Abstract
Background
Ibrutinib is a Bruton’s tyrosine kinase inhibitor that has shown to be a superior choice in the treatment of chronic lymphocytic leukemia (CLL) and a simple, oral alternative to other chemoimmunotherapies. The standard dose is 420 mg daily; however, its irreversible binding mechanism allows adequate target blockade at much lower doses due to prolonged effect. Dose reductions or interruptions are often used in clinical practice to limit its distinct side effects, including diarrhea, bleeding and atrial fibrillation and emerging evidence exists that these do not hinder efficacy. Using a retrospective clinical audit of a single-center outpatient hematology clinic, we aimed to examine outcomes and toxicities of a reduced frequency dose regimen of ibrutinib in patients beyond the confines of a clinical trial.
Methods
A small pilot study was conducted on 16 voluntary CLL patients that had achieved partial or complete remission on standard dose ibrutinib and were considering cessation due to side effects. Patients were consented and prescribed a 420 mg thrice weekly regimen and side effects and outcomes were recorded on routine review. A retrospective clinical audit from 2015 to 2018 was then conducted to compare pilot participants to patients that had remained on standard dosing and results from the extended follow-up of the landmark RESONATE trial.
Results
None of the 16 patients in the pilot relapsed or died during the study period equating to a 100% progression free and overall survival. There was resolution or reduction in all side effects reported following switchover; however, the study was too small to establish a statistical relationship.
Conclusion
This is the first study to demonstrate use of a thrice weekly regimen to reduce ibrutinib-related toxicities whilst preserving safety and efficacy in patients following complete or partial remission on standard dose therapy. Higher powered, prospective studies are required to establish positive health and financial implications in the elderly and vulnerable CLL demographic.
Keywords: Ibrutinib, CLL, Dose
Introduction
Ibrutinib inhibits action of Bruton’s tyrosine kinase (BTK), an essential enzyme in the B-cell receptor signalling pathway. It is approved for first and second-line treatment of chronic lymphocytic leukemia (CLL) and small lymphocytic leukemia. The highly bioavailable oral agent administered once daily has been shown to offer a simple and efficacious alternative to other therapies [1-5]. However, the impact of the novel agent’s unique toxicities limiting tolerability is an emerging clinical issue [5, 6].
CLL patients represent a particularly complex and vulnerable demographic when it comes to selecting treatments. Epidemiological data from the United States (US) show the median age at diagnosis is 72 years and the number of elderly individuals affected projected to rise as populations age [7]. Data from the Mayo Clinic show 89% of newly diagnosed CLL patients have comorbidities with 46% having at least one major comorbidity [8]. Despite ibrutinib offering a relatively safer and more accessible oral alternative compared to other chemotherapy or infusion-based regimens for elderly patients, ibrutinib hosts a distinctive set of prescribing challenges [5, 6]. Of particular concern is the significantly increased risk of bleeding and incident atrial fibrillation (AF) in those with underlying cardiovascular conditions and the impact of consequent heart failure or stroke on patient and health system. The paradoxical increased bleeding risk secondary to effect on platelet pathways in the setting of anticoagulation further complicates the issue [9], as does concurrent prescription of common CYP3A4 inhibitors, which significantly increase ibrutinib levels and lead to more adverse events and worse overall survival (OS) rates [10]. Moreover, high rates of gastrointestinal side effects (SEs) and respiratory tract infections can lead to decompensation and significantly impact quality of life in older individuals. A recent retrospective review of both commercial and clinical trials found toxicity to be the leading cause of discontinuation of ibrutinib in CLL accounting for 63% in frontline and 50% in relapsed/refractory settings [6]. In the extended follow-up of the RESONATE trial which compared efficacy of ibrutinib to ofatumumab in previously treated CLL, the most common SEs reported were diarrhea in over half of patients, followed by bleeding in 43%, then fatigue, nausea and pyrexia. Respiratory tract infections were the most common infections, occurring in 25%. Cytopenias were also a common finding with both neutropenia and anemia reported in 25%. Incident AF was recorded in 7% [11].
The standard dose of ibrutinib is 420 mg daily based on evidence showing adequate BTK blockade, and well-established efficacy [1-4]. Ibrutinib’s half-life is very short at only 4 h; however, its duration of effect is prolonged and sufficient BTK blockade achieved at lower doses secondary to irreversible binding [12, 13]. We know dose reductions to manage ibrutinib toxicities are relatively common in clinical practice [14] and a number of retrospective reviews have shown equivalent efficacy [14-17]. However, a gap remains in our knowledge and approach toward rationale-based dosing that could significantly reduce drug burden as well as cost to patient and health system.
This is the first published study to use thrice weekly dosing as opposed to dose reduction or treatment breaks to manage SEs. We hypothesized reduced frequency dosing (RFD) of ibrutinib would limit SEs but preserve efficacy based on drug pharmacokinetics [12, 13] and mounting clinical evidence [14-17]. Secondary aims of the study were to examine rates of SEs and outcomes beyond the restrictions of a clinical trial.
Materials and Methods
The small pilot study was conducted in a private hematology outpatient clinic. Patients were recruited on an opportunistic and voluntary basis between 2015 and 2018. Patients who had achieved complete remission (CR) or partial remission (PR) according to the International Workshop on Chronic Lymphocytic Leukemia consensus criteria (iwCLL) [18] on single-agent, standard dose ibrutinib (420 mg daily) and considering treatment cessation secondary to SEs, were offered an RFD regimen of 420 mg three times a week (Monday, Wednesday and Friday). Sixteen patients were consented to the intervention arm and data on SEs were collected using routine physician review, serial blood testing and electrocardiograms.
A retrospective review was then conducted on 40 patients on single-agent ibrutinib therapy including the 16 in the pilot. Subjects were not matched. Variables recorded were: basic demographic data, total time on ibrutinib therapy, time on RFD, SEs, and outcomes including OS, progression-free survival (PFS) and death. Prevalence of toxicities was recorded prior to switch over, at the time of switchover and after switchover to RDF regimen and expressed as proportions and percentages. OS and PFS were defined by iwCLL criteria [18] and measured by Kaplan-Meier survival analysis.
The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration including provision of informed consent obtained from all participants in the pilot. Institutional review board approval was granted.
Results
Patient characteristics
Mean age of all subjects in the audit was 72 years and median age was 73 years (range: 56 - 87 years). Median ages of patients in the intervention (RFD) arm and standard dose arm were 70 and 73 years, respectively and 62.5% of patients were male. All patients included in the audit had previously received more than one therapy for CLL. Three patients (18%) in the RFD arm had the p53 mutation. Median total time on standard dose ibrutinib therapy was 10.5 months (range: 1 - 44 months) in the standard dose arm, 20.5 months in the RFD arm prior to switchover and 21 months on RFD following switchover.
Disease outcomes
Overall patient outcomes are summarized in Table 1. Of the 40 patients included in the audit, 22 patients or just over half continued on ibrutinib therapy beyond the study period including all in RFD arm. The most common cause of drop-out was death in seven patients (five secondary to Richter’s transformation). Only four (10%) required other therapies for refractory disease and three were lost to follow-up or elected to discontinue.
Table 1. Treatment Outcomes of CLL Patients on Single-Agent Ibrutinib.
| Forty CLL patients on ibrutinib | Twenty-two (55%) continued therapy | Sixteen (40%) on RFD thrice weekly regimen (three with p53 mutation) |
| Six (15%) continued on standard dose (two with p53 mutation) | ||
| Eighteen (45%) ceased therapy | Seven (17.5%) died (five from Richter’s transformation with p53) | |
| Four (10%) required other therapies | ||
| Four (10%) have not required other therapy | ||
| Three (7.5%) lost to follow-up or refused therapy |
CLL: chronic lymphocytic leukemia; RFD: reduced frequency dosing.
PFS and OS
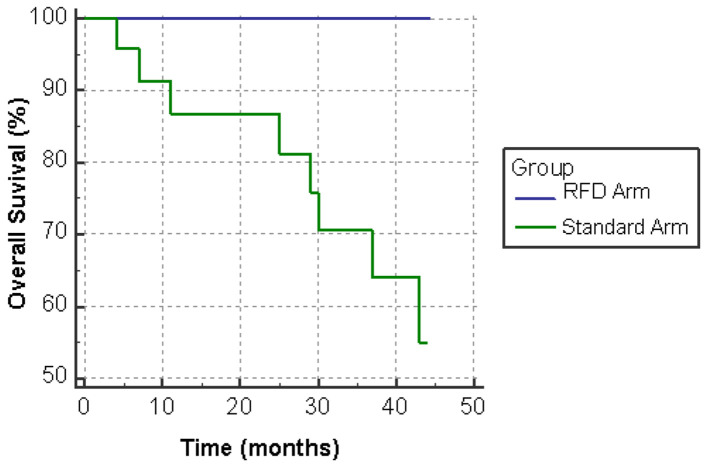
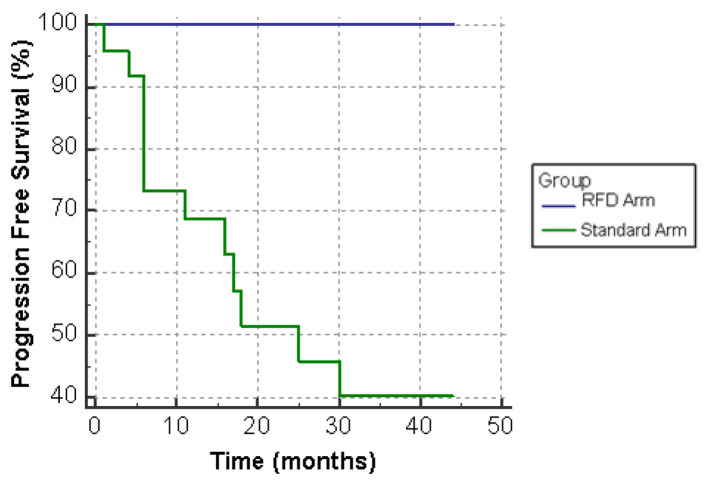
All 16 patients in the pilot study achieved PFS at extended follow-up of 42 months. In the patients which remained on standard dose therapy, 21 of 24 (88%) patients survived to 24 months and 17 of 24 (71%) were alive at 42 months (Fig. 1). PFS was achieved by 14 of 24 (58%) patients at 24 months and 13 of 24 (54%) patients beyond 30 months (Fig. 2).
Figure 1.
Overall survival (OS) on standard dose versus reduced frequency dosing (RFD) ibrutinib.
Figure 2.
Progression-free survival (PFS) on standard dose versus reduced frequency dosing (RFD) ibrutinib.
Ibrutinib-related toxicities
Prevalence rates of SEs in standard dosing group, RFD group prior to switchover, and post switchover are expressed as the first three columns in Table 2. Frequencies of SEs are expressed in comparison to frequency (from highest to lowest) identified in the extended follow-up of the RESONATE paper. The most common SEs reported in the audit overall were skin lesions and nail changes (pitting and softening with breaks), identified in 14 of 40 (35%) patients. These SEs resolved in two of three patients following switchover to RFD. Other common SEs overall were fatigue, upper respiratory tract infection and cough, with baseline percentages almost equivalent to RESONATE and decreased following RFD. Conversely diarrhea and bleeding occurred at much lower rates than reported in RESONATE (10% vs. 53.8% and 12.5% vs. 43%, respectively). Proportions of patients found to have incident AF were higher at all testing points in the audit compared to RESONATE [11].
Table 2. Rates of Ibrutinib-Related Side Effects in Audit Versus RESONATE Extended Follow-Up.
| Side effects | All patients in audit (n = 40) | Reduced frequency dosing arm (n = 16) |
RESONATE (n = 195) | |
|---|---|---|---|---|
| Prior to switchover | After switchover | |||
| Median follow-up (months) | 34 | 20.5 | 21 | 19 |
| Diarrhea | 4 (10%) | 1 (6.25%) | 0 | 105 (53.8%) |
| Bleeding | 5 (12.5%) | 1 (6.25%) | 0 | 84 (43%) 19 (10%) > 18 m |
| Fatigue | 14 (35%) | 6 (37.5%) | 4 (25%) | 67 (34.4%) |
| Nausea | 2 (5%) | 1 (6.25%) | 0 | 61 (31.3%) |
| Pyrexia | 4 (10%) | 0 | 0 | 58 (29.7%) |
| Cough | 20% (8) | 0 | 0 | 51 (26.2%) |
| Neutropenia | 2 (5%) | 0 | 0 | 50 (25.6%) |
| Anemia | 2 (5%) | 0 | 0 | 49 (25.1%) |
| Upper respiratory tract infection | 10 (25%) | 2 (12.5%) | 1 (6.25%) | 49 (25.1%) |
| Peripheral edema | 0 | 0 | 0 | 38 (19.5%) |
| Sinusitis | 0 | 0 | 0 | 37 (19.0%) |
| Arthralgia | 4 (10%) | 0 | 0 | 36 (18.5%) |
| Muscle spasms | 0 | 0 | 0 | 36 (18.5%) |
| Constipation | 2 (5%) | 0 | 0 | 35 (17.9%) |
| Headache | 4 (10%) | 2 (12.5%) | 2 (12.5%) | 33 (16.9%) |
| Pneumonia | 2 (5%) | 0 | 0 | 33 (16.9%) |
| Thrombocytopenia | 1 (2.5%) | 0 | 0 | 33 (16.9%) |
| Vomiting | 0 | 0 | 0 | 33 (16.9%) |
| Hypertension | 1 (2.5%) | 1 (6.25%) | 0 | 10 (5%) 4 (2%) > 18 m |
| Atrial fibrillation | 4 (10%) | 2 (12.5%) | 1 (6.25%) | 8 (4%) 0 > 18 m |
| Skin/nail lesions | 14 (35%) | 3 (18.75%) | 1 (6.25%) | Not reported |
Discussion
In the last decade, novel targeted agents such as ibrutinib have been replacing chemotherapy for the treatment of B-cell malignancies such as CLL. Ibrutinib has been marketed as a safe and simple oral alternative [1, 2, 4]; however, the burden of its distinct and serious SEs in the elderly CLL demographic is generating clinical interest. Dose reduction or interruption to manage toxicities is well documented and shown to not adversely affect disease-related outcomes [14-17]. This study is the first to demonstrate that switchover to a thrice weekly dose regimen following standard daily dose induction could offer a safe and efficacious option in CLL patients that have achieved CR or PR.
We aimed to examine a representative sample of the CLL demographic separated from the restrictions of a clinical trial. The single-center pilot was conducted at a specialist hematology outpatient clinic in a regional area of New South Wales, Australia. There were no exclusion criteria for comorbidities or performance status; patient recruitment was opportunistic and voluntary. The median age of subjects was 73 years, almost equivalent to the median age of diagnosis of CLL in the US [7], but older than the RESONATE trial which was 67 years. Median follow-up of the 16 subjects in the intervention arm was equivalent to the extended follow-up of RESONATE [11].
Disease-related outcomes
The audit demonstrated significantly improved OS and PFS in the RFD arm with no patients experiencing relapse or death. Overall PFS in our standard dose arm was 58% compared to 74% in the RESONATE trial at 24 months, perhaps reflecting the older age, comorbidities and lower performance status of subjects compared to RESONATE. However, OS was almost equivalent to RESONATE [11].
Ibrutinib-related SEs
The most common SEs reported in the audit were skin lesions and nail changes experienced in 14 patients (35%). There was a reduction to one in three patients following switch to RFD. In terms of mechanism, ibrutinib is also known to inhibit other kinases including epidermal growth factor, collagen and collagen-related peptide-induced platelet aggregation as off-target effects and in a dose dependent manner [19, 20]. Skin toxicity was not listed as an SE in the RESONATE paper though it has been listed as a frequent SE and cause for discontinuation in other reviews [6, 15]. Several case studies have described varied skin reactions which resolve with treatment cessation [20, 21].
Atrial fibrillation is an established AE, cause of morbidity and common reason for ibrutinib discontinuation [6, 9, 22]. To date, the mechanism has not been elucidated although BTK at Tec targets is expressed in cardiac tissue. The prevalence of AF has previously been reported as 5-9% in CLL patients, increasing to 16% in older patients [22]. There was an elevated proportion of AF across our subject groups compared to RESONATE, likely reflecting the healthier and younger subjects included in a clinical trial. AF resolved in only one of the two patients in our RFD arm, limiting our ability to interpret these results.
Bleeding, said to be mediated by BTK and Tec inhibition of platelet aggregation pathways [19], is a common and paradoxically dangerous SE in the setting of anticoagulation and concomitant use of CYP3A4 inhibitors [10]. It was reported in 44% with major events in 1-2% of cases in RESONATE. Another center found bleeding rate of 47%, but 17% with major bleeding events, although this is perhaps partially explained by 9.7% of patients being co-administered vitamin K antagonists [23]. Only five patients (12.5%) in our audit reported bleeding and it resolved in the single patient who reported it following switchover to RFD. Conversely, the rates of the other most common SEs in the audit (fatigue, upper respiratory tract infection and cough) were almost equivalent to RESONATE. No patients in the RFD group ceased therapy secondary to SEs compared to 18 (45%) in the audit overall and 7% in RESONATE [11].
We did not utilize rigorous questionnaires that would feature in clinical trials and this may account for the significantly lower rate of embarrassing gastrointestinal SEs as opposed to those that use objective measures such as incident AF.
The pathophysiological relationship between various kinase blockade and SEs suggests a dose-dependent relationship may exist. Further knowledge surrounding in vitro and in vivo effects of RFD could lead way to major clinical implications such as curtailing rates of heart failure, disabling stroke and life-threatening bleeding. At a population health level, this regimen would significantly lower drug cost on patient and health system. In Australia, the government pays $8,785.41 for 1-month supply of ibrutinib per patient. The regimen proposed would save over $60,000 per patient, per year [24]. These costs are similar in other countries.
Limitations
As the study was preliminary, it is limited by its small sample size (n = 40) with only 16 patients recruited to the pilot arm. The pilot’s inclusion criteria of CR or PR on standard dose ibrutinib may also have selected out-patients that were both sensitive and tolerant to ibrutinib. A much higher powered prospective study is required to establish if SEs are dose responsive, given numerous SEs were only reported by one or two subjects in the intervention arm. Investigators and subjects were not blinded, which may explain reductions in SEs following switchover. Finally, subjects were not matched to controls and baseline comorbidities and demographics were collected but not adjusted for in analysis due to the limited sample size. This would be important in future studies where stratification of variables such as age, comorbidities and medications, e.g. anticoagulants or CYP3A4 inhibitors, could guide a rationale-based dosing approach.
Conclusion
This study is the first to demonstrate an RFD regimen of thrice weekly ibrutinib could offer safe and efficacious option in CLL patients who have achieved CR or PR following standard dose induction. Whilst RFD also resulted in reduction of serious and minor SEs, higher powered, prospective studies are required to establish statistical significance. We propose further investigation into rationale-based dosing is important for limiting toxicities, extending treatment and enhancing response rates in the vulnerable CLL demographic. Not to mention the positive financial implications on patient and health system.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from all participants in the pilot.
Author Contributions
William Alexander: study design, recruited participants, collected data; analyzed data, statistical analysis, wrote manuscript, final article approval prior to submission. Sarah Davis: analyzed data, statistical analysis, wrote manuscript, final article approval prior to submission. Raj Ramakrishna: study design, recruited participants, analyzed data, critical revision of manuscript for important intellectual content, final article approval prior to submission. Arumugam Manoharan: recruited participants, analyzed data, critical revision of manuscript for important intellectual content, final article approval prior to submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
References
- 1.Deeks ED. Ibrutinib: A review in chronic lymphocytic leukaemia. Drugs. 2017;77(2):225–236. doi: 10.1007/s40265-017-0695-3. [DOI] [PubMed] [Google Scholar]
- 2.Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, Bairey O. et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, Coutre S. et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, Grant B. et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nuttall E, Tung J, Trounce E, Johnston R, Chevassut T. Real-world experience of ibrutinib therapy in relapsed chronic lymphocytic leukemia: results of a single-center retrospective analysis. J Blood Med. 2019;10:199–208. doi: 10.2147/JBM.S202286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mato AR, Nabhan C, Thompson MC, Lamanna N, Brander DM, Hill B, Howlett C. et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874–879. doi: 10.3324/haematol.2017.182907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Cancer Institute. Cancer stat facts: leukemia - chronic lymphocytic leukemia (CLL). http://seer.cancer.gov/statfacts/html/clyl.html. Accessed on Apr 10, 2020.
- 8.Thurmes P, Call T, Slager S, Zent C, Jenkins G, Schwager S, Bowen D. et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49(1):49–56. doi: 10.1080/10428190701724785. [DOI] [PubMed] [Google Scholar]
- 9.Leong DP, Caron F, Hillis C, Duan A, Healey JS, Fraser G, Siegal D. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138–140. doi: 10.1182/blood-2016-05-712828. [DOI] [PubMed] [Google Scholar]
- 10.Kittai A, Maniar A, Gordon M, Churnetski M, Rivera X, Alqahtani H, Hoff S. et al. Effect of concurrent CYP3A4 interacting medications on ibrutinib outcomes in patients with CLL. J Clin Onc. 2018;36(15_suppl):e19514. doi: 10.1200/JCO.2018.36.15_suppl.e19514. [DOI] [Google Scholar]
- 11.Brown JR, Hillmen P, O'Brien S, Barrientos JC, Reddy NM, Coutre SE, Tam CS. et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018;32(1):83–91. doi: 10.1038/leu.2017.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, Kolibaba KS. et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31(1):88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LS, Bose P, Cruz ND, Jiang Y, Wu Q, Thompson PA, Feng S. et al. A pilot study of lower doses of ibrutinib in patients with chronic lymphocytic leukemia. Blood. 2018;132(21):2249–2259. doi: 10.1182/blood-2018-06-860593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parikh SA, Achenbach SJ, Call TG, Rabe KG, Ding W, Leis JF, Kenderian SS. et al. The impact of dose modification and temporary interruption of ibrutinib on outcomes of chronic lymphocytic leukemia patients in routine clinical practice. Cancer Med. 2020;9(10):3390–3399. doi: 10.1002/cam4.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forum UC. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101(12):1563–1572. doi: 10.3324/haematol.2016.147900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akhtar OS, Attwood K, Lund I, Hare R, Hernandez-Ilizaliturri FJ, Torka P. Dose reductions in ibrutinib therapy are not associated with inferior outcomes in patients with chronic lymphocytic leukemia (CLL) Leuk Lymphoma. 2019;60(7):1650–1655. doi: 10.1080/10428194.2018.1554862. [DOI] [PubMed] [Google Scholar]
- 17.Mato AR, Timlin C, Ujjani C, Skarbnik A, Howlett C, Banerjee R, Nabhan C. et al. Comparable outcomes in chronic lymphocytic leukaemia (CLL) patients treated with reduced-dose ibrutinib: results from a multi-centre study. Br J Haematol. 2018;181(2):259–261. doi: 10.1111/bjh.14540. [DOI] [PubMed] [Google Scholar]
- 18.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, Hillmen P. et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 19.Ysebaert L, Levade M, Cedric G, Michallet A-S, Tam C, Pierre S, Payrastre B. Elucidation of mild bleeding disorders reported under ibrutinib (Imbruvica(R)) therapy: implications for optimal clinical management. Blood. 2014;124:21. doi: 10.1182/blood.V124.21.3296.3296. [DOI] [Google Scholar]
- 20.Mannis G, Wu D, Dea T, Mauro T, Hsu G. Ibrutinib rash in a patient with 17p del chronic lymphocytic leukemia. Am J Hematol. 2015;90(2):179. doi: 10.1002/ajh.23775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulvey JJ, Nuovo GJ, Magro CM. Cutaneous, purpuric painful nodules upon addition of ibrutinib to RCVP therapy in a CLL patient: a distinctive reaction pattern reflecting iatrogenic Th2 to Th1 milieu reversal. Am J Dermatopathol. 2016;38(7):492–498. doi: 10.1097/DAD.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 22.Thompson PA, Levy V, Tam CS, al Nawakil C, Goudot FX, Quinquenel A, Michallet A-S. et al. The impact of atrial fibrillation on subsequent survival of patients receiving ibrutinib as treatment of chronic lymphocytic leukemia (CLL): an international study. Blood. 2016;128:22. doi: 10.1182/blood.V128.22.3242.3242. [DOI] [Google Scholar]
- 23.Castelli R, Schiavon R, Preti C, Deliliers GL. Ibrutinib related bleeding complications in elderly patients with B cell malignancies. J Thromb Thrombolysis. 2019;48(4):694–696. doi: 10.1007/s11239-019-01907-9. [DOI] [PubMed] [Google Scholar]
- 24. Australian Government Department of Health. The Pharmaceutical Benefits Scheme -Ibrutinib. http://www.pbs.gov.au/medicine/item/11213E. Accessed on March 1, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.