Abstract
Objectives:
The aim of this study is to estimate the cutoff length for stereomicroscopically visible white core (SVWC) required for the pathological diagnosis of subepithelial lesions (SELs) from samples obtained using a novel 22-G Franseen biopsy needle and determine the sensitivity using the SVWC cutoff length.
Patients and Methods:
Thirty patients with SELs requiring pathological diagnoses were included. EUS-guided fine-needle biopsies (EUS-FNBs) were performed using a novel 22G Franseen biopsy needle. SVWC cutoff lengths were measured using sample isolation processing by stereomicroscopy (SIPS). The utility of the calculated SVWC cutoff lengths was measured.
Results:
The procedural success and SVWC sampling rates were both 100%. The median SVWC length was 14.5 mm. Pathological examinations identified 16 patients with gastrointestinal stromal tumors, 7 with schwannomas, 6 with leiomyomas, and 1 with an ectopic pancreas. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for diagnosing malignancy using EUS-FNB were all 100%. The final diagnostic accuracy was 100%. Regarding the final diagnosis, based on the receiver operating characteristic curves calculated using the SVWC length, the area under the curve was 0.958 (95% confidence interval: 0.897–1.020, P < 0.001) and the cutoff length was 4 mm. The sensitivity of the new SVWC cutoff length was 98.7%.
Conclusions:
Diagnostic results of EUS-FNBs using a novel 22-G Franseen biopsy needle were significantly better with SVWC cutoff lengths ≥4 mm. Performing the SIPS procedure with a cutoff value of 4 mm as an index may be especially useful for successful pathological diagnosis of SELs at institutions where rapid on-site evaluation cannot be performed.
Keywords: EUS, fine-needle biopsy, histopathology, sample isolation processing by stereomicroscopy, subepithelial lesion
INTRODUCTION
Rapid on-site evaluation (ROSE) significantly improves the sensitivity and accuracy of tissue diagnoses using EUS-guided fine-needle aspiration biopsy (EUS-FNAB).[1,2,3,4] ROSE reduced the number of inadequate samples obtained and needle passes.[5] However, ROSE is not possible at all institutions where EUS-FNAB is performed owing to the lack of financial, other hospital resources, and cytopathologists. Results of recent well-designed trials and meta-analyses have questioned the effectiveness of ROSE.[6,7,8] To resolve this, we reported that the pathological diagnostic efficacy significantly improved when cutoff lengths for stereomicroscopically visible white cores (SVWCs) obtained by EUS-FNAB using 22G needles were ≥3.5 mm for subepithelial lesions (SELs) and ≥11 mm for pancreatic neoplasms.[9] Our results may provide new indices useful for EUS-FNAB samples.
The Franseen biopsy needle, a fine-needle biopsy (FNB) needle developed to collect multiple tissue samples for obtaining a histological diagnosis, has attracted substantial attention recently owing to its advanced diagnostic capabilities and superiority relative to conventional fine-needle aspiration (FNA) needles.[10,11,12,13,14,15,16,17] Advanced diagnostic capabilities of new-generation biopsy needles may even eliminate the need for ROSE.[18]
Although our previous study demonstrated the efficacy of EUS-FNAB using a conventional FNA needle under-sample isolation processing by stereomicroscopy (SIPS) as an alternative to ROSE, it did not evaluate the efficacy when using the Franseen needle. Therefore, the present prospective exploratory study aimed to use stereomicroscopy to examine the required SVWC cutoff length for the pathological diagnosis of samples obtained using the novel 22-G Franseen biopsy needle, sensitivity using the SVWC cutoff length, accuracy, the presence of microscopic tissue, and procedure-related adverse events.
PATIENTS AND METHODS
Study design
In this single-center prospective study, we enrolled consecutive patients who underwent EUS-FNB for SELs in the upper gastrointestinal tract at the Kitasato University Hospital between March 2018 and January 2019. Inclusion criteria were the age ≥20 years, SELs in the upper gastrointestinal tract that required EUS-FNB, and the presence of a pathological diagnosis required to guide treatment. We excluded patients in whom the risk of puncturing the cystic part of the lesion was high, those with a hemorrhagic tendency, and those deemed unsuitable by an investigator for any reason.
The primary outcome was the new SVWC cutoff length using the novel 22-G Franseen biopsy needle. Secondary outcomes included sensitivities of EUS-FNB when using the new SVWC cutoff length and the previously reported cutoff length (3.5 mm),[9] accuracy, presence of microscopic tissue, and procedure-related adverse events.
All patients provided prior written informed consent to participate in this study, which had been approved by our Institutional Review Board, based on its ethical, scientific, and medical validity. The study conforms to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013). The study is registered at http://www.umin.ac.jp (UMIN000023013).
EUS-guided fine-needle aspiration biopsy
EUS was performed using a linear scanning video echoendoscope (GF-UCT260, TGF-260J; Olympus Medical Systems, Tokyo, Japan), and a 22-G Franseen needle (Acquire™; Boston Scientific Corp., Natick, MA, USA) was used as the EUS-FNB needle in all cases. The 22-G Franseen needle has three novel symmetric heels designed to maximize tissue capture and minimize fragmentation. Electropolished strain-resistant cutting edges are fully formed to maximize needle sharpness and to cut the tissue from three different angles, creating a circular cut. Following stylet withdrawal, 10–20 strokes were made with the needle inside the lesion using a 20-ml syringe under negative pressure, and three-needle passes were performed in all lesions. Patients were checked twice for adverse events: Once at 3 h after EUS-FNB and again the following morning. Following the report of the American Society for Gastrointestinal Endoscopy Workshop,[19] the incidence of adverse events up to 30 days after EUS-FNB was evaluated during medical examinations in the outpatient clinic.
Sample isolation processing by stereomicroscopy
SIPS was performed following our previously reported method[9] [Figure 1]. The sample in the puncture needle was initially pushed out onto a Petri dish by compressing the air in the syringe and then using a stylet. The vermiform tissue component from the Petri dish was immersed in 10% neutral buffered formalin solution in another previously prepared Petri dish. The tissue component in the Petri dish was examined under a stereomicroscope (×30, SZX10; Olympus Medical Systems), and white and red samples were dissected using injection needles. SVWC lengths were measured using a scale on the microscope monitor. White and red samples were closely aligned on separate filter papers, placed in vessels containing 10% neutral buffered formalin, and sent for pathological analyses. SIPS procedures were performed for each pass of the needle by one of the two designated endoscopists (K. O. or H. M.).
Figure 1.
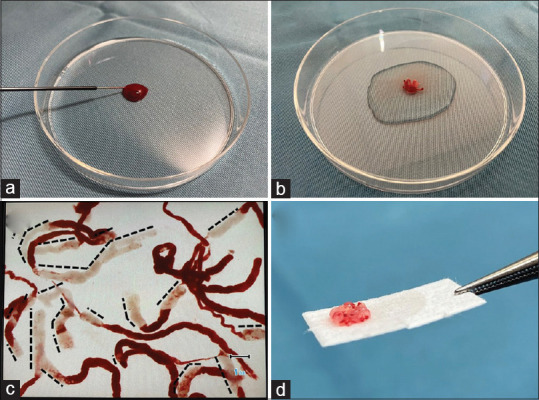
The sample isolation processing by stereomicroscopy process for a EUS-fine-needle biopsy sample from a patient with gastrointestinal stromal tumor. (a) The sample in the puncture needle was initially pushed out onto a Petri dish by compressing the air in the syringe and then using a stylet. (b) The vermiform tissue component from the Petri dish was immersed in 10% neutral buffered formalin solution in another previously prepared Petri dish. (c) The stereomicroscopically visible white core lengths (black dashed lines) were measured using a scale on the stereomicroscope monitor screen. (d) The white and red samples were dissected using injection needles and closely aligned on separate filter papers. The photo is a white sample. SIPS: Sample isolation processing by stereomicroscope, EUS-FNB: EUS-guided fine-needle biopsy, GIST: Gastrointestinal stromal tumor, SVWC: Stereomicroscopic visible white core
Pathological examination and tissue diagnosis
Following formalin fixation, h and e (HE)-stained specimens of white and red samples were prepared separately from formalin-fixed and paraffin-embedded tissues. If immunohistochemical staining was required for diagnosis, it was performed at the discretion of a specialized pathologist. Pathological examinations were checked twice by two or more doctors, qualified as specialized pathologists. Among patients who underwent surgical resection following EUS-FNB, the final diagnosis was considered correct if it was consistent with the diagnosis from the pathological examination of the resected specimen. For patients with unresected malignancies or benign conditions, the subsequent clinical course was monitored, and diagnostic imaging was performed. If results were consistent with those of EUS-FNB, the final diagnosis was considered correct. Patients with benign conditions were monitored for ≥6 months after EUS-FNB.
Statistical analyses
Owing to the study’s exploratory nature, power calculation was not performed. Therefore, an achievable target of 30 patients was selected. The presence of SVWCs was classified as positive if white samples from the target lesion were visible under stereomicroscopy. Tissue sampling rates were classified as positive if the tissue from the target lesion was visible under optical microscopy. Results of EUS-FNB were based only on histological diagnoses and did not incorporate cytologic findings. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated for the final diagnosis of malignancy. Regarding final diagnoses, receiver operating characteristic (ROC) curves for SVWC length were plotted, and the accuracy of the area under the curve (AUC) for diagnostic yield was calculated. The optimal cutoff length required to obtain a final diagnosis was calculated using the Youden index (sensitivity + specificity − 1).[20] Statistical comparisons were made using Fisher’s exact test for categorical variables. Statistical analyses were performed using the Statistical Package SPSS Base 17.0 (SPSS Inc., Chicago, IL, USA). Values of P < 0.05 were considered statistically significant.
RESULTS
Patient characteristics
Table 1 shows the characteristics of the 30 enrolled patients. The cohort comprised 19 male and 11 female patients, and the median age was 67 (range, 23–84) years. The median maximum diameter of the lesions was 24 mm (range, 14–55 mm). Lesions were located in the esophagus, stomach, and duodenum in 4, 24, and 2 patients, respectively.
Table 1.
Characteristics of the patients and lesions
| n (%) | |
|---|---|
| Number of patients | 30 |
| Median age, years (range) | 67 (23-84) |
| Male/female | 19/11 (63.3/36.7) |
| Number of lesions | 30 |
| Maximum lesion diameters: median, mm (range) | 24 (14-55) |
| Location of lesion | |
| Esophagus | 4 (13.3) |
| Stomach | 24 (80) |
| Duodenum | 2 (6.7) |
EUS-fine-needle biopsy biopsy
The procedural success rate for EUS-FNB using the 22G Franseen needle was 100%. For the needle passes, three punctures were performed in all lesions with the suction method. An adverse event (hemorrhage from the puncture site during EUS-FNB [severity grade: Mild]) occurred in two patients (6.7%) [Table 2].
Table 2.
Results of EUS-FNB
| n (%) | |
|---|---|
| Technical success | 30/30 (100) |
| Number of passes per lesion 3 passes | 30 (100) |
| Number of total passes | 90 |
| Adverse events | |
| Bleeding, mild | 2 (6.7) |
EUS-FNB: EUS-guided fine-needle aspiration biopsy
Sample isolation processing by stereomicroscopy
Table 3 displays the results of the SIPS for SVWCs and EUS-FNBs. In total, 89 of 90 needle passes yielded SVWCs (98.9%) in all 30 patients (100%) who underwent EUS-FNB using the 22-G Franseen needle. In the entire cohort, the median SVWC length was 14.5 mm (range, 1–89 mm).
Table 3.
Results of sample isolation processing by stereomicroscopy
| Stereomicroscopic assessments | % (n) |
|---|---|
| Presence of SVWC | |
| Per pass | 98.9 (89/90) |
| Per lesion | 100 (30/30) |
| Length of SVWC median, mm (range) | |
| Per pass | 14.5 (1-89)* |
*n=89. SVWC: Stereomicroscopic visible white core
Pathological examination and tissue diagnosis
As shown in Table 4, tissue sampling rates were 97.8% (per pass analysis) and 100% (per lesion analysis). The sensitivity, specificity, PPV, NPV, accuracy of EUS-FNBs for malignant diagnoses (per lesion analysis), and accuracy of the final diagnoses were all 100%. The diagnostic rates of the first, second, and third passes were 93.3% (28/30), 90% (27/30), and 96.7% (29/30), respectively. The cumulative diagnostic rate for up to two passes was 96.7% (by lesion) and 100% (by lesion) for up to three passes, showing that diagnostic performance slightly increased with each pass.
Table 4.
Results of the pathological examination and tissue diagnosis
| % (n) | |
|---|---|
| Pathological assessments | |
| Presence of microscopic tissue | |
| Per pass | 97.8 (88/90) |
| Per lesion | 100 (30/30) |
| Diagnosis rates | |
| Per pass malignancy diagnosis | |
| 1st pass | 93.3 (28/30) |
| 2nd pass | 90 (27/30) |
| Cumulative diagnostic rate for up to 2 passes (per lesion) | 96.7 (29/30) |
| 3rd pass | 96.7 (29/30) |
| Cumulative diagnostic rate for up to 3 passes (per lesion) | 100 (30/30) |
| Per lesion malignant diagnosis | |
| Sensitivity | 100 |
| Specificity | 100 |
| PPV | 100 |
| NPV | 100 |
| Accuracy | 100 |
| Per lesion final diagnosis | |
| Accuracy | 100 |
PPV: Positive predictive value; NPV: Negative predictive value.
A total of 16, 7, 6, and 1 patient(s) had gastrointestinal stromal tumors (GISTs,) schwannomas, leiomyomas, and an ectopic pancreas, respectively [Table 5]. All lesions were diagnosed by histology with immunohistochemical staining except for the ectopic pancreas, which was diagnosed using HE-stained specimens. Throughout the study duration, 15 patients (14 patients with GIST and 1 patient with leiomyoma) underwent surgical resection. Due to serious complications, 2 patients with GISTs did not undergo surgical resection. The final diagnoses in the other 13 patients with benign diseases (5 leiomyomas, 7 schwannomas, and 1 ectopic pancreas) were determined by monitoring their clinical courses for >6 months throughout the study duration.
Table 5.
Final diagnosis
| Location | Final diagnosis | n (%) |
|---|---|---|
| Esophagus | GIST | 1* (3.3) |
| Leiomyoma | 2 (6.7) | |
| Schwannoma | 1 (3.3) | |
| Stomach | GIST | 13* (43.3) |
| Leiomyoma | 4 (13.3) | |
| Schwannoma | 6 (20) | |
| Ectopic pancreas | 1 (3.3) | |
| Duodenum | GIST | 2* (6.7) |
*Final diagnosis of malignancy. GIST: Gastrointestinal stromal tumor
Calculation of cutoff lengths and their utility
Regarding final diagnoses, from the ROC curves calculated using the SVWC length, the AUC was 0.958 (95% confidence interval: 0.897–1.020, P < 0.001), and the cutoff length was 4 mm [Figure 2]. The sensitivity of EUS-FNB performed using the newly calculated SVWC cutoff length (4 mm) and the previously reported length (3.5 mm)[6] are presented in Table 6. Both sensitivities were 98.7% according to the cutoff lengths (per pass analyses) in the entire cohort.
Figure 2.
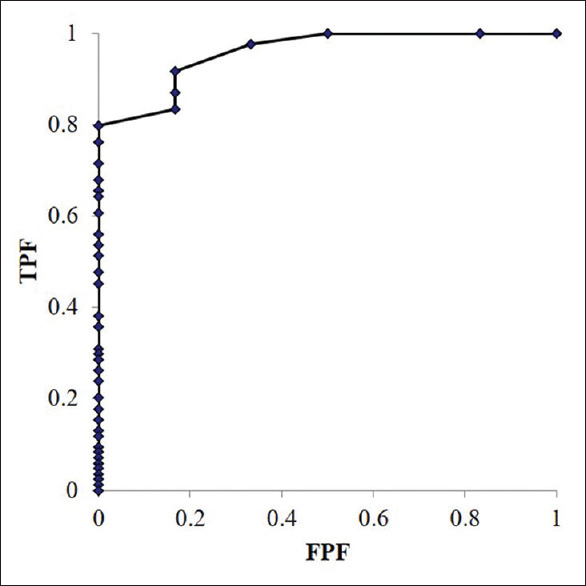
Receiver operating characteristic curves calculated from the stereomicroscopically visible white core lengths with respect to the diagnostic accuracy. The overall area under the curve is 0.958 (95% confidence interval: 0.897–1.020, P < 0.001). AUC: Area under the curve, CI: Confidence interval, ROC: Receiver operating characteristic, SVWC: Stereomicroscopic visible white core
Table 6.
Results of EUS-FNB using a novel 22-G Franseen biopsy needle according to the cutoff length
| Positive (n) | Negative (n) | Sensitivity (%) | P | |
|---|---|---|---|---|
| Cutoff length: 4 mm | ||||
| Per pass analysis | ||||
| SVWC ≥4 mm | 77 | 1 | 98.7 | <0.001 |
| SVWC <4 mm | 7 | 5 | 58.3 | |
| Cutoff length: 3.5 mm | ||||
| Per pass analysis | ||||
| SVWC ≥3.5 mm | 77 | 1 | 98.7 | <0.001 |
| SVWC <3.5 mm | 7 | 5 | 58.3 | |
EUS-FNB: EUS-guided fine-needle aspiration biopsy; SVWC: Stereomicroscopic visible white core
DISCUSSION
In this study, the cutoff length of 4 mm calculated by the Youden index yielded good sensitivities in EUS-FNB using the Franseen needles. However, the sensitivity was the same even when the cutoff length was 3.5 mm, which we had previously identified using conventional FNA needles. In other words, when the obtained sample was longer than the SVWC cutoff length, SIPS had a superior histological diagnostic ability for SELs regardless of whether Franseen needles or conventional FNA needles were used.
We devised and reported the utility of SIPS previously as an alternative method to be used in institutions where ROSE cannot be performed.[6] Nevertheless, there are two persistent issues with SIPS. The first is a dissociation observed between the SVWC and tissue sampling rates. Although this was lower than those of previous studies (95.2% vs. 93.1%), a dissociation of 1.1% (one sample) still emerged in this study. Specifically, an SVWC of 1 mm was determined to have been obtained by SIPS in one case of leiomyoma. However, when examined under an optical microscope, the sample contained only fibrin and a small quantity of red blood cells with no tissue. In that case, the remaining two samples were diagnosed with leiomyomas (SVWCs of 7 and 3 mm). Even if a stereomicroscope is used, accurate macroscopic evaluation of a sample for the presence of tissue remains difficult. Rather than performing a sensory evaluation with the human eye, quantifying the characteristics of a whitish specimen (intensity of hue, transparency, and hardness) using computer analysis software and clarifying the standard for a good sample could resolve this slight dissociation. Thus, an error rate of one sample of 90 samples is low enough to be accepted. The second issue with SIPS is that it is unclear whether the isolation of SVWC and non-SWVC red components is necessary when performing the SIPS procedure. In the present study, 77 red samples with SVWCs removed were obtained, for which the accurate diagnosis rate was 64.9% (50/77). In nearly all red samples for which histopathology was not obtained, the optical microscope images mainly showed red blood cells and fibrin. Considering this result, we believe that separating SVWCs and the red components remove most red blood cells and fibrin and enable the creation of better-quality specimens. Nevertheless, proving this hypothesis requires a prospective comparison and verification with two cohorts (one in which SWVCs and red components are separated and one in which they are not). Confirming that the separation of SVWCs and red components are unnecessary would reduce the effort to process specimens and would be a more convenient method that can be performed at any institution.
This study has several limitations. First, this research was an exploratory study performed at a single institution with a small sample size. Nevertheless, as we have also previously reported, we found that EUS-FNB under SIPS provides an extremely advanced histological capability for SELs, the calculated newly cutoff values for SVWCs were nearly identical, and sensitivity using the cutoff values was also satisfactory. Furthermore, the results of this trial suggest a high diagnostic rate using the 22G Franseen needle in the setting of SELs. These results require re-examination in a multicenter prospective study with a larger sample size. Second, we clarified the utility of the cutoff value regardless of the characteristics of the needle being used, but only as an index to evaluate the histological diagnosis of SELs and not for the quantity of tissue. Given the slight divergence (0.5 mm) between the cutoff values in the two prospective exploratory studies, the FNB needles had a higher cutoff value, and we cannot conclude that the FNB needles collect less tissue than do the FNA needles. The next challenge will be to compare and verify the tissue quantity for each needle used with a cutoff value.
CONCLUSIONS
We obtained improved diagnostic results using the new cutoff value even when using the newer EUS-FNB needles. Performing SIPS with the cutoff value as an index may be especially useful in institutions where ROSE cannot be performed.
Financial support and sponsorship
Nil.
Clinical trial registration
Clinical trial registration number: UMIN000030773.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chang KJ, Katz KD, Durbin TE, et al. Endoscopic ultrasound-guided fine-needle aspiration. Gastrointest Endosc. 1994;40:6949. [PubMed] [Google Scholar]
- 2.Klapman JB, Logrono R, Dye CE, et al. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289–94. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]
- 3.Cleveland P, Gill KR, Coe SG, et al. An evaluation of risk factors for inadequate cytology in EUS-guided FNA of pancreatic tumors and lymph nodes. Gastrointest Endosc. 2010;71:1194–9. doi: 10.1016/j.gie.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 4.Haba S, Yamao K, Bhatia V, et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol. 2013;48:973–81. doi: 10.1007/s00535-012-0695-8. [DOI] [PubMed] [Google Scholar]
- 5.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–10. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 6.Wani S, Mullady D, Early DS, et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: A prospective multicenter randomized controlled trial. Am J Gastroenterol. 2015;110:1429–39. doi: 10.1038/ajg.2015.262. [DOI] [PubMed] [Google Scholar]
- 7.Kong F, Zhu J, Kong X, et al. Rapid on-site evaluation does not improve endoscopic ultrasound-guided fine needle aspiration adequacy in pancreatic masses: A meta-analysis and systematic review. PLoS One. 2016;11:e0163056. doi: 10.1371/journal.pone.0163056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan MA, Grimm IS, Ali B, et al. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: Diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017;5:E363–75. doi: 10.1055/s-0043-101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masutani H, Okuwaki K, Kida M, et al. On-site stereomicroscope quality evaluations to estimate white core cutoff lengths using EUS-FNA biopsy sampling with 22-gauge needles. Gastrointest Endosc. 2019;90:947–56. doi: 10.1016/j.gie.2019.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Bang JY, Hebert-Magee S, Hasan MK, et al. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: Initial assessment. Dig Endosc. 2017;29:338–46. doi: 10.1111/den.12769. [DOI] [PubMed] [Google Scholar]
- 11.Fujita A, Ryozawa S, Kobayashi M, et al. Diagnostic ability of a 22G Franseen needle in endoscopic ultrasound-guided fine needle aspiration of subepithelial lesions. Mol Clin Oncol. 2018;9:527–31. doi: 10.3892/mco.2018.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukai S, Itoi T, Yamaguchi H, et al. A retrospective histological comparison of EUS-guided fine-needle biopsy using a novel Franseen needle and a conventional end-cut type needle. Endosc Ultrasound. 2019;8:50–7. doi: 10.4103/eus.eus_11_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung Ki EL, Lemaistre AI, Fumex F, et al. Macroscopic onsite evaluation using endoscopic ultrasound fine needle biopsy as an alternative to rapid onsite evaluation. Endosc Int Open. 2019;7:E189–94. doi: 10.1055/a-0770-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugiura R, Kuwatani M, Yane K, et al. Prospective, multicenter, observational study of tissue acquisition through EUS-guided fine-needle biopsy using a 25G Franseen needle. Endosc Ultrasound. 2019;8:321–8. doi: 10.4103/eus.eus_66_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asokkumar R, Yung Ka C, Loh T, et al. Comparison of tissue and molecular yield between fine-needle biopsy (FNB) and fine-needle aspiration (FNA): A randomized study. Endosc Int Open. 2019;7:E955–63. doi: 10.1055/a-0903-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuno J, Ogura T, Kurisu Y, et al. Prospective comparison study of Franseen needle and standard needle use for pancreatic lesions under EUS guidance. Endosc Ultrasound. 2019;8:412–7. doi: 10.4103/eus.eus_38_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mita N, Iwashita T, Uemura S, et al. Endoscopic ultrasound-guided fine needle biopsy using 22-gauge Franseen needle for the histological diagnosis of solid lesions: A multicenter prospective pilot study. Dig Dis Sci. 2020;65:1155–63. doi: 10.1007/s10620-019-05840-y. [DOI] [PubMed] [Google Scholar]
- 18.Bang JY, Hebert-Magee S, Navaneethan U, et al. Randomized trial comparing the Franseen and Fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2018;87:1432–8. doi: 10.1016/j.gie.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]


