Abstract
Background
Atrial fibrillation (AF) affects quality of life and prognosis of patients with cardiovascular disease. Resistin plays an important role in inflammatory response to internal and external factors. The aim of this study is to evaluate the association between resistin and permanent AF (PAF) in patients with cardiovascular disease.
Methods
In our study, we included 146 patients with cardiovascular disease. Plasma resistin concentrations and demographic characteristics of patients were recorded. The patients were divided in two groups: 118 patients without a history of PAF (NonAF group), and 28 patients with a history of PAF (AF group). Association of resistin with PAF and other variables was examined by parametric and non-parametric tests, and multivariable linear and univariable logistic regression analysis.
Results
No differences of demographic characteristics (gender, age and body mass index (BMI)) between two groups were observed (P > 0.05). Higher median levels of resistin were observed in group AF than in group NonAF (6.90 ng/mL vs. 5.83 ng/mL, P = 0.03). Multivariate linear regression analysis (adjusted to gender, age, BMI, hypertension, diabetes mellitus, coronary artery disease, and mitral valve disease) showed that resistin was associated with PAF (β = 0.79, 95% confidence interval (CI): 0.08 to 1.51, P = 0.03).
Conclusions
Our analysis showed that plasma resistin was associated with PAF, and resistin concentration was higher in patients with AF compared to those without AF.
Keywords: Resistin, Atrial fibrillation, Cardiovascular disease
Introduction
Cardiovascular disease (CVD) is a complex pathologic mechanism which includes genetic predisposition, lifestyle and diet, metabolic and autoimmune diseases. Although in the last year there are more studies that have shown that genetic predisposition plays one of the most significant roles in the onset of clinical manifestations and worsening of CVD, the impact of the environment and lifestyle remains consistently variable as a risk factor for CVD. The above also applies to atrial fibrillation (AF), which significantly affects the morbidity and mortality rate of patients with CVD [1, 2]. Early detection of biomarkers in the peripheral blood of patients who have not shown clinical laboratory findings of any types of AF including permanent AF (PAF) could be considered as diagnostic and prognostic indicators for predicting the occurrence of PAF and preventing the severe adverse events due to AF (stroke, heart failure and admission rate in hospital). On the other hand AF is a multifactor disease in which many pathological mechanisms are involved, although not clearly understood [1]. Unfortunately, the diagnosis of AF is often confirmed after clinical manifestations, and early detection of biomarkers in patients’ blood (even without the classic predisposing factors) could give the opportunity to predict the onset of AF in asymptomatic patients, to modify and control the risk factors with more regular monitoring of these patients, and possible administration of anticoagulant and antiarrhythmic medication (after clinical and laboratory test and risk assessment), and reduce the likelihood of adverse events (such as stroke) [1, 2]. Various risk factors have been suggested for the occurrence of AF. AF is associated with structural heart disease and especially with mitral valve disease (stenosis or insufficiency), arterial hypertension (AH), heart failure (HF), heart valve disease (HVD), diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), chronic renal failure (CRF) and obesity.
Human resistin is a protein that is formed from 106 amino acids, which has a crystalline form, and its weight is 12.5 kDa [3]. The human resistin gene is located on chromosome 19 [4]. The presence and action of resistin were initially studied mainly in patients with metabolic diseases such as DM type II, in whom elevated levels of resistin in the blood were found and which is secreted mainly by adipocytes of adipose tissue [3]. Other disease in which increasing the resistin concentration has been observed is obesity, which is associated with decreasing insulin function and cell resistance to insulin action. Elevated resistin levels are associated not only with chronic inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease or metabolic diseases (DM and obesity), but as recent studies have shown with CVD such as atherosclerosis [5-10].
The aim of this study is to evaluate the association of resistin with PAF, in 146 patients with CVD.
Materials and Methods
Study population
Between 2016 and 2017, 146 patients with CVD (coronary artery disease and/or peripheral vascular disease and/or HVD and/or AF) were enrolled in our study. For the study purpose, the patients were divided into two groups depending on the presence and absence of PAF from their medical history. Permanent AF was defined according to the American Heart Association and European Society of Cardiology guidelines, the atrial fibrillation which was accepted by the patients and physicians [1, 2]. All demographic data were recorded. Patients with acute heart disease or acute illness or known oncology disease were not included in this study.
Peripheral blood samples (10 mL) were taken after peripheral vena puncture during assessment of patients in outpatient clinic. The blood samples were processed within 1 - 2 h of collection. After the centrifugation of the blood, the blood plasma was isolated (200 µL) and maintained at -80 °C. In continuous, blood plasma analysis for resistin concentration was performed by the enzyme-linked immunosorbent assay (ELISA) multiplex method in FlexMAP3D, Luminex Corporation. The unit of resistin measurement concentration in blood plasma samples was expressed by ng/mL.
Statistical analysis
The data were presented with mean and standard deviation (SD) (mean ± SD) or with intermediate value (median) or number (n). The normality of distribution of variables and data (normality of distribution) was examined by Shapiro-Wilk test and Q-Q plot. Differences between characteristics of patients and the association of resistin with CVD or other parameters and PAF were examined by parametric (Student’s t-test) or non-parametric test (Mann-Whitney test, Kruskal-Wallis test, Chi-square or Fisher’s exact test). Also, the correlation strength of resistin with other variables (BMI, left ventricular ejection fraction (LVEF)) and correlation between other variables which was included in analysis were expressed with Spearman (rs) or Pearson (r) correlation coefficient, and it was depending on the type of the variables. In addition, univariable logistic regression and uni- and multivariable linear regression analysis was used to analyze the predictive value of resistin for PAF. Depending on the regression model, the prognostic value of resistin for PAF was expressed by odds ratio (OR), and association of PAF with resistin was expressed by linear regression coefficient (β). In all tests the confidence interval (CI) was set at 95% (95% CI). The statistically significant difference were considered if P < 0.05. IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, USA) were used for the data analysis.
Ethical compliance
This study was followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975. Informed consents were obtained from all individual participants included in the study, and this study had been approved by our Hospital Institutional Review Board.
Results
The 146 patients included in the study were divided into two groups depending on presence and absence of PAF. The group without PAF was defined as NonAF group with 118 patients, while the group with PAF was defined as AF group with 28 patients. Comparison of the demographic data of the two groups showed that there was no difference between them (gender, age, and BMI; P > 0.05). Patients in the AF group had a higher incidence of mitral valve disease than the NonAF group (P < 0.001). There were 10.1% of patients (9/89 patients) with coronary artery disease history, while 5.6% of patients (2/36 patients) with a history of acute coronary syndrome (> 90 days). There was also no difference between the two groups (NonAF vs. AF) regarding smoking, history of high blood pressure and DM (P > 0.05). The median LVEF did not differ between two groups (55% vs. 55%, P = 0.25). The highest levels of resistin were observed in AF patients compared to the NonAF group (median: 6.90 ng/mL vs. 5.83 ng/mL, P = 0.03) (Fig. 1). The above data are presented in detail in Table 1.
Figure 1.
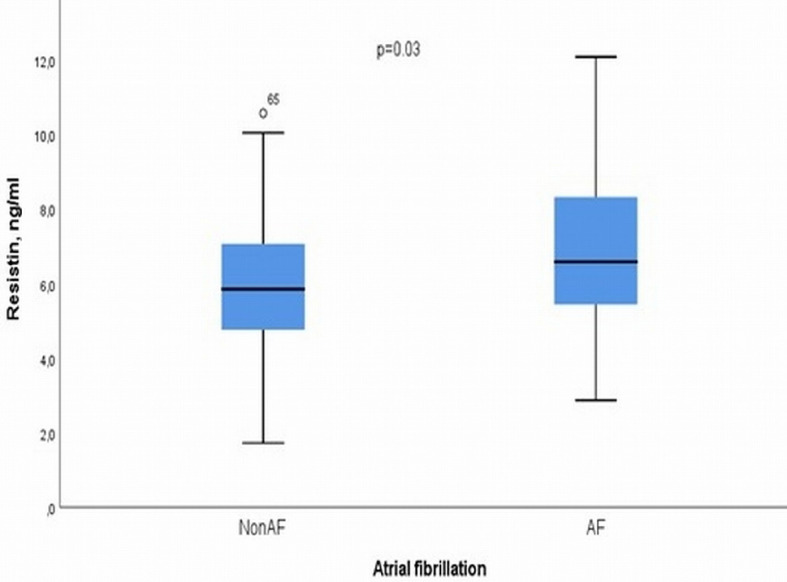
Boxplot graph for the levels of resistin in the blood plasma of patients with and without permanent atrial fibrillation.
Table 1. Demographic Characteristics and Blood Plasma Resistin Levels in Two Groups.
| Variables | NonAF (n = 118) | AF (n = 28) | P value |
|---|---|---|---|
| Age, years, median (IQR) | 69 (62 - 76) | 69.5 (58 - 74.5) | 0.62 |
| Gender, female, n (%) | 27 (22.9) | 9 (32.1) | 0.30 |
| Body mass index, kg/m2, median (IQR) | 28.1 (26.1 - 31.6) | 28 (23.2 - 29.6) | 0.27 |
| Hypertension, n (%) | 78 (66.1) | 17 (60.7) | 0.71 |
| Smoking, n (%) | 85 (72) | 16 (57) | 0.17 |
| Hypercholesterolemia, n (%) | 72 (61) | 14 (50) | 0.35 |
| Diabetes mellitus, n (%) | 0.10 | ||
| Type I | 1 (0.8) | 2 (7.1) | |
| Type II | 40 (33.9) | 8 (28.6) | |
| Chronic obstructive pulmonary disease, n (%) | 27 (22.9) | 5 (17.8) | 0.62 |
| Coronary artery disease, n (%) | 80 (67.8) | 9 (32.1) | < 0.01* |
| Previous acute coronary syndrome (> 90 days), n (%) | 34 (28.8) | 2 (7.1) | 0.03* |
| Mitral valve disease, n (%) | < 0.01* | ||
| Regurgitation | 20 (17) | 11 (39.2) | |
| Stenosis | 7 (5.9) | 2 (7.1) | |
| Mixed mitral valve disease | 0 (0) | 5 (17.8) | |
| Aortic valve disease, n (%) | 0.05 | ||
| Stenosis | 37 (31.3) | 11 (39.3) | |
| Regurgitation | 6 (5) | 5 (17.8) | |
| Left atrium diameter, mm (IQR) | 42 (38.8 - 46) | 51 (44 - 55) | < 0.001* |
| LVEF, %, median (IQR) | 55 (45 - 60) | 55 (42.5 - 60.0) | 0.25 |
| Creatinine, mg/dL, median (IQR) | 1.0 (0.8 - 1.1) | 1.0 (0.8 - 1.3) | 0.16 |
| Resistin, ng/mL, median (IQR) | 5.83 (4.96 - 7.13) | 6.90 (5.85 - 8.33) | 0.03* |
NonAF group: patients without permanent atrial fibrillation. AF group: patients with permanent atrial fibrillation. IQR: interquartile range; n: number; LVEF: left ventricular ejection fraction. *P < 0.05 was considered statistically significant.
An interesting finding in our analysis was that patients without DM and with or without PAF had a small difference in resistin levels in the blood, while patients with DM and PAF had higher levels of resistin in the blood than those without DM and without PAF (Fig. 2). The above result could support the idea that DM is involved in the systemic inflammatory response due to hyperglycemia and insulin resistance in the pathological mechanism of PAF. Resistin has often been linked with obesity, and in the present study we tried to investigate whether the BMI of patients with CVD of our sample is related to resistin. The analysis of the data showed that the BMI was not correlated with PAF (r = -0.02, P = 0.77 and β = 0.001, 95% CI: -0.06 to 0.05, P = 0.78) which is shown by Figure 3. We tested the possible relationship of resistin with the LVEF in the sample of our study patients. It has been shown that LVEF was not correlated significantly with resistin blood levels (r = -0.08, P = 0.40 and β = -0.01, 95% CI: -0.04 to 0.01, P = 0.37) (Fig. 4).
Figure 2.
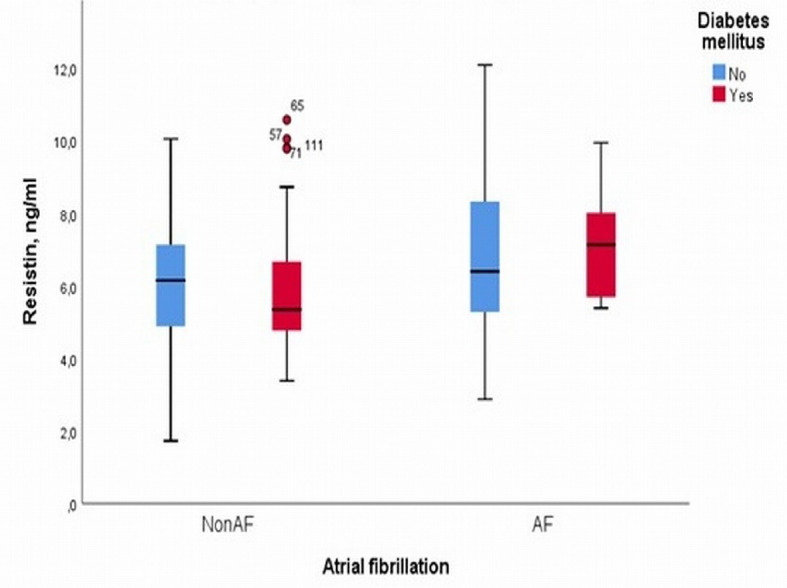
Boxplot graph for the levels of resistin in the blood plasma of patients with and without permanent atrial fibrillation depending to diabetes mellitus.
Figure 3.
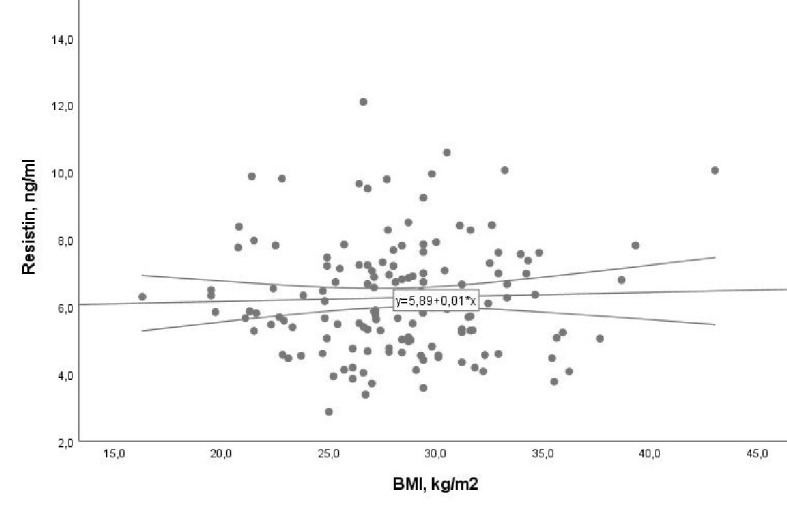
Association of resistin with body mass index (BMI) with 95% confidence interval (CI) (β = 0.001, 95% CI: -0.06 to 0.05, P = 0.78).
Figure 4.
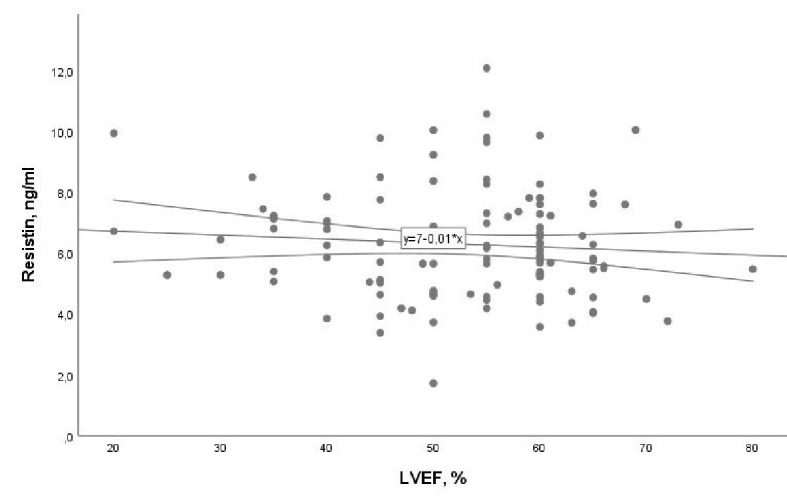
Association of resistin with left ventricular ejection fraction (LVEF) with 95% confidence interval (CI) (β = -0.01, 95% CI: -0.04 to 0.01, P = 0.37).
In addition, a multivariable linear regression (adjusted to gender, age, hypertension, DM, coronary artery disease, mitral valve disease, aortic valve disease) showed that PAF was associated with blood plasma resistin (β = 0.99, 95% CI: 0.16 to 1.81, P = 0.03) (Table 2). Furthermore the prognostic value of resistin as a biomarker for PAF in patients with CVD has been studied. Univariable logistic regression analysis was applied for this, and the predictive value of resistin for PAF was OR = 1.3, 95% CI: 1.02 to 1.64, P = 0.02.
Table 2. Multivariate Linear Regression Analysis of Resistin With Permanent Atrial Fibrillation.
| Variables | P value | β | 95% Confidence interval |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 0.13 | 0.02 | -0.008 | 0.06 |
| Hypertension | 0.52 | 0.21 | -0.45 | 0.88 |
| Aortic valve disease | 0.93 | 0.03 | -0.77 | 0.83 |
| Mitral valve disease | 0.69 | 0.14 | -0.58 | 0.87 |
| Atrial fibrillation | 0.03* | 0.99 | 0.16 | 1.81 |
| Plasma creatinine | < 0.001* | 0.68 | 0.38 | 0.98 |
| Left ventricular ejection fraction | 0.73 | -0.005 | -0.03 | 0.02 |
| Gender | 0.96 | 0.02 | -0.72 | 0.76 |
| Coronary artery disease | 0.56 | -0.22 | -0.99 | 0.54 |
β: linear regression coefficient. *P < 0.05 was considered statistically significant.
Discussion
AF is the most common persistent heart arrhythmia that is observed in the general population and especially in patients with CVD. AF is usually diagnosed after the onset of symptoms, and the most common laboratory finding is electrocardiographic disturbances with arrhythmic atrial and ventricular frequency, with that atrial frequency can reach about 300 beats/min. The incidence of AF in the general population, according to recent studies, ranges from 1% to 4% [1]. The increase in the number of patients with PAF is probably due to the discovery and diagnosis of “silent” PAF and increase of the average age of the population in developing and developed countries. The incidence of AF does not differ between male versus female, on the other hand the female is an additional factor for the occurrence of stroke [1].
Structural heart disease activates various inflammatory mechanisms and reactions in human organism resulting in the activation of fibroblasts and macrophages, connective tissue deposition, fibrosis and cardiac myocyte necrosis within the myocardium. The above factors cause heart rhythm disturbance, which further aggravates the already burdened cardiac function. Cardiac remodeling due to structural heart disease (mitral regurgitation, etc.) contributes to disruption of the cohesion of cardiomyocytes and cardiac conduction system. CVD disease and microangiopathy is caused by early necrosis and apoptosis of myocardial cells (cardiomyocytes). Obesity due to inflammatory reaction is considered a cause of local damage in the conduction cardiac system [11-14]. The turbulence blood flow in the left atrium in patients with AF added an additional risk factor that contributes to the clots formation in the heart.
The response to AF is expressed by acute (postoperative) or chronic inflammatory reaction, and it is due to dilatation of the left atrium, left atrium fibrosis, oxidative stress and pro-thrombotic and thrombotic predisposition [15-19]. The above pathophysiological mechanisms of myocardial fibrosis contribute to the cardiac remodeling which occurs in atria and ventricles. Coronary artery disease, hypertension and other predisposing factors create complicated pathological mechanisms, and help enhance inflammation in myocardial cells. Left atrium dilation due to other structural heart diseases such as mitral valve disease contributes on the pathophysiological mechanism of AF. PAF causes chronic damage to the left ventricular wall by fibrosis and replacement of myocardial cells with fibroblasts. The deposition of fibroblasts in the atrium wall due to the dilation of the left atrium and chronic volume overload of systemic circulation and chronic local inflammation in left atrium and ventricles causes an increase in matrix metallopeptidase-9 (MMP-9), MMP-2 and transforming growth factor beta (TGF-β1) levels [20, 21].
Recent studies on resistin demonstrated effects on the endocrine system. In addition, resistin also plays an important role in inflammatory response to internal and external factors [22-27]. The presence of adipocytes in the left atrium wall may lead to the fatty infiltration of the atrial wall, and especially in obese patients it could be the center of interaction of adipocytes with cardiac conduction system [28]. The presence of resistin in adipocytes could mediate the paracrine action of adipocytes and cause of local inflammation in the myocardial cells through pro-inflammatory and inflammatory cytokines resulting in either necrosis or dysfunction of the cell membrane. Similar effect appears to be present at the point where the adipose tissue comes into contact with the epicardial coronary arteries. Chronic local inflammation could probably also affect the development of fibrous tissue and cause coagulation disorders of the atrial endocardium. On the other hand, chronic stress also affects the function of the sympathetic nervous system, resulting in increased overproduction of stress hormones and increased blood glucose levels. Resistin is elevated in patients with DM (mainly in obese patients), and increased and regulated blood glucose concentration is maintained for a long time. This mechanism could be considered as the local action as well as the systemic action of resistin in the atrial myocardium and may be a factor which mainly associated with PAF, since this arrhythmia persists for a long time [11, 29]. The above suspicion and theory could also be combined with the fact that obesity is considered a risk factor for the occurrence of AF. This is supported by another study showing that adipose tissue is associated with the development of postoperative AF in cardiac surgery patients [30].
The mechanism of fibrous tissue growth in the atrial wall is most likely due to chronic inflammation that develops due to chronic pressure on the walls that results from chronic volume overload which is observed in patients mainly with PAF [24]. Resistin is the substance involved in immune response and is a protein of inflammation, its action on the left ventricular remodeling mechanism due to PAF could be the explanation for its increased concentration in the blood of patients with PAF even if other CVD is coexisted [12, 17]. Secretion of resistin by monocytes stimulates the secretion of inflammatory cytokines by the endothelium and macrophages resulting in the activation of fibroblasts and the replacement of the gaps created between the myocytes and the location of dead fibrous cells by fibrous tissue.
Some studies have suggested that resistin is involved as a mediator in the human body’s immune response to chronic left ventricular myocardial damage with resulting left ventricule dysfunction. Many researchers have tried to associate the increased concentration of resistin in patients with CVD and left ventricular dysfunction and heart failure [16, 18]. On the other hand, mitochondria play a central role in the function of cardiac myocyte and many of the pathophysiological processes involved in AF are related with the mitochondria function, including reactive oxygen species (ROS) formation and the changes in oxygen consumption in the myocardial cells of the heart [30-34]. Oxidative stress and inflammation are closely linked because inflammation can lead to ROS production and ROS could also promote the synthesis of pro-inflammatory cytokines such as interleukin (IL)-6, IL-8, IL-10, IL-12 and tumor necrosis factor alpha (TNF-α) [33]. Elevated ROS levels, such as H2O2 and peroxide, have been associated with AF as a result of reduced NO bioavailability. Increased oxygen needs of the myocardium under conditions of tachycardia and arrhythmia cause the damage of metabolism and myocyte function. The consequence of pathological necrosis is a remodeling of the atrium, which practically inactivates the contractile capacity of the atria and thus burdens the circulation in the heart cavities. On the other hand, resistin in the context of inflammatory response is produced by the monocytes that in turn activate the endothelium for the secretion of intercellular adhesion molecules-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) [34, 35]. Resistin actively participates in the mechanism of immune response to chronic stress due to AF and on the other hand oxidative stress activates pathophysiological mechanisms that cause the remodeling of the heart and the formation of blood clots in the left atrium.
Study limitations
Our study included the small number of patients and we did not include in our study the patients without CVD as control group. Paroxysmal AF may be observed in past in patients group without AF, which was asymptomatic and did not record in patient’s medical history.
Conclusions
We concluded that resistin could be considered as a biomarker which characterizes PAF in patients with CVD. The concentration of resistin is higher in patients with PAF than that in patients without PAF. To confirm the above study results randomized trials with a larger number of patients should be designed to draw safer conclusions regarding the diagnostic and prognostic value of resistin as a biomarker for PAF.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consents were obtained.
Author Contributions
George Samanidis: conceptualization, methodology, software, data curation, writing (original and revision draft preparation), visualization, investigation, validation, and statistical analysis. Anastasios Gkogkos: equal contribution to writing (original draft), investigation, and methodology. Stefanos Bousounis: investigation. Leonidas Alexopoulos: peripheral blood analysis. Despina N. Perrea: supervision. Konstantinos Perreas: conceptualization, writing (original draft preparation), investigation, and supervision.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
References
- 1.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB. et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199–267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR. et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Ambrosi J, Fruhbeck G. Evidence for the involvement of resistin in inflammation and cardiovascular disease. Curr Diabetes Rev. 2005;1(3):227–234. doi: 10.2174/157339905774574392. [DOI] [PubMed] [Google Scholar]
- 5.Bakker MF, Cavet G, Jacobs JW, Bijlsma JW, Haney DJ, Shen Y, Hesterberg LK. et al. Performance of a multi-biomarker score measuring rheumatoid arthritis disease activity in the CAMERA tight control study. Ann Rheum Dis. 2012;71(10):1692–1697. doi: 10.1136/annrheumdis-2011-200963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka N, Kusunoki N, Kusunoki Y, Hasunuma T, Kawai S. Resistin is associated with the inflammation process in patients with systemic autoimmune diseases undergoing glucocorticoid therapy: comparison with leptin and adiponectin. Mod Rheumatol. 2013;23(1):8–18. doi: 10.3109/s10165-012-0623-z. [DOI] [PubMed] [Google Scholar]
- 7.Angel San Miguel Hernandez, Maria San Miguel Rodriguez, Angel San Miguel Rodriguez, Sara Martin Armentia, Jesus Pachon. Clinical significance of the resistin in clinical practice. Clin and Med Rep. 2018;1:1–5. doi: 10.15761/CMR.1000130. [DOI] [Google Scholar]
- 8.Zhang Y, Li Y, Yu L, Zhou L. Association between serum resistin concentration and hypertension: A systematic review and meta-analysis. Oncotarget. 2017;8(25):41529–41537. doi: 10.18632/oncotarget.17561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norman G, Norton GR, Libhaber CD, Michel F, Majane OH, Millen AM, Sareli P. et al. Independent associations between resistin and left ventricular mass and myocardial dysfunction in a community sample with prevalent obesity. Int J Cardiol. 2015;196:81–87. doi: 10.1016/j.ijcard.2015.05.184. [DOI] [PubMed] [Google Scholar]
- 10.Gencer B, Auer R, de Rekeneire N, Butler J, Kalogeropoulos A, Bauer DC, Kritchevsky SB. et al. Association between resistin levels and cardiovascular disease events in older adults: The health, aging and body composition study. Atherosclerosis. 2016;245:181–186. doi: 10.1016/j.atherosclerosis.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeClercq V, Taylor C, Zahradka P. Adipose tissue: the link between obesity and cardiovascular disease. Cardiovasc Hematol Disord Drug Targets. 2008;8(3):228–237. doi: 10.2174/187152908785849080. [DOI] [PubMed] [Google Scholar]
- 12.Calabro P, Golia E, Maddaloni V, Malvezzi M, Casillo B, Marotta C, Calabro R. et al. Adipose tissue-mediated inflammation: the missing link between obesity and cardiovascular disease? Intern Emerg Med. 2009;4(1):25–34. doi: 10.1007/s11739-008-0207-2. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010;2010:535918. doi: 10.1155/2010/535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and Atrial Fibrillation Prevalence, Pathogenesis, and Prognosis: Effects of Weight Loss and Exercise. J Am Coll Cardiol. 2017;70(16):2022–2035. doi: 10.1016/j.jacc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Brugts JJ, Akin S, Helming AM, Loonstra S, van den Bos EJ, Kofflard MJ. The predictive value of cardiac biomarkers in prognosis and risk stratification of patients with atrial fibrillation. Curr Opin Cardiol. 2011;26(5):449–456. doi: 10.1097/HCO.0b013e3283499ed3. [DOI] [PubMed] [Google Scholar]
- 16.Chae CW, Kwon YW. Cell signaling and biological pathway in cardiovascular diseases. Arch Pharm Res. 2019;42(3):195–205. doi: 10.1007/s12272-019-01141-0. [DOI] [PubMed] [Google Scholar]
- 17.Hogas S, Bilha SC, Branisteanu D, Hogas M, Gaipov A, Kanbay M, Covic A. Potential novel biomarkers of cardiovascular dysfunction and disease: cardiotrophin-1, adipokines and galectin-3. Arch Med Sci. 2017;13(4):897–913. doi: 10.5114/aoms.2016.58664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Streb W, Mitrega K, Szymala M, Wozniak A, Podolecki T, Kalarus Z. The intracardiac concentrations of the N-terminal-pro B-type natriuretic peptide (NT-proBNP) and the determinants of its secretion in patients with atrial fibrillation. Kardiol Pol. 2018;76(2):433–439. doi: 10.5603/KP.a2017.0206. [DOI] [PubMed] [Google Scholar]
- 19.Smekal A, Vaclavik J. Adipokines and cardiovascular disease: A comprehensive review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161(1):31–40. doi: 10.5507/bp.2017.002. [DOI] [PubMed] [Google Scholar]
- 20.Vilchez JA, Roldan V, Hernandez-Romero D, Valdes M, Lip GY, Marin F. Biomarkers in atrial fibrillation: an overview. Int J Clin Pract. 2014;68(4):434–443. doi: 10.1111/ijcp.12304. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima A, Yokoyama K, Kawanami D, Ohkido I, Urashima M, Utsunomiya K, Yokoo T. Association between resistin and fibroblast growth factor 23 in patients with type 2 diabetes mellitus. Sci Rep. 2018;8(1):13999. doi: 10.1038/s41598-018-32432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanciu AE, Vatasescu RG, Stanciu MM, Serdarevic N, Dorobantu M. The role of pro-fibrotic biomarkers in paroxysmal and persistent atrial fibrillation. Cytokine. 2018;103:63–68. doi: 10.1016/j.cyto.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Fontana A, Spadaro S, Copetti M, Spoto B, Salvemini L, Pizzini P, Frittitta L. et al. Association between resistin levels and all-cause and cardiovascular mortality: a new study and a systematic review and meta-analysis. PLoS One. 2015;10(3):e0120419. doi: 10.1371/journal.pone.0120419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Y, Liu L, Wang J. Adiponectin and the risk of new-onset atrial fibrillation: a meta-analysis of prospective cohort studies. Biosci Rep. 2019;39(6):BSR20182284. doi: 10.1042/BSR20182284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, Darbar D. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7(4):438–444. doi: 10.1016/j.hrthm.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79(3):495–502. doi: 10.1253/circj.CJ-15-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abhishek Maan, Moussa Mansour, Ruskin JN, Kevin Heist E. Role of epicardial fat in atrial fibrillation pathophysiology and clinical implications. The Journal of Innovations in Cardiac Rhythm Management. 2013;4:1077–1082. [Google Scholar]
- 28.Ermakov S, Azarbal F, Stefanick ML, LaMonte MJ, Li W, Tharp KM, Martin LW. et al. The associations of leptin, adiponectin and resistin with incident atrial fibrillation in women. Heart. 2016;102(17):1354–1362. doi: 10.1136/heartjnl-2015-308927. [DOI] [PubMed] [Google Scholar]
- 29.Peller M, Kaplon-Cieslicka A, Rosiak M, Tyminska A, Ozieranski K, Eyileten C. et al. Are adipokines associated with atrial fibrillation in type 2 diabetes? Endokrynol Pol. 2020;71:34–41. doi: 10.5603/EP.a2019.0059. [DOI] [PubMed] [Google Scholar]
- 30.Rachwalik M, Obremska M, Zysko D, Matusiewicz M, Sciborski K, Jasinski M. The concentration of resistin in perivascular adipose tissue after CABG and postoperative atrial fibrillation. BMC Cardiovasc Disord. 2019;19(1):294. doi: 10.1186/s12872-019-1254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Yang X, Li Y, Yuan M, Tian C, Yang Y, Zhang X. et al. Mitochondria and the pathophysiological mechanism of atrial fibrillation. Curr Pharm Des. 2018;24(26):3055–3061. doi: 10.2174/1381612824666180903125300. [DOI] [PubMed] [Google Scholar]
- 32.Dilaveris P, Antoniou CK, Manolakou P, Tsiamis E, Gatzoulis K, Tousoulis D. Biomarkers Associated with Atrial Fibrosis and Remodeling. Curr Med Chem. 2019;26(5):780–802. doi: 10.2174/0929867324666170918122502. [DOI] [PubMed] [Google Scholar]
- 33.Samman Tahhan A, Sandesara PB, Hayek SS, Alkhoder A, Chivukula K, Hammadah M, Mohamed-Kelli H. et al. Association between oxidative stress and atrial fibrillation. Heart Rhythm. 2017;14(12):1849–1855. doi: 10.1016/j.hrthm.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li JY, He Y, Ke HH, Jin Y, Jiang ZY, Zhong GQ. Plasma oxidative stress and inflammatory biomarkers are associated with the sizes of the left atrium and pulmonary vein in atrial fibrillation patients. Clin Cardiol. 2017;40(2):89–94. doi: 10.1002/clc.22633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren X, Wang X, Yuan M, Tian C, Li H, Yang X, Li X. et al. Mechanisms and treatments of oxidative stress in atrial fibrillation. Curr Pharm Des. 2018;24(26):3062–3071. doi: 10.2174/1381612824666180903144042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.


