Abstract
Background and Objective:
EUS-guided pancreatic drainage (EUS-PD) is an efficacious, acceptable risk option for patients with pancreatic duct obstruction who fail conventional ERCP. The aim of this study was to define the learning curve (LC) for EUS-PD.
Methods:
Consecutive patients undergoing EUS-PD by a single operator were included from a dedicated registry. Demographics, procedural info, adverse events, and follow-up data were collected. Nonlinear regression and cumulative sum (CUSUM) analyses were conducted for the LC.
Results:
Fifty-six patients were included (54% of male, with a mean age of 58 years). Technical success was achieved in 47 patients (84%). Stent placement was antegrade in 36 patients (77%) and retrograde in 11 (23%). Clinical success was achieved in 46/47 (98%) patients who achieved technical success. Adverse events were seen in 13 patients (6 of whom did not achieve technical success) and included bleeding requiring embolization (n = 5), bleeding treated with clips peri-procedurally (n = 1), pancreatitis (n = 5), and a pancreatic fluid collection drained via EUS-drainage (n = 2). The median procedural time was 80 min (range 49–159 min). The CUSUM chart showed that 80-min procedural time was achieved at the 27th procedure. Durations further reduced 40th procedure onward, reaching a plateau indicating proficiency (nonlinear regression P < 0.0001).
Conclusion:
Endoscopists experienced in EUS-PD are expected to achieve a reduction in procedural time over successive cases, with efficiency reached at 80 min and a learning rate of 27 cases. Continued improvement is demonstrated with additional experience, with plateau indicating mastery suggested at the 40th case. EUS-PD is probably one of the hardest therapeutic endosonographic procedures to learn.
Keywords: EUS-guided pancreatic drainage, pancreatic stricture, pancreaticogastrostomy, pancreatico-jejunostomy, therapeutic EUS
INTRODUCTION
EUS-guided pancreatic drainage (EUS-PD) is a minimally invasive alternative for patients who fail conventional endoscopic retrograde pancreatography (ERP).[1,2,3,4,5,6] EUS-PD has gained increasing recognition as an alternative to surgery or percutaneous interventions for patients who fail conventional ERP.[7,8,9,10,11,12,13,14,15,16]
EUS-PD is a challenging procedure that requires access to the main pancreatic duct (MPD), creation of a fistulous tract, and deployment of a decompressing stent across the tract.[8] Numerous technical challenges have been identified in performing EUS-PD.[17,18] Accessing the MPD requires traversing fibrotic changes in the pancreatic parenchyma.[19,20] Other technical challenges identified are the lack of stability of the EUS endoscope in the stomach, the ability to move a guidewire through a needle to create a fistulous tract, and the ability to dilate the fistulous tract with either a balloon-dilating catheter or cautery.[19,21,22] No study has been performed on the learning curve (LC) for performing EUS-PD. The aim of this study is to define the LC for performing EUS-PD based on a single-operator experience.
METHODS
Study overview
Over a 14-year span, 56 patients underwent EUS-PD by a single endoscopist (MK) with expertise in therapeutic EUS and ERCP. All patients were included in a prospective registry. Data on patient demographics, clinical indications for procedure, procedural techniques, and follow-up information were collected. Technical success was defined as successful stent placement. Clinical success was defined as resolution of procedural indication. Adverse effects including bleeding, pancreatic fluid collections, and pancreatitis were recorded.
Procedural technique
All patients were done under general anesthesia and using CO2 insufflation. Antibiotics were given to all patients peri-procedurally.
A linear echoendoscope was advanced into the stomach or small bowel. Following localization of the MPD and the use of color Doppler imaging to identify regional vasculature, the MPD was punctured with a 19G fine-needle aspiration needle. A pancreatogram was performed by injecting contrast through the 19G needle, and wire access was obtained by advancing a guidewire through the EUS needle into the MPD under fluoroscopic guidance. A 0.035” hydrophilic wire was used in all cases. Fistula track creation and dilation was performed using cautery with a needle-knife catheter or 10 Fr cystotome followed by a 4-mm dilating balloon after which a stent was deployed.
Stent placement
The terminology and techniques used to describe stent placement have been previously reported by our group.[17] Antegrade stent placement is in the direction toward the head of the pancreas and retrograde is toward the tail of the pancreas. In the majority of cases, one plastic double-pigtail stent was deployed; in several cases, a fully covered metal stent was deployed.
Statistical analysis
Nonlinear regression was performed to evaluate the effect of number of procedures on procedural time. LC-Cumulative sum (CUSUM) analysis was used to assess the LC for performing EUS-PD. LC-CUSUM analysis was performed similar to the modeling demonstrated by Biau et al.[23]
The following parameters were applied to this series: for the LC, the hypothesis H0 was set with P0 = 0.175 (failure rate 17.5%; process out of control) and H1 with P1 = 0.1 (failure rate 10%; process in control) as per standard ERCP failures rates. Type I (α) and Type II (β) error rates were set at 0.1. A control limit of h = 2.0 was chosen. All descriptive and statistical analyses were conducted using MedCalc V18.9 (MedCalc Software, Ostend, Belgium).
RESULTS
In total, 56 patients (54% men; mean age, 58.0 ± 18.2 years) were included. The majority of patients (n = 48, 86%) had benign disease: 26 patients (46%) had chronic pancreatitis, 16 patients (29%) had a surgical stricture, and 6 patients (11%) had pancreatic divisum [Table 1]. The remaining eight patients (14%) had malignant obstruction. Twenty-five patients (45%) had undergone prior surgical intervention (Whipple n = 22, Roux-en-Y n = 3). The procedures were evenly spread over the 14-year period.
Table 1.
EUS-pancreatic drainage learning curve (n=56)
| Characteristic | n (%) |
|---|---|
| Age, mean (SD) | 58 (18.2) |
| Gender – male, n (%) | 29/56 (54) |
| Indication for EUS-PD, n (%) | |
| Chronic pancreatitis | 26 (46) |
| Surgical stricture | 16 (29) |
| Malignant stricture | 8 (14) |
| Pancreas divisum | 6 (11) |
| Technical success | 47/56 (84) |
| Approach, n (%) | |
| Antegrade | 36 (77) |
| Retrograde | 11 (23) |
| Type of stent | |
| Plastic | 46/47 |
| Metal | 1/47 |
| Adverse events, n (%) | 13/56 (24) |
| Bleeding | 6 |
| Pancreatitis | 5 |
| PFC with cystogastrostomy | 2 |
| Clinical success, n (%) | 46/47 (98) |
| Procedural time (min) | 80 |
| Reintervention required | 2 |
| Mean follow-up time (months) | 13.2 |
PD: Pancreatic drainage; SD: Standard deviation; PFC: Pancreatic fluid collection
Technical success
Technical success was achieved in 84% of patients (n = 47) [Table 1]. MPD was accessed in a transgastric orientation in 38 patients (81%) and a transduodenal orientation in 5 patients (11%). The stent was deployed in an anterograde manner in 36 of the 47 patients (77%): 4 anterograde transpapillary or transanastomotic and 32 anterograde transgastric. The stent was deployed in a retrograde manner in 11 patients (23%), with 5 retrograde transgastric and 6 retrograde transenteric. Except for one metal stent, all stents were double-pigtail plastic stents (range 5 F–10 F). Two patients who did not achieve technical success had a repeat attempt at EUS-PD and achieved technical success (not included in this article’s technical success rate); one patient underwent surgery and four patients underwent celiac plexus block for symptom control. All the technical failures were attempted via transgastric puncture.
Clinical success
Clinical success was achieved in 84% of the total patients (46/56) and in 98% of patients who achieved technical success (46/47) [Table 1]. Two patients required reintervention due to stent migration with successful repeat EUS-PD. The median follow-up period was 13 months (range 1–41), with 50% of patients having follow-up for ≥12 months.
Adverse events
The overall adverse event rate was 24% (n = 13). Six of the 13 patients who experienced adverse effects did not achieve technical success. Adverse events included bleeding requiring embolization (n = 5), bleeding requiring peri-procedural clips (n = 1), pancreatitis (n = 5), and a pancreatic fluid collection requiring EUS-guided drainage (n = 2).
Learning curve
The median procedural time was 80 min (range 49–159 min). The CUSUM plot showed a progressing reduction in procedural time, indicating that performance proficiency consistently improved. At the 27th procedure, a total procedural time of 80 min was achieved, indicating procedural efficiency.
Figure 1 shows the nonlinear regression curve between procedural time and number of cases. The downward slope of the curve shows progressive reduction in procedural time. Procedural durations further reduced 40th procedure onward, reaching a plateau indicating proficiency (nonlinear regression P < 0.0001).
Figure 1.
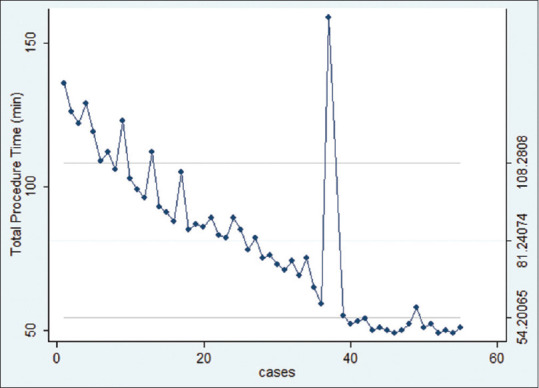
Nonlinear regression curve of EUS-PD between procedural time and number of cases
DISCUSSION
EUS-PD has been described as a potential alternative for patients who fail conventional ERCP. Initial studies demonstrated a wide range of efficacy and adverse effect rates, ranging from 50% to 100% and 7% to 55%, respectively.[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]
With technological improvement and increasing operator experience, EUS-PD’s efficacy and safety rates have increased as well. A large, international multicentric study reported a technical success of 89%, a clinical success of 81%, and an overall adverse effect rate of 20% in eighty patients undergoing EUS-PD.[17] Chen et al.’s study compared EUS-PD with enteroscopy-assisted ERP in patients with post-Whipple anatomy and reported a technical success of 92.5%, clinical success of 87.5%, and an overall adverse effect rate of 35% in forty patients undergoing EUS-PD.[18]
Despite improvements in technology and increased time spent doing therapeutic EUS procedures, EUS-PD remains a technically challenging procedure for proficient echoendoscopists. Limited data exist on the LC associated with technically difficult advanced endoscopy procedures, including EUS-PD. Fujii et al. reported a procedural duration mean of 142 min (standard deviation [SD]: 59 min) in 32 patients who underwent successful stenting with EUS-PD and 154 min (SD: 54 min) in 11 patients who failed stenting, although the authors suggested that the reported duration times may be an overestimate due to limitations of their procedural database.[6] In their report, it was suggested that increasing experience of the endoscopists led to increasing technical success and fewer adverse effects.[6] Chen et al. reported a procedural duration of 55.1 min in forty patients with post-Whipple anatomy undergoing EUS-PD in an international, multicentric study.[18] In our study, CUSUM analysis showed a reduction in procedural time over successive cases, with efficiency achieved at around the 27th case. In addition, continued improvement was demonstrated with additional experience, with proficiency reached around 40 cases. CUSUM analysis has been used to evaluate the LC for trainees performing ERCP, trainees performing EUS in gastric cancer T staging, and an advanced endoscopist performing EUS-guided hepaticogastrostomy with transmural stenting.[24,25,26,27]
Our study has a few limitations. This study reports the LC of a single operator with expertise in both EUS and ERCP at a large tertiary academic center. Our study also does not separately assess the LC between patients who had benign indication for the procedure and those with malignant disease and between patients with normal anatomy and those with surgically altered anatomy based on the stent placement technique, as the procedural duration times are directly impacted by disease indication, stent placement technique, and anatomy.
CONCLUSION
In conclusion, our study is the first study to assess the LC of therapeutic EUS of PD for various indications. Our data may form the basis for recommending the minimum number of EUS-PD procedures needed to achieve competency and mastery. Further studies with more operators, and further separation of LC s by disease indication, would help produce competency estimates and form a credentialing standard for EUS-PD training.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tessier G, Bories E, Arvanitakis M, et al. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc. 2007;65:233–41. doi: 10.1016/j.gie.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 2.François E, Kahaleh M, Giovannini M, et al. EUS-guided pancreaticogastrostomy. Gastrointest Endosc. 2002;56:128–33. doi: 10.1067/mge.2002.125547. [DOI] [PubMed] [Google Scholar]
- 3.Brauer BC, Chen YK, Fukami N, et al. Single-operator EUS-guided cholangiopancreatography for difficult pancreaticobiliary access (with video) Gastrointest Endosc. 2009;70:471–9. doi: 10.1016/j.gie.2008.12.233. [DOI] [PubMed] [Google Scholar]
- 4.Vila JJ, Pérez-Miranda M, Vazquez-Sequeiros E, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest Endosc. 2012;76:1133–41. doi: 10.1016/j.gie.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc. 2004;59:100–7. doi: 10.1016/s0016-5107(03)02300-9. [DOI] [PubMed] [Google Scholar]
- 6.Fujii LL, Topazian MD, Abu Dayyeh BK, et al. EUS-guided pancreatic duct intervention: Outcomes of a single tertiary-care referral center experience. Gastrointest Endosc. 2013;78:854–640. doi: 10.1016/j.gie.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Kahaleh M, Yoshida C, Yeaton P. EUS anterograde pancreatography with gastropancreatic duct stent placement: Review of two cases. Gastrointest Endosc. 2007;65:224–30. doi: 10.1016/s0016-5107(03)02297-1. [DOI] [PubMed] [Google Scholar]
- 8.Kahaleh M, Hernandez AJ, Tokar J, et al. EUS-guided pancreaticogastrostomy: Analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest Endosc. 2007;65:224–30. doi: 10.1016/j.gie.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Ergun M, Aouattah T, Gillain C, et al. Endoscopic ultrasound-guided transluminal drainage of pancreatic duct obstruction: Long-term outcome. Endoscopy. 2011;43:518–25. doi: 10.1055/s-0030-1256333. [DOI] [PubMed] [Google Scholar]
- 10.Shah JN, Marson F, Weilert F, et al. Single-operator, single-session EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointest Endosc. 2012;75:56–64. doi: 10.1016/j.gie.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Kurihara T, Itoi T, Sofuni A, et al. Endoscopic ultrasonography-guided pancreatic duct drainage after failed endoscopic retrograde cholangiopancreatography in patients with malignant and benign pancreatic duct obstructions. Dig Endosc. 2013;25(2):109–16. doi: 10.1111/den.12100. [DOI] [PubMed] [Google Scholar]
- 12.Will U, Fueldner F, Thieme AK, et al. Transgastric pancreatography and EUS-guided drainage of the pancreatic duct. J Hepatobiliary Pancreat Surg. 2007;14:377–82. doi: 10.1007/s00534-006-1139-8. [DOI] [PubMed] [Google Scholar]
- 13.Kinney TP, Li R, Gupta K, et al. Therapeutic pancreatic endoscopy after Whipple resection requires rendezvous access. Endoscopy. 2009;41:898–901. doi: 10.1055/s-0029-1215081. [DOI] [PubMed] [Google Scholar]
- 14.Barkay O, Sherman S, McHenry L, et al. Therapeutic EUS-assisted endoscopic retrograde pancreatography after failed pancreatic duct cannulation at ERCP. Gastrointest Endosc. 2010;71:1166–73. doi: 10.1016/j.gie.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 15.Will U, Fueldner F, Goldmann B, et al. Successful transgastric pancreaticography and endoscopic ultrasound-guided drainage of a disconnected pancreatic tail syndrome. Therap Adv Gastroenterol. 2011;4:213–8. doi: 10.1177/1756283X10394232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Will U, Füldner F, Reichel A, et al. EUS-guided drainage of the pancreatic duct (EUPD)--promising therapeutic alternative to surgical intervention in case of symptomatic retention of the pancreatic duct and unsuccessful ERP. Zentralbl Chir. 2014;139:318–25. doi: 10.1055/s-0033-1350868. [DOI] [PubMed] [Google Scholar]
- 17.Tyberg A, Sharaiha RZ, Kedia P, et al. EUS-guided pancreatic drainage for pancreatic strictures after failed ERCP: A multicenter international collaborative study. Gastrointest Endosc. 2017;85:164–9. doi: 10.1016/j.gie.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 18.Chen YI, Levy MJ, Moreels TG, et al. An international multicenter study comparing EUS-guided pancreatic duct drainage with enteroscopy-assisted endoscopic retrograde pancreatography after Whipple surgery. Gastrointest Endosc. 2017;85:170–7. doi: 10.1016/j.gie.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Devière J. EUS-guided pancreatic duct drainage: A rare indication in need of prospective evidence. Gastrointest Endosc. 2017;85:178–80. doi: 10.1016/j.gie.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 20.Will U, Reichel A, Fueldner F, et al. Endoscopic ultrasonography-guided drainage for patients with symptomatic obstruction and enlargement of the pancreatic duct. World J Gastroenterol. 2015;21:13140–51. doi: 10.3748/wjg.v21.i46.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii-Lau LL, Levy MJ. Endoscopic ultrasound-guided pancreatic duct drainage. J Hepatobiliary Pancreat Sci. 2015;22:51–7. doi: 10.1002/jhbp.187. [DOI] [PubMed] [Google Scholar]
- 22.Itoi T, Yasuda I, Kurihara T, et al. Technique of endoscopic ultrasonography-guided pancreatic duct intervention (with videos) J Hepatobiliary Pancreat Sci. 2014;21:E4–9. doi: 10.1002/jhbp.43. [DOI] [PubMed] [Google Scholar]
- 23.Biau DJ, Williams SM, Schlup MM, et al. Quantitative and individualized assessment of the learning curve using LC-CUSUM. Br J Surg. 2008;95:925–9. doi: 10.1002/bjs.6056. [DOI] [PubMed] [Google Scholar]
- 24.Waller HM, Connor SJ. Cumulative sum (CUSUM) analysis provides an objective measure of competency during training in endoscopic retrograde cholangio-pancreatography (ERCP) HPB (Oxford) 2009;11:565–9. doi: 10.1111/j.1477-2574.2009.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wani S, Hall M, Wang AY, et al. Variation in learning curves and competence for ERCP among advanced endoscopy trainees by using cumulative sum analysis. Gastrointest Endosc. 2016;83:711–9. doi: 10.1016/j.gie.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Park CH, Park JC, Kim EH, et al. Learning curve for EUS in gastric cancer T staging by using cumulative sum analysis. Gastrointest Endosc. 2015;81:898–905. doi: 10.1016/j.gie.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Oh D, Park DH, Song TJ, et al. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap Adv Gastroenterol. 2017;10:42–53. doi: 10.1177/1756283X16671671. [DOI] [PMC free article] [PubMed] [Google Scholar]


