I, Gabrilovac J, Kusec R, Boranic M. Methionine enkephalin suppresses metabolic activity of a leukemic cell line (NALM-1) and enhances CD10 expression. Int Immunopharmacol. 2003;3(5):707–711. doi: 10.1016/S1567-5769(03)00058-4. [DOI] [PubMed] [Google Scholar]
- 94.Tarchalska-Krynska B, Zawisza E, Samolinski B. [Influence of morphine and naloxone on the blast transformation of lymphocytes] Otolaryngol Pol. 1989;43(1):12–18. [PubMed] [Google Scholar]
- 95.Singh A, Jayanthan A, Farran A, Elwi AN, Kim SW, Farran P, Narendran A. Induction of apoptosis in pediatric acute lymphoblastic leukemia (ALL) cells by the therapeutic opioid methadone and effective synergy with Bcl-2 inhibition. Leuk Res. 2011;35(12):1649–1657. doi: 10.1016/j.leukres.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 96.Mernenko OA, Blishchenko EY, Mirkina, II, Karelin AA. Met-enkephalin induces cytolytic processes of apoptotic type in K562 human erythroid leukemia cells. FEBS Lett. 1996;383(3):230–232. doi: 10.1016/0014-5793(96)00208-6. [DOI] [PubMed] [Google Scholar]
- 97.Perez-Alvarez S, Cuenca-Lopez MD, de Mera RM, Puerta E, Karachitos A, Bednarczyk P, Kmita H. et al. Methadone induces necrotic-like cell death in SH-SY5Y cells by an impairment of mitochondrial ATP synthesis. Biochim Biophys Acta. 2010;1802(11):1036–1047. doi: 10.1016/j.bbadis.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 98.Seifert R, Burde R, Schultz G. Lack of effect of opioid peptides, morphine and naloxone on superoxide formation in human neutrophils and HL-60 leukemic cells. Naunyn Schmiedebergs Arch Pharmacol. 1989;340(1):101–106. doi: 10.1007/BF00169214. [DOI] [PubMed] [Google Scholar]
- 99.Takeuchi R, Hoshijima H, Nagasaka H, Chowdhury SA, Kikuchi H, Kanda Y, Kunii S. et al. Induction of non-apoptotic cell death by morphinone in human promyelocytic leukemia HL-60 cells. Anticancer Res. 2006;26(5A):3343–3348. [PubMed] [Google Scholar]
- 100.Shipp MA, Stefano GB, D'Adamio L, Switzer SN, Howard FD, Sinisterra J, Scharrer B. et al. Downregulation of encephalin-mediated inflammatory responses by CD10/neutral endopeptidase 24.11. Nature. 1990;347:94–396. doi: 10.1038/347394a0. [DOI] [PubMed] [Google Scholar]
- 101.Sorensen AN, Claesson MH. Effect of the opioid methionine enkephalinamide on signal transduction in human T-lymphocytes. Life Sci. 1998;62(14):1251–1259. doi: 10.1016/S0024-3205(98)00055-1. [DOI] [PubMed] [Google Scholar]
- 102.Sergeeva MG, Grishina ZV, Varfolomeyev SD. Morphine effect on proliferation of normal and tumor cells of immune origin. Immunol Lett. 1993;36(2):215–218. doi: 10.1016/0165-2478(93)90055-7. [DOI] [PubMed] [Google Scholar]
- 103.Bosshart H. Morphine and cancer progression: hydrogen peroxide points to need for more research. J Opioid Manag. 2011;7(2):93–96. doi: 10.5055/jom.2011.0051. [DOI] [PubMed] [Google Scholar]
- 104.Zhou Z, Liu T, Zhang J. Morphine activates blast-phase chronic myeloid leukemia cells and alleviates the effects of tyrosine kinase inhibitors. Biochem Biophys Res Commun. 2019;520(3):560–565. doi: 10.1016/j.bbrc.2019.10.067. [DOI] [PubMed] [Google Scholar]
- 105.Ishikawa M, Tanno K, Kamo A, Takayanagi Y, Sasaki K. Enhancement of tumor growth by morphine and its possible mechanism in mice. Biol Pharm Bull. 1993;16(8):762–766. doi: 10.1248/bpb.16.762. [DOI] [PubMed] [Google Scholar]
- 106.Vig S, Mishra S, Rustagi K, Bhan S. Opioid toxicity with underlying tumour lysis syndrome in a patient with CMML: a diagnostic and therapeutic challenge. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2018-225646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Watson RR, Prabhala RH, Darban HR, Yahya MD, Smith TL. Changes in lymphocyte and macrophage subsets due to morphine and ethanol treatment during a retrovirus infection causing murine AIDS. Life Sci. 1988;43(6):v–xi. doi: 10.1016/0024-3205(88)90145-2. [DOI] [PubMed] [Google Scholar]
- 108.Kang SM, Rosales JL, Meier-Stephenson V, Kim S, Lee KY, Narendran A. Genome-wide loss-of-function genetic screening identifies opioid receptor mu1 as a key regulator of L-asparaginase resistance in pediatric acute lymphoblastic leukemia. Oncogene. 2017;36(42):5910–5913. doi: 10.1038/onc.2017.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Campana G, Sarti D, Spampinato S, Raffaeli W. Long-term intrathecal morphine and bupivacaine upregulate MOR gene expression in lymphocytes. Int Immunopharmacol. 2010;10(9):1149–1152. doi: 10.1016/j.intimp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 110.Chen GJ, Watson RR. Modulation of tumor necrosis factor and gamma interferon production by cocaine and morphine in aging mice infected with LP-BM5, a murine retrovirus. J Leukoc Biol. 1991;50(4):349–355. doi: 10.1002/jlb.50.4.349. [DOI] [PubMed] [Google Scholar]
- 111.Tubaro E, Avico U, Santiangeli C, Zuccaro P, Cavallo G, Pacifici R, Croce C. et al. Morphine and methadone impact on human phagocytic physiology. Int J Immunopharmacol. 1985;7(6):865–874. doi: 10.1016/0192-0561(85)90049-9. [DOI] [PubMed] [Google Scholar]
- 112.Juneja R. Opioids and cancer recurrence. Curr Opin Support Palliat Care. 2014;8(2):91–101. doi: 10.1097/SPC.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 113.Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. 2016;124(1):69–79. doi: 10.1097/ALN.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 114.Portenoy RK, Sibirceva U, Smout R, Horn S, Connor S, Blum RH, Spence C. et al. Opioid use and survival at the end of life: a survey of a hospice population. J Pain Symptom Manage. 2006;32(6):532–540. doi: 10.1016/j.jpainsymman.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 115.Boland JW, Pockley AG. Influence of opioids on immune function in patients with cancer pain: from bench to bedside. Br J Pharmacol. 2018;175(14):2726–2736. doi: 10.1111/bph.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.