Abstract
Robust data in favor of clear superiority of 22G fine-needle biopsy (FNB) over 22G FNA for an echoendoscopic-guided sampling of pancreatic masses are lacking. The objective of this study is to compare the diagnostic outcomes and sample adequacy of these two needles. Computerized bibliographic search on the main databases was performed and restricted to only randomized controlled trials. Summary estimates were expressed regarding risk ratio (RR) and 95% confidence interval. A total of 11 trials with 833 patients were analyzed. The two needles resulted comparable in terms of diagnostic accuracy (RR 1.02, 0.97–1.08; P = 0.46), sample adequacy (RR 1.01, 0.96–1.06; P = 0.61), and histological core procurement (RR 1.01, 0.89–1.15; P = 0.86). Pooled sensitivity in the diagnosis of pancreatic cancer was 93.1% (87.9%–98.4%) and 90.4% (86.3%–94.5%) with biopsy and aspirate, respectively, whereas specificity for detecting pancreatic cancer was 100% with both needles. Analysis of the number of needle passes showed a nonsignificantly positive trend in favor of FNB (mean difference: −0.32, −0.66–0.02; P = 0.07). Our meta-analysis stands for a nonsuperiority of 22G FNB over 22G FNA; hence, no definitive recommendations on the use of a particular device can be made.
Keywords: Accuracy, EUS, FNA, fine-needle biopsy, pancreas, sensitivity
INTRODUCTION
EUS represents a valuable and accurate diagnostic technique for the morphological characterization of the pancreatic lesions; furthermore, EUS allows sampling of the pancreatic tissue for cytopathological and histological diagnosis using FNA and more recently, fine-needle biopsy (FNB).[1,2]
In spite of the good results observed with EUS-FNA and the recent developments in this field, such as the use of rapid on-site evaluation (ROSE),[3] contrast-enhanced guided FNA[4] or tissue elastography,[5] and diagnostic sensitivity remains an issue. Among the most commonly adopted devices, 22G and 25G FNA needles have proved equally effective and safe in clinical practice, and consequently, they represent the current standard of care when performing sampling of pancreatic masses.[6,7]
Cellular acquisition through EUS-FNA does not necessarily retain the stroma or associated architecture of the surrounding tissue, which may be necessary to provide a definitive diagnosis. EUS-FNB, which typically uses a core biopsy needle and preserves the cellular architecture, has become an increasingly useful tool in establishing a definitive diagnosis of malignancy.[8] Obtaining a complete histologic sample is, particularly of the interest in light of the advent of molecular profiling and novel personalized oncologic therapies; furthermore, EUS-FNB accuracy seems less prone to be influenced by the absence of ROSE or to be dependent on a high number of needle passes.[8]
Two recent meta-analyses reached the conclusion that EUS-FNB needles show comparable diagnostic accuracy and sample adequacy in comparison to EUS-FNA but with the need of a lower number of passes.[9,10]
However, these meta-analyses included both randomized controlled trials (RCTs) and retrospective studies, and they did not focus only on pancreatic masses but considered also other abdominal lesions or lymph nodes; moreover, subgroup analysis according to the needle size was not performed; hence, heterogeneity of the results calls for a note of caution in interpreting their findings. Since EUS-FNB of the pancreatic lesions is commonly performed with 22G needle and currently available RCTs tested prevalently this specific device, we decided to compare 22G EUS-FNB with 22G EUS-FNA for the diagnosis of the pancreatic solid lesions. To maximize the reliability of our conclusions and to obviate to the heterogeneity found in previous systematic reviews,[10,11] we decided to restrict our analysis to only RCTs comparing these two-needle sizes.
Primary endpoint was diagnostic accuracy. Secondary outcomes were sample adequacy, optimal histological core procurement, the mean number of needle passes, and pooled specificity and sensitivity. Safety data and technical success rate were also analyzed.
MATERIALS AND METHODS
Inclusion and exclusion criteria
Only studies meeting the following criteria were included: (1) RCTs comparing EUS-FNA and EUS-FNB with 22G needle of pancreatic solid lesions; (2) Studies published in English; and (3) Articles reporting at least one of the following outcomes: diagnostic accuracy (or data useful for its calculation), sample adequacy (or data useful for its calculation), and histologic core procurement.
Search strategy
Figure 1 reports the search strategy followed in the meta-analysis.
Figure 1.
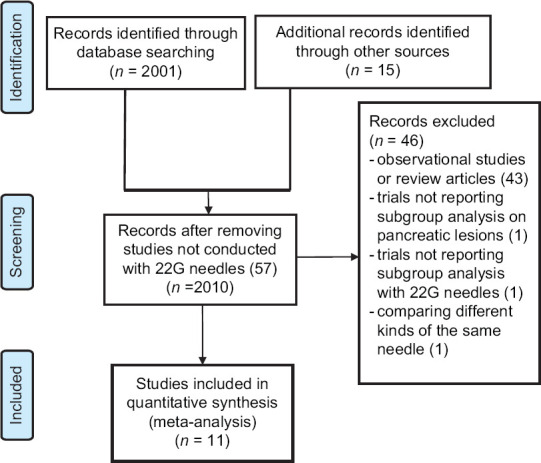
Flow chart of included studies
Bibliographic research was conducted on PubMed, EMBASE, Cochrane Library, and Google Scholar, including all studies fulfilling inclusion criteria published until November 2018. The following search strategy was adopted: ([[[EUS [MeSH Terms]] AND eus [MeSH Terms]] OR biopsy [MeSH Terms]] OR fna [MeSH Terms]) AND pancreas (MeSH Terms).
Relevant reviews and meta-analyses on the use of EUS in pancreatic solid lesions were examined for potential suitable studies. Authors of included studies were contacted to obtain full text or further information when needed.
Data extraction was conducted by two reviewers (atrial fibrillation [AF] and HSB) using a standardized approach (the PRISMA statement).[12] The quality of included studies was assessed by two authors independently (AF, KM) according to the currently accepted criteria described elsewhere.[13] Disagreements were solved by discussion and following a third opinion (NM).
Statistical analysis
Chi-square and I2 tests were used for across studies comparison of the percentage of variability attributable to heterogeneity beyond chance. A value of P < 0.10 for Chi-square test and I2 <20% were interpreted as low-level heterogeneity.
As recommended by recent Cochrane guidelines, random-effects model with DerSimonian–Laird test was chosen a priori for all analyses (regardless of the level of heterogeneity), and then fixed-effect model by means of Mantel–Haenszel test was performed as a sensitivity test.[14] Summary estimates were expressed regarding risk ratio (RR) and 95% confidence interval (CI).
Primary endpoint was diagnostic accuracy, defined as true positive + true negative/total number of patients.
Among secondary outcomes, sample adequacy (rate of adequate samples on the total number of patients), optimal histological core procurement rate, and the mean number of needle passes were compared between the two groups, whereas specificity (true negative/true negative + false positive) and sensitivity (true positive/true positive + false negative) were separately pooled for each needle. Safety data and technical success rate were inconsistently reported; hence, they were analyzed descriptively.
The probability of publication bias was assessed using funnel plots and with Begg and Mazumdar test. Sensitivity analysis was conducted according to the quality of included studies, study design (parallel vs. cross-over), and several technical characteristics (use of stylet, ROSE, suction, and type of needle).
All statistical analyses were conducted using RevMan version 5 (the Cochrane Collaboration, Oxford, UK) and Open Meta (Analyst) software (freely available at the site: http://www.cebm.brown.edu/openmeta). For all calculations, a two-tailed P < 0.05 was considered statistically significant.
RESULTS
Characteristics of included studies
As shown in Figure 1, out of 2001 studies initially identified, after preliminary exclusion of papers not fulfilling inclusion criteria, 14 potentially relevant RCTs were examined. Among these studies, three were excluded because they did not report subgroup analysis on pancreatic lesions[15] or with 22G needles[16] or due to comparison of different kinds of the same needle.[17]
Finally, 11 RCTs[18,19,20,21,22,23,24,25,26,27,28] with 833 patients (239 sampled with 22 G EUS-FNB, 271 with 22 G EUS-FNA, and 323 with both needles in cross-over trials) were included in the meta-analysis.
The main characteristics of included studies are reported in Table 1.
Table 1.
Characteristics of included randomized controlled trials
| Study | Arm | Sample size | Study period/design | Country | Age | Gender male | Lesion size (cm) | Location head/uncinate | Stylet use | Suction | ROSE | Needle |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alatawi et al., 2015[18] | FNB FNA | 50 50 | 2012-2013/parallel | France | 67.8±13.1 68±11.2 | 28 (56%) 35 (70%) | 3.2±0.5 3.3±0.2 | 34 (68%) 38 (76%) | No | Yes | No | ProCore® Echo Ultra® |
| Bang et al., 2012[19] | FNB FNA | 28 28 | 2011/parallel | USA | 65±15.4 65.4±11.1 | 15 (53.6%) 16 (57.1%) | 3.2±0.9 3.3±0.7 | 20 (71.4%) 20 (71.4%) | No | Only in FNB group | Yes | ProCore® Expect® |
| Bang et al., 2018[20] | FNB FNA | 4646 | Cross-over | USA | 67.9±14.7 | 28 (60.9%) | 2.9±0.8 | 28 (60.9%) | NR | No | Yes | Acquire® Expect® |
| Cheng et al., 2018[21]* | FNB FNA | 123 126 | 2014-2016/parallel | China | 58.3±11.1 58.3±12.2 | 59.3% 63.6% | 2.91 2.95 | NR | Only at first two passes | Only at 3 and 4 passes | No | ProCore® EchoTip® |
| Ganc et al., 2014[22]a | FNB FNA | 30 30 | Cross-over | Brazil | NR | NR | NR | NR | NR | NR | No | ProCore® EchoTip® |
| Hucl et al., 2013[23]* | FNB FNA | 69 69 | 2011-2012/cross-over | India | 51.7±13.6 | 37 (53.6%) | 4.19±1.7 | 54% | No | Yes | No | ProCore® EchoTip® |
| Lee et al., 2017[24]* | FNB FNA | 9 7 | 2013-2014/parallel | Korea | 69 (26-85) 66 (36-81) | 62% 75.8% | 4.4±3.2 3.7±2 | NR | Yes | Partially | No | ProCore® EchoTip® |
| Noh et al., 2018[25] | FNB FNA | 60 60 | 2013-2015/cross-over | Korea | 61.6±10 | 35 (58.3%) | 3.1±0.8 | 23 (38.4%) | No | No Yes | No | ProCore® EZShot 2® |
| Othman et al., 2017[26] | FNB FNA | 29 60 | 2013-2014/parallel | USA | 67.9±10.3 63.4±10 | 16 (55.1%) 27 (45%) | NR | 16 (55.1%) 30 (50%) | No | Yes | Yes | ProCore® EZShot 2®/Expect® |
| Sterlacci et al., 2016[27]* | FNB FNA | 38 38 | 2011-2013/cross-over | Germany | 68±12 | 51.8% | 3.3±1.2 | NR | No | Yes | No | ProCore® EchoTip® |
| Vanbiervliet et al., 2014[28] | FNB FNA | 80 80 | 2012/cross-over | France | 67.1±11.1 | 49 (61.2%) | 3.3±1 | 50 (62.5%) | No | Yes | No | ProCore® EchoTip® |
*Trials including either pancreatic and extra-pancreatic masses. Only pancreatic lesions were reported in the table and included in the analysis, aConference abstract, bThree-arm trial comparing two different FNA needles and FNB. Data from the two FNA arms were merged. FNB: Fine-Needle Biopsy; ROSE: Rapid on-site evaluation; NR: Not reported
The recruitment period ranged from 2011 to 2016. Five RCTs[18,19,21,24,26] were parallel trials and six[20,22,23,25,27,28] were cross-over studies (thus meaning that the same lesion was sampled with both needles in a randomized order). Four RCTs were conducted in Asia[21,23,24,25] and all studies presented two well-balanced arms regarding lesion features (location and size) and clinical-demographical characteristics [Table 1]. ROSE was available in three studies, all conducted in the USA.[19,20,26] FNB needle was ProCore® in all studies except that by Bang et al.[20] where Acquire® was used.
Quality was deemed high in five RCTs,[18,19,20,24,28] and moderate or low in the other six trials.[21,22,23,25,26,27]
Details on methodological characteristics and quality of included articles are shown in Supplementary Figure 1a (808.4KB, tif) and b (808.4KB, tif) .
Diagnostic accuracy, sensitivity, and specificity
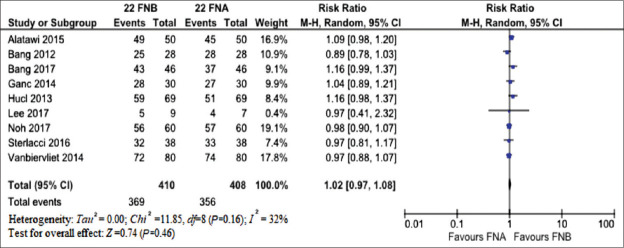
As depicted in Figure 2, the two needles resulted comparable regarding diagnostic accuracy (RR 1.02, 95% CI 0.97–1.08; P = 0.46) with moderate evidence of heterogeneity (I2 = 32%). There was no evidence of publication bias [Supplementary Figure 2 (897.1KB, tif) ]. The findings of main analysis were confirmed in the sensitivity analysis performed according to the study design, quality, and several technical characteristics of the procedure [Table 2]. Of note, ROSE was the main responsible of heterogeneity found as restricting the analysis to only studies not using ROSE led to robust results with I2 = 0 [Table 2 and Supplementary Figure 3a (1.1MB, tif) and b (1.1MB, tif) ].
Figure 2.
Meta-analysis comparing the diagnostic accuracy of 22G fine-needle biopsy and 22G FNA. The two needles resulted comparable regarding diagnostic accuracy (risk ratio 1.02, 95% confidence interval 0.97–xs1.08; P = 0.46) with moderate evidence of heterogeneity (I2 = 32%)
Table 2.
Sensitivity analysis
| Subgroup | Number of studies | RR (95% CI) | Within-group heterogeneity (I2), % | P |
|---|---|---|---|---|
| Study design | ||||
| Parallel | 3 | 0.99 (0.84-1.18) | 59 | 0.95 |
| Cross-over | 6 | 1.03 (0.96-1.1) | 31 | 0.44 |
| ROSE | ||||
| Yes | 2 | 1.02 (0.78-1.33) | 84 | 0.90 |
| No | 7 | 1.02 (0.97-1.07) | 0 | 0.41 |
| Stylet | ||||
| Yes | 1 | 0.97 (0.41-2.32) | - | 0.95 |
| No | 8 | 1.02 (0.96-1.08) | 41 | 0.47 |
| Suction | ||||
| Yes | 5 | 1.01 (0.93-1.1) | 52 | 0.78 |
| No | 4 | 1.03 (0.95-1.12) | 17 | 0.45 |
| Quality | ||||
| High | 5 | 1.02 (0.93-1.12) | 52 | 0.67 |
| Low/moderate | 4 | 1.02 (0.95-1.1) | 19% | 0.61 |
Pooled risk ratio of diagnostic accuracy obtained according to (a) Study design, (b) ROSE, (c) Use of stylet; (d) Use of suction, and (e) Study quality. Numbers in parentheses indicate 95% CIs. Significances are reported in bold. CIs: Confidence intervals; ROSE: Rapid on-site evaluation; RR: Risk ratio
Four trials[18,22,27,28] reported sensitivity and specificity; hence, their results were pooled as depicted in Supplementary Figures 4 (1,012.6KB, tif) and 5 (1MB, tif) . In details, pooled sensitivity in the diagnosis of pancreatic cancer of 22G FNB needle was 93.1% (87.9%–98.4%), whereas 22G FNA needle showed 90.4% (86.3%–94.5%) sensitivity [Supplementary Figure 4a (1,012.6KB, tif) and b (1,012.6KB, tif) ]. On the other hand, pooled specificity for detecting pancreatic cancer was 100% with both needles [Supplementary Figures 5a (1MB, tif) and b (1MB, tif) ].
Sample adequacy and optimal histologic core procurement
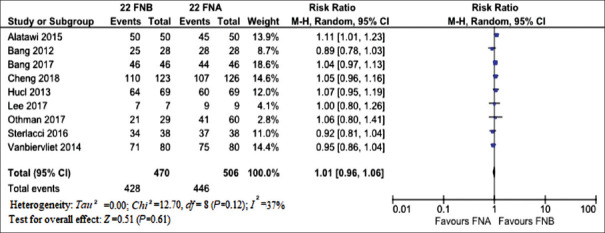
The forest plot of the comparison of sample adequacy is reported in Figure 3. RR for sample adequacy was very close to 1 with only a slight increase in favor of 22G FNB (1.01, 0.96–1.06; P = 0.61). Moderate evidence of heterogeneity was observed (I2 = 37%, Chi2 = 12.7, df = 8; P = 0.12) [Figure 3].
Figure 3.
Meta-analysis comparing sample adequacy of 22G fine-needle biopsy and 22G FNA. Risk ratio for sample adequacy was very close to 1 with only a slight increase in favor of 22G FNB (P = 0.61). Moderate evidence of heterogeneity was observed (I2 = 37%)
No evidence of publication bias was observed, as confirmed with Begg and Mazumdar test (P = 0.64).
Similar results were observed when comparing histologic core procurement rate [Supplementary Figure 6 (728.9KB, tif) ]. In fact, RR was 1.01 (0.89–1.15; P = 0.86) with high evidence of heterogeneity (I2 = 77%). Of note, analysis restricted to the only trial using Franseen biopsy needle (Acquire®)[20] showed significant benefit regarding higher quality histologic yield (RR 1.18, 1.03–1.36; P = 0.02).
Number of passes and adverse events
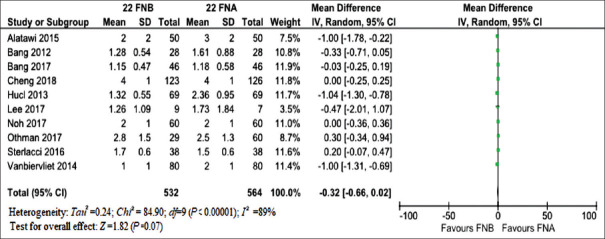
Analysis of a number of needle passes needed to obtain adequate sample showed a nonsignificantly positive trend in favor of FNB (mean difference: −0.32, −0.66–0.02; P = 0.07) with high evidence of heterogeneity (I2 = 89%) [Figure 4]. Again, ROSE was the main responsible of heterogeneity as restricting the analysis to trials using on-site cytologic evaluation[19,20,26] confirmed main results but with a significant drop in heterogeneity (I2 = 38%) [Supplementary Figure 7a (1.1MB, tif) and b (1.1MB, tif) ].
Figure 4.
Meta-analysis comparing mean number of needle passes of 22G fine-needle biopsy and 22G FNA. Analysis of number of needle passes needed to obtain adequate sample showed a nonsignificantly positive trend in favor of FNB (mean difference: −0.32, −0.66–xs0.02; P = 0.07) with high evidence of heterogeneity (I2 = 89%)
Details on the safety profile of the two devices are reported in Supplementary Table 1. Out of a total of six adverse events reported, five were experienced by patients treated with 22G FNA needles while only one event was caused by a 22G FNB needle. Of note, all of the reported adverse events (mainly bleeding) were mild and did not impact on patient outcomes.
Supplementary Table 1.
Adverse events reported in the included trials
| Study, Year | Adverse events | |
|---|---|---|
| FNB | FNA | |
| Alatawi 2015 | None | None |
| Bang 2012 | 1 event (3.6%) | 1 event (3.6%) |
| Bang 2017 | None | None |
| Cheng 2017 | None | 2 mild bleeding |
| Ganc 2014 | Not reported | Not reported |
| Hucl 2013 | Not reported | Not reported |
| Lee 2017 | None | None |
| Noh 2017 | None | None |
| Othman 2017 | None | 1 bleeding |
| Sterlacci 2016 | None | None |
| Vanbiervliet 2014 | None | 1 mild bleeding |
Technical failure was observed twice with FNB needle (both in the study by Othman et al.[26]) and only once with FNA needle.[19]
DISCUSSION
EUS-guided tissue sampling plays a pivotal role in the diagnostic algorithm of solid pancreatic lesions, but its diagnostic accuracy is strictly dependent on a series of tumor-related features (such as lesion size, number, and histological type) and technical variables such as needles adopted, number of passes or availability of an on-site pathologist in the endoscopic room for evaluation of sample adequacy. To overcome at least partially these limitations and given the pressing need of adequate histological samples for molecular analysis, biopsy needles have been developed and introduced in the clinical practice.
The first flexible biopsy needle (Quick-Core®) was introduced in the early 2000s and adapted from the Tru-Cut design, but its performances were impaired by several technical issues such as challenges in deploying the spring-loaded tray when in torqued positions within the duodenum, as well as loss of specimen when the needle was withdrawn.[29] As a consequence, tru-cut needle failed to determine a significant increase in diagnostic outcomes as compared to standard FNA needles, thus limiting its use worldwide.
Although ProCore® biopsy needle seems to address most of the limitations of previous biopsy devices, thanks to the addition of a reverse bevel just distal to the tip promoting collection of a core sample, no significant differences in adequate tissue acquisition, diagnostic accuracy, and rate of histological core specimen acquisition were seen with a significantly lower number of passes being the only advantage observed with FNB as compared to standard FNA.[9,30]
Given the recent development of novel FNB needle designs (such as Acquire®) and since previous meta-analyses provided highly heterogeneous findings due to different study designs, needle sizes and locations of the lesions sampled, there is a clear need to address properly this issue (which represents a quality item of paramount importance in EUS)[31] with a systematic review of only RCTs focused on pancreatic masses and comparing an univocal needle size, namely, 22G which is far the most tested in the literature.
With a meta-analysis of 11 RCTs selectively comparing 22G FNB and 22G FNA in the pancreatic masses, we made several key observations. First, the two needles resulted in comparable regarding either diagnostic accuracy (RR 1.02, P = 0.46) and sample adequacy (RR 1.01, P = 0.61). Similarly, histologic core procurement rate (RR 1.01, P = 0.86) and pooled sensitivity were similar with both devices (93.1% with FNB and 90.4% with FNA). As expected, pooled specificity was 100% with both needles. Second, sensitivity analysis confirmed these findings in all settings and availability of ROSE was found to be major source of heterogeneity observed in main analysis. However, FNA led to competitive results in comparison to FNB even in the absence of ROSE (not available in most non-American series). Third, while ProCore® did not result in a clear advantage regarding accuracy and adequate tissue sampling, Franseen biopsy needle (Acquire®) showed significant benefit concerning high quality histologic yield (RR 1.18, P = 0.02) although this finding is based on a single American study[20] and requires further confirmation. Fourth, unlike the aforementioned meta-analyses[9,30] where a number of needle passes was found to be significantly lower with FNB, our analysis showed only a nonsignificantly positive trend (mean difference:−0.32, P = 0.07) with high evidence of heterogeneity; again, ROSE was the main source of heterogeneity. Finally, both devices were safe and easy to use as adverse events and technical failure were rarely reported and did not impact on the patient clinical course.
As our analysis was restricted to RCTs; hence not influenced by any selection or outcome reporting bias, the theoretical advantages of 22G FNB needles do not result significantly in clinical practice and the diagnostic outcomes of the two devices can, therefore, be considered comparable, even concerning histological core procurement and number of passes which should be the main advantages of FNB.
The promising results observed with the Franseen needle (Acquire®) may be due to its three-point cutting surface designed to provide improved control at the puncture site and stability at the tip, allowing for enhanced penetration.[8] Other new-generation FNB needles are actually available in clinical practice such as Fork-tip needle (SharkCore®) which was proved to be competitive with Franseen device in a recent head-to-head clinical trial.[17] The same striking results were confirmed in a retrospective real-life American series comparing both needles with standard FNA.[32] Again, it should be stated expressly that our results apply to 22G needles in pancreatic masses, while further studies with other needle calipers and in different abdominal lesions are warranted to draw more general assumptions.
There are some limitations to the present study. First, the low number of included studies and enrolled patients require particular caution in interpreting our findings. However, we deliberately decided to restrict inclusion criteria to only RCTs comparing specifically 22G needles to provide more robust and homogenous outcome estimates. Moreover, all main outcomes (sensitivity, specificity, diagnostic accuracy, sample adequacy, histologic procurement, and safety profile) were explored, and this aspect represents a nearly unique analysis in this field. Second, there are other technical aspects such as the use of stylet, ROSE availability, or a number of passes which may influence diagnostic accuracy of the procedure. By the way, all of these technical features were explored as eventual sources of heterogeneity through dedicated sensitivity analyses and as expected, ROSE availability was found as a potential cause of heterogeneity. As previously commented, ProCore® needle failed to lead to significant accuracy improvement even in the absence of ROSE, when FNA would be expected to result more poorly performant.
CONCLUSION
Despite these weaknesses, our meta-analysis stands for a nonsuperiority of 22G FNB over 22G FNA; hence, no definitive suggestions on the use of a particular device may be actually provided. New-generation devices seem very promising but need to be explored in further RCTs.
Supplementary Materials
Supplementary information is linked to the online version of the paper on the Endoscopic Ultrasound website.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Risk of bias across included studies. a) Risk of bias graph, b) Risk of bias summary
Funnel plot for assessing the risk of publication bias concerning diagnostic accuracy analysis
Subgroup analysis for diagnostic accuracy restricted to studies using rapid on-site cytological evaluation (A) and not using rapid on-site cytological evaluation (B)
Pooled sensitivity of 22 FNB needle (A) and 22 FNA needle (B)
Pooled specificity of 22 FNB needle (A) and 22 FNA needle (B)
Pairwise meta-analysis for optimal histologic core procurement rate between 22 G FNB and 22 G FNA
Subgroup analysis for number of needle passes restricted to studies using rapid on-site cytological evaluation (A) and not using rapid on-site cytological evaluation (B)
REFERENCES
- 1.Jani BS, Rzouq F, Saligram S, et al. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic lesions: A systematic review of technical and procedural variables. N Am J Med Sci. 2016;8:1–11. doi: 10.4103/1947-2714.175185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsubayashi H, Matsui T, Yabuuchi Y, et al. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: Clinical aspects to improve the diagnosis. World J Gastroenterol. 2016;22:628–40. doi: 10.3748/wjg.v22.i2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iglesias-Garcia J, Lariño-Noia J, Abdulkader I, et al. Rapid on-site evaluation of endoscopic-ultrasound-guided fine-needle aspiration diagnosis of pancreatic masses. World J Gastroenterol. 2014;20:9451–7. doi: 10.3748/wjg.v20.i28.9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou X, Jin Z, Xu C, et al. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of solid pancreatic lesions: A retrospective study. PLoS One. 2015;10:e0121236. doi: 10.1371/journal.pone.0121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Facciorusso A, Martina M, Buccino RV, et al. Diagnostic accuracy of fine-needle aspiration of solid pancreatic lesions guided by endoscopic ultrasound elastography. Ann Gastroenterol. 2018;31:513–8. doi: 10.20524/aog.2018.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Facciorusso A, Stasi E, Di Maso M, et al. Endoscopic ultrasound-guided fine needle aspiration of pancreatic lesions with 22 versus 25 gauge needles: A meta-analysis. United European Gastroenterol J. 2017;5:846–53. doi: 10.1177/2050640616680972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) technical guideline – March 2017. Endoscopy. 2017;49:989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 8.James TW, Baron TH. A comprehensive review of endoscopic ultrasound core biopsy needles. Expert Rev Med Devices. 2018;15:127–35. doi: 10.1080/17434440.2018.1425137. [DOI] [PubMed] [Google Scholar]
- 9.Khan MA, Grimm IS, Ali B, et al. Ameta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: Diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017;5:E363–75. doi: 10.1055/s-0043-101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh HC, Kang H, Lee JY, et al. Diagnostic accuracy of 22/25-gauge core needle in endoscopic ultrasound-guided sampling: Systematic review and meta-analysis. Korean J Intern Med. 2016;31:1073–83. doi: 10.3904/kjim.2016.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Li W, Zhou QY, et al. Fine needle biopsy is superior to fine needle aspiration in endoscopic ultrasound guided sampling of pancreatic masses: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2018;97:e0207. doi: 10.1097/MD.0000000000010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 510 The Cochrane Collaboration. 2011. [Last updated on 2011 Mar 21; Last accessed on 2018 Nov 02]. Available from: http://wwwcochrane-handbookorg .
- 15.Nagula S, Pourmand K, Aslanian H, et al. Comparison of endoscopic ultrasound-fine-needle aspiration and endoscopic ultrasound-fine-needle biopsy for solid lesions in a multicenter, randomized trial. Clin Gastroenterol Hepatol. 2018;16:1307–130. doi: 10.1016/j.cgh.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Lee YN, Moon JH, Kim HK, et al. Core biopsy needle versus standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: A randomized parallel-group study. Endoscopy. 2014;46:1056–62. doi: 10.1055/s-0034-1377558. [DOI] [PubMed] [Google Scholar]
- 17.Bang JY, Hebert-Magee S, Navaneethan U, et al. Randomized trial comparing the Franseen and fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2018;87:1432–8. doi: 10.1016/j.gie.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 18.Alatawi A, Beuvon F, Grabar S, et al. Comparison of 22G reverse-beveled versus standard needle for endoscopic ultrasound-guided sampling of solid pancreatic lesions. United European Gastroenterol J. 2015;3:343–52. doi: 10.1177/2050640615577533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bang JY, Hebert-Magee S, Trevino J, et al. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76:321–7. doi: 10.1016/j.gie.2012.03.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bang JY, Hebert-Magee S, Navaneethan U, et al. EUS-guided fine needle biopsy of pancreatic masses can yield true histology. Gut. 2018;67:2081–4. doi: 10.1136/gutjnl-2017-315154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng B, Zhang Y, Chen Q, et al. Analysis of fine-needle biopsy vs.fine-needle aspiration in diagnosis of pancreatic and abdominal masses: A prospective, multicenter, randomized controlled trial. Clin Gastroenterol Hepatol. 2018;16:1314–21. doi: 10.1016/j.cgh.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Ganc R, Colaiacovo R, Carbonari A, et al. Endoscopic ultrasonography-fine-needle aspiration of solid pancreatic lesions: A prospective, randomized, single-blinded, comparative study using the 22 gauge EchoTip® ProCoreTM HD (A) and the 22 gauge EchoTip® ultra HD (B) endoscopic ultrasound needles. Endosc Ultrasound. 2014;3:S11. [PMC free article] [PubMed] [Google Scholar]
- 23.Hucl T, Wee E, Anuradha S, et al. Feasibility and efficiency of a new 22G core needle: A prospective comparison study. Endoscopy. 2013;45:792–8. doi: 10.1055/s-0033-1344217. [DOI] [PubMed] [Google Scholar]
- 24.Lee BS, Cho CM, Jung MK, et al. Comparison of histologic core portions acquired from a core biopsy needle and a conventional needle in solid mass lesions: A prospective randomized trial. Gut Liver. 2017;11:559–66. doi: 10.5009/gnl16284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noh DH, Choi K, Gu S, et al. Comparison of 22-gauge standard fine needle versus core biopsy needle for endoscopic ultrasound-guided sampling of suspected pancreatic cancer: A randomized crossover trial. Scand J Gastroenterol. 2018;53:94–9. doi: 10.1080/00365521.2017.1390597. [DOI] [PubMed] [Google Scholar]
- 26.Othman MO, Abdelfatah MM, Padilla O, et al. The cellularity yield of three different 22-gauge endoscopic ultrasound fine needle aspiration needles. Diagn Cytopathol. 2017;45:426–32. doi: 10.1002/dc.23689. [DOI] [PubMed] [Google Scholar]
- 27.Sterlacci W, Sioulas AD, Veits L, et al. 22-gauge core vs.22-gauge aspiration needle for endoscopic ultrasound-guided sampling of abdominal masses. World J Gastroenterol. 2016;22:8820–30. doi: 10.3748/wjg.v22.i39.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanbiervliet G, Napoléon B, Saint Paul MC, et al. Core needle versus standard needle for endoscopic ultrasound-guided biopsy of solid pancreatic masses: A randomized crossover study. Endoscopy. 2014;46:1063–70. doi: 10.1055/s-0034-1377559. [DOI] [PubMed] [Google Scholar]
- 29.Muthusamy VR. Endoscopic ultrasound-guided fine-needle aspiration vs.fine-needle biopsy. Gastroenterol Hepatol (N Y) 2017;13:496–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–49. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 31.Facciorusso A, Buccino RV, Muscatiello N. How to measure quality in endoscopic ultrasound. Ann Transl Med. 2018;6:266. doi: 10.21037/atm.2018.03.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bang JY, Kirtane S, Krall K, et al. In memoriam: Fine-needle aspiration, birth: Fine-needle biopsy: The changing trend in endoscopic ultrasound-guided tissue acquisition. Dig Endosc. 2018 doi: 10.1111/den.13280. doi: 101111/den13280 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk of bias across included studies. a) Risk of bias graph, b) Risk of bias summary
Funnel plot for assessing the risk of publication bias concerning diagnostic accuracy analysis
Subgroup analysis for diagnostic accuracy restricted to studies using rapid on-site cytological evaluation (A) and not using rapid on-site cytological evaluation (B)
Pooled sensitivity of 22 FNB needle (A) and 22 FNA needle (B)
Pooled specificity of 22 FNB needle (A) and 22 FNA needle (B)
Pairwise meta-analysis for optimal histologic core procurement rate between 22 G FNB and 22 G FNA
Subgroup analysis for number of needle passes restricted to studies using rapid on-site cytological evaluation (A) and not using rapid on-site cytological evaluation (B)