Abstract
Numerous clinical pathways exist for patients presenting with a suspicious pancreatic mass. These range from direct surgical intervention following staging, with preoperative cross-sectional imaging, EUS with or without fine-needle aspiration or fine-needle core biopsy; neoadjuvant chemotherapy and/or radiation therapy; or palliation. Although international guidelines exist for pancreas cancer management, the ideal workup and treatment for a suspicious pancreas mass is unclear. During its annual meeting in September 2017 (The Forum for Canadian Endoscopic Ultrasonography), the Canadian Society of Endoscopic Ultrasound organized a working group of experienced endosonographers and hepatobiliary surgeons from across Canada to achieve this goal.
Keywords: diagnosis, staging, pancreas, cancer, endosonograophy
INTRODUCTION
Pancreatic adenocarcinoma is the fourth-leading cause of death among all malignancies in Canada and has the lowest 5-year survival rate (8%).[1] Data from 2017 estimated that 5500 Canadians were diagnosed with pancreatic cancer (PC), 4800 died from PC. An estimated 1 male in 74 and 1 female in 72 will develop PC during their lifetime.
Numerous risk factors have been identified for PC, none stronger than smoking and family history.[2,3] Smoking is thought to cause of 20%–30% of all PCs, with a two-fold-increased risk compared to nonsmokers. The risk increases with the duration of years smoked and the number of cigarettes and decreases after one quits smoking.[4] African–Americans have a higher incidence (16.4/100,000) compared to Caucasians (10.8/100,000) and other races (9.8/100,000).[5] Obesity, diabetes,[6,7] rare inherited conditions such as hereditary pancreatitis, Peutz–Jeghers syndrome, Lynch syndrome, hereditary breast and ovarian cancer syndrome, and familial malignant melanoma and PC syndrome all significantly increase the risk of PC.[3]
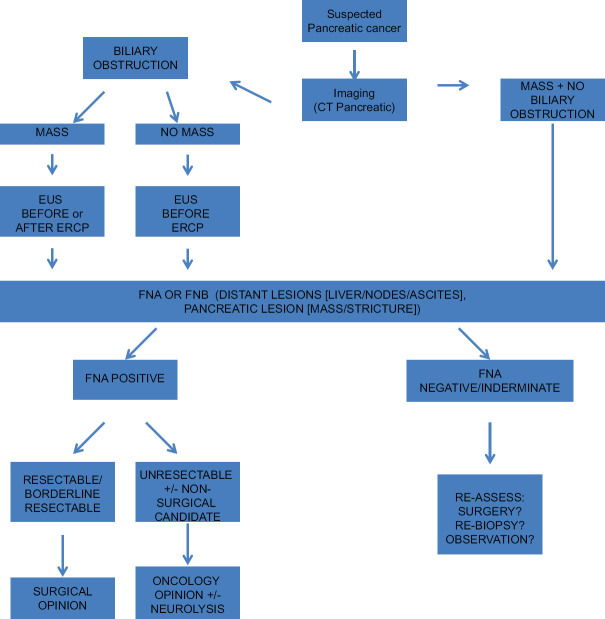
Numerous clinical pathways exist for patients presenting with a suspicious pancreatic mass. These range from direct surgical intervention following staging, with preoperative cross-sectional imaging, EUS with or without fine-needle aspiration (FNA) or fine-needle core biopsy (FNB); neoadjuvant chemotherapy and/or radiation therapy; or palliation. Although international guidelines exist for pancreas cancer management,[8] the ideal workup and treatment for a suspicious pancreas mass are unclear. During its annual meeting in September 2017 (The Forum for Canadian Endoscopic Ultrasonography), the Canadian Society of Endoscopic Ultrasound organized a working group of experienced endosonographers and hepatobiliary surgeons from across Canada to achieve this goal [Figure 1].
Figure 1.
Proposed algorithm for management of suspected pancreatic cancer
SHOULD EUS BE ROUTINELY USED IN CONJUNCTION WITH CROSS SECTIONAL IMAGING FOR ASSESSMENT AND TISSUE SAMPLING OF SUSPICIOUS PANCREAS MASSES?
With technological advancements, the pancreas protocol computed tomography (CT) accuracy for the detection and staging of PC continues to improve, and it remains the cornerstone for the initial assessment of suspicious lesions. Access to quality EUS is also improving, along with its unique ability to safely detect and sample suspicious pancreatic and extrapancreatic lesions (e.g., liver, nodes) for cytology or core biopsy, with a sensitivity and specificity approaching 100%.
However, EUS is still not considered “standard of care” for the management of suspected PC. This is exemplified in the National Comprehensive Cancer Network (NCCN) for Pancreas Adenocarcinoma Guidelines.[8] EUS is not routinely recommended as a staging tool but may be considered complementary. They recommend staging using “multi-detector CT angiography with preferably sub-millimeter axial sections and multi-planar reconstruction.” Even with this level of imaging, the sensitivity for CT for small hepatic metastases and peritoneal implants is limited. Furthermore, the guidelines concede that NCCN member institutions vary in the use of additional technologies and that, besides tissue acquisition, “sometimes additional diagnostic information is acquired” by EUS. EUS can also provide additional information when an “initial scan shows no lesion or whose lesions have questionable involvement of blood vessels or lymph nodes.”
A recent single-center retrospective study specifically addressed the NCCN recommendation that EUS should not be routinely used for vascular staging of PC.[9] This is in the context of a meta-analysis showing higher postoperative mortality and lower long-term survival with the need for portal or superior mesenteric vein resection (VR).[10] All patients deemed to have borderline resectable pancreatic cancer (BRPC) had undergone pancreas protocol CT and EUS staging at diagnosis (and required tissue by FNA or FNB to receive neoadjuvant chemotherapy), then underwent surgery with curative intent. Sixty-two patients underwent surgery. Patients were borderline resectable by EUS alone in 29%, CT alone in 23%, and both modalities in 48% of patients. Of 34 with planned VR, EUS correctly identified 88% and CT 67% of cases. EUS recognized 11 patients as BRPC that CT did not, with only four recognized by CT but not EUS. EUS had higher sensitivity and specificity than CT to identify the need for VR. The concept of complementarity of EUS following CT scan for staging is strongly reinforced as a result.
Another recent study directly compared EUS and CT for the assessment of the pancreas masses.[11] Ninety-three patients underwent a pancreas protocol CT and EUS for which 75 (80%) had adenocarcinoma. This study highlighted the only modest agreement between tests on mass detection, mass size, vascular involvement, and LN involvement with Cohen’s kappa scores of 0.45, 0.70, 0.42, and 0.54, respectively. The authors admit that although NCCN guidelines recommend pancreas protocol CT with EUS in specific cases only, caution should be used to avoid using single modalities to make complex management decisions in pancreas cancer. Each test can provide independent information, and the importance of proper staging for the aggressive disease may involve routine, high-quality EUS.
EUS is uniquely sensitive for small pockets of ascites that are undetectable by CT. One study performed EUS ± FNA in all patients following CT to procure tissue if unresectable or to confirm the patient should not be upstaged.[12] Ascites was detected by EUS in 14% (19/136) of patients not seen on CT. Ascites detected by EUS was the only independent predictive factor of carcinomatosis (odds ratio = 11 [confidence interval 95%: 3–40]). Ascites at EUS was associated with shorter survival 7.3 versus 14.2 months (P = 0.018). EUS detection of ascites is a crucial factor that alters prognosis and management. Another single-center study of 60 patients underwent EUS-FNA of ascites, of which 28 had no ascites seen on CT. Despite the fact that 22 of these aspirations were in benign disease, this reinforces the ability of EUS to detect ascites despite negative CT scans.[13]
A study involving 58 patients set out to explore pancreatic head carcinomas with a component of “diagnostic dilemma” and compare CT, EUS, and histopathological stage.[14] In comparing CT and EUS in the 25 patients undergoing resection, kappa for T and N stages was 0.250 (P = 0.05) and −0.080 (P = 0.288), respectively, for CT but 0.738 (P = 0.0001) and 0.606 (P = 0.0001), respectively for EUS. In this study, EUS was significantly more accurate for both T and N staging.
In a retrospective analysis, 42 patients with malignancy and prior imaging, seventeen (41%) were detected by EUS-FNA to have liver metastasis not previously recognized. Thirteen patients had CT alone, one ultrasound, and 3 had both CT and ultrasound. Mean size of the 17 lesions was 12.6 mm (range 3–26 mm).[15]
A less common but state-of-the-art staging concern involves remote malignant intravascular thrombi (RMT). A retrospective analysis of a prospective database found 8 newly diagnosed pancreas cancer patients with RMT. Of these CT did not detect the intravascular thrombus in 5 (63%), of whom 3 patients had positive or suspicious intravascular EUS-FNA cytology. EUS-FNA upstaged 3 patients (37.5%) and converted 2 (25%) from CT resectable to unresectable disease.[16]
Besides NCCN guidelines discussed earlier, the American Society of Gastrointestinal Endoscopy firmly places EUS ± FNA in the algorithm for suspected pancreas cancer and recommends that imaging evaluation of patients with suspected solid pancreatic neoplasia include EUS and multi-detector pancreas protocol CT scans with selective use of MRI and PET-CT when appropriate.[17] The European Society of Medical Oncology guidelines state “Aside from allowing the diagnosis of pancreas cancer, this technique [EUS] also permits the sampling of atypical lymph nodes (portocaval especially) to check for tumours with distant metastasis, a finding which would contraindicate radical resection. Incidental hepatic metastases can also be sampled during the same procedure without introducing any major risk.” They deem EUS as a complimentary modality to CT.[18] The Japanese Pancreas Society guidelines recommend the use of EUS for resectability as needed.[19] Finally, a recent Cochrane Review examined the role of EUS assessing pancreas cancer found resectable on CT.[20] Only two studies (from 2001 and 2003) with a meager 34 patients total were included.[21,22] The authors conclude that as a result of methodological and other problems found, there is too much uncertainty in the utility of EUS to recommend that it be performed routinely.
However, in patients with suspected pancreatic adenocarcinoma by CT, alternate diagnoses, for which pancreatic resection may be entirely inappropriate, are not infrequent. In a recent study of 132 patients, of whom 99 had pancreas lesions (and the rest extrapancreatic), the accuracy of EUS-FNB for pancreatic lesions was 97.9%.[23] Diagnoses other than adenocarcinoma were found in 25/99 (25%) of patients (12 patients neuroendocrine tumors, 5 benign lesions, 1 solid pseudo-papillary tumor, and 7 pancreatic metastases). Gerritsen et al. reported a 6.6% pancreaticoduodenectomy (PD) for benign disease in 2014,[24] and in 2012, van Heerde et al. found PD performed in 8.4% of suspected pancreatic malignancy ultimately found to have benign disease.[25] One-third of these benign cases carried a diagnosis of autoimmune pancreatitis (AIP). What is the acceptable rate of inappropriate surgery for presumed adenocarcinoma where a benign (or other) diagnosis is found?
As will be discussed in a later section, neo-adjuvant chemotherapy and/or radiation therapy are used with increasing frequency. Given the pretreatment requirement for histological confirmation of adenocarcinoma, the debate concerning the preoperative value of EUS-guided tissue acquisition may become moot.
Given the above, it would appear reasonable to attempt to obtain EUS in all cases of masses suspicious for PC of pancreatic masses and staging of presumed adenocarcinoma, and should be placed in an appropriate algorithm [Figure 1]. The risks of the test are extremely low, and upstaging should be based on biopsy of lesions (e.g., liver metastases, distant nodes, suspicious ascites), eliminating false positives. The morbidity and mortality of a PD are not negligible, and the impact on patient management, if even 1% of such “resectable by CT” cancers are upstaged, would be dramatic.
In addition, more definitive benign or alternative diagnoses (AIP, pancreatic metastasis) can be made with newer core biopsy needles. Many studies have shown benefit of EUS beyond FNA of the pancreatic mass in question.
WHEN BILE DUCT OBSTRUCTION IS PRESENT, SHOULD EUS BE SYSTEMATICALLY PERFORMED BEFORE ERCP WITH STENTING?
It may be clinically challenging to distinguish benign and malignant biliary. Conventionally, ERCP is used as a first-line diagnostic and therapeutic procedure in patients with suspicious biliary strictures.[26] The accuracy of ERCP can be influenced by stricture morphology, tissue acquisition technique, and endoscopist experience.[27] The sensitivity of brush cytology is generally <50%.[28] ERCP-guided cholangioscopy and biopsy may improve yield, may be time-consuming, expensive, and technically demanding.[29]
EUS has been increasingly employed in patients with obstructive jaundice.[30,31] As discussed previously, EUS allows tissue acquisition using FNA or FNB provides high diagnostic yield for solid pancreatic masses.[32,33] Comparing ERCP and EUS in tissue acquisition for malignant biliary strictures has shown to trend toward enhanced yield of EUS over ERCP.[34,35,36] Weilert et al. prospectively evaluated ERCP versus EUS-FNA for malignant strictures. The overall sensitivity and accuracy for EUS-FNA was 94% and 94%, and 50% and 53% for ERCP sampling.[35]
There have been conflicting data on whether a biliary plastic stent influences the accuracy of EUS-FNA for pancreatic head masses. One study found the presence of a biliary stent associated with a significant decrease in the accuracy of EUS-FNA for histologic diagnosis of PC from 89% to 77%, while accuracy increased when a cytopathologist was onsite and when FNB was utilized.[37] A contradicting, retrospective study, determined the presence of biliary stent does not influence the tissue sampling adequacy and accuracy of pancreatic head masses performed with core biopsies (FNB).[38] A combined approach of EUS and ERCP with biliary stenting, under the same sedation, yielded a 25 min increase in procedure length when compared to separate procedures but similar high yields for diagnostic EUS. A trend toward lower biliary stent placement success rates with combined EUS/ERCP was noted but nonsignificant.[39]
The significant trend toward enhanced yield of tissue acquisition of EUS over ERCP enables one to surmise that EUS should be used as a primary diagnostic tool to evaluate patients with obstructive jaundice first and then proceed with ERCP for biliary stenting. Whether performed under the same or separate sessions depend on the resources available.
WHAT SHOULD THE MULTIDISCIPLINARY TEAM CONSIDER WITH RESPECT TO DEFINING RESECTABLE VS. NONRESECTABLE PANCREATIC CANCER?
Prior to summarizing the details of which PCs are oncologically and technically resectable, it is critical to remember that pancreas adenocarcinoma is a systemic disease, meaning that it is unlikely that surgery alone will cure most PCs. More specifically, even in patients with an R0 resection, 5-year survival approximates only 20%.[40] This reality must be coupled; however, with the concurrent understanding that surgical resection is also the only potentially curative treatment currently available for pancreas cancer. It is also clear that amongst various risk factors for recurrence following resection, microscopic involvement of the resection margin (R1) is one of the strongest predictors of both local and distant recurrence, and therefore the prognostic outcome of patients.[41] R1 resections have been reported in up to 83% of patients depending on the variation in both operative techniques and, most importantly, pathological inking/sectioning techniques.[42]
Factors which determine resectability may include, application of the most recent (and often modified) committee staging scheme,[43,44] fidelity of the preoperative imaging (and therefore the accuracy of the staging), surgeon experience, the cultural milieu of the institution (based on numerous factors including the outcomes of previous anecdotal cases and surgeon “resectional aggression”), as well as patient preference and comorbidities.
From a purely technical perspective, nearly every locally advanced tumor is resectable, with or without some form of collateral risk and reconstruction. Among the 30% of patients who present with nonmetastatic, locally advanced disease (i.e., some of whom are “borderline” resectable), vascular resections and/or reconstructions are often mandatory in the consideration of resectability.[45,46] These may include the superior mesenteric, portal, and splenic veins, as well as the celiac axis, hepatic arteries, and superior mesenteric artery. Despite the technical possibility of resecting these structures, the assessment conversation must always include the realities of pancreas cancer as a systemic disease. More specifically, when the risk of multi-visceral and/or vascular resections exceeds the litmus test of reasonable behavior within the context of a biologically challenging cancer, the surgeon and team must ask themselves if resection is truly in the patient’s best interest. To this end, pancreatic adenocarcinomas that directly involve the aorta, celiac axis, superior mesenteric artery, hepatic artery, and inferior vena cava are generally considered unwise to resect. The involvement of the superior mesenteric vein and/or portal vein are often technically resectable; however, they require a detailed evaluation by an experienced pancreatic surgeon. While multivisceral resections are more common for tumors of the body and tail (i.e., concurrent resection of the stomach, spleen, and colon), they are also occasionally warranted for cancers in the head and uncinate of the gland (i.e., colon).
Clearly, patients with overt evidence of metastatic disease must be ruled out for initial surgical resection (even in the case of a technically resectable primary lesion). This includes distant metastases, as well as preoperative regional lymphadenopathy. Given the recent evidence that neoadjuvant folfirinox improves R0 resection rates in borderline resectable patients in up to 70% of cases,[47,48,49] the traditional dogma of accepting a high potential for an R1 margin following initial surgical resection of some tumours may also be changing. Despite this potentially exciting development, it is clear that even the definition of “borderline” resectability is difficult to consolidate. Although tumor approximation to the lateral wall of the superior mesenteric artery is relatively consistent, multiple societies and associations continue to propagate differing criteria.[42] It is evident, however that the granularity and accuracy of the initial diagnostic imaging studies remain critical in defining precise staging (and hence resectability) of pancreas cancers. While most patients continue to benefit from an isolated cross-sectional imaging series utilizing CT with intravenous contrast, EUS in experienced hands is particularly adept at the detailed evaluation of regional lymph nodes, as well as defining the relationship between tumour margins and adjacent vascular structures. As long as there is no additional delay in the interval between the initial diagnosis on a CT scan and subsequent evaluation by an experienced pancreatic surgeon, some patients will benefit from undergoing EUS.[50,51]
SHOULD NEOADJUVANT THERAPY BE APPLIED TO ALL PATIENTS WITH PANCREATIC CANCER?
Despite advances in the safety and availability of pancreas cancer surgery in the past 40 years, long-term survival remains rare and most patients eventually succumb to their malignancy.[52] At diagnosis, only 10%–20% of patients are considered resectable.[53] Recurrence can occur early after surgery and is nearly universal, with the incidence of newly diagnosed cases of pancreas cancer mirroring the annual death rate.[54] Indeed, over the past 30–40 years, the number of patients being offered surgery and the overall safety of pancreas resections has increased; however, during the same time period, pancreas cancer survival has remained level at approximately 24 months in large institutional databases.[54]
Until now, the gold standard treatment for potentially curable and removable PC has been surgery for full resection of the tumor followed by adjuvant chemotherapy. This strategy has been bolstered by recent randomized controlled trials demonstrating longer overall survival improvements with median overall survival in excess of 4 years in prospective studies.[55,56] The challenge with chemotherapy following surgery is that between 40% and 60% of patients never receive chemotherapy after resection of PC.[57] This may be due to deconditioning postsurgery, complications, or other factors.[57] Since most of these patients will ultimately die of recurrence, an optimal treatment strategy should include systemic therapy.
For the aforementioned reasons, a neoadjuvant approach involving chemotherapy and possibly radiation for potentially curable or resectable pancreas cancer has been advocated.[58] The rationale for this approach is multifactorial but includes: the high risk of systemic failure of surgery alone, or before chemotherapy; increased delivery of full treatment; the avoidance of nontherapeutic surgery in patients who progress on treatment (selection of favorable tumor biology); as well as the increased likelihood of R0 resection, lower rates of lymph node positivity, and decreased local recurrence.[57] A chemotherapy up-front approach has already become the gold standard for borderline-resectable, or locally-advanced pancreas cancers.[59] Indeed, patients with tumours considered unresectable may become resectable in 10%–20% of cases with an upfront treatment strategy with chemotherapy with or without radiation.[59] At the 2018 American Society of Clinical Oncology meeting a prospective randomized controlled trial comparing chemotherapy and radiation prior to surgery, to upfront surgery for resectable pancreas cancer demonstrated improvement in median overall survival, time until PC recurrence, and the chance of surviving longer than 2 years was higher with preoperative treatment.[60] This raises interesting possibilities in expanding neoadjuvant treatments for pancreas cancer. The benefit of chemotherapy versus chemotherapy and radiation before surgery is not clear, and future studies may answer this question. Nonetheless, with recent advances in novel chemotherapy, and the recently presented findings regarding neoadjuvant therapy and improved survival in pancreas cancer, the role of upfront treatment will likely expand and may become the gold standard.
There are barriers to broad application of a neoadjuvant approach. All patients subjected to this approach require high-quality tissue diagnosis. The gold standard for diagnosis of primary pancreas cancer is EUS and tissue sampling.[61] If a neoadjuvant strategy for resectable pancreas cancer is more broadly embraced, greater access to EUS will be necessary.
CONCLUSION
The diagnosis, staging, and treatment of pancreatic masses suspicious for PC continue to evolve. Treatment options for pancreatic adenocarcinoma have failed to achieve a significant mortality benefit despite surgical advances. Neoadjuvant chemotherapy (and/or radiation therapy) currently shows the most promise in achieving a survival benefit by approaching pancreas cancer as a systemic disease. We conclude that strong consideration should be given to performing EUS and EUS-guided biopsy to provide the most precise diagnosis possible (including excluding benign conditions that mimic cancer), to maximize the chance of identifying distant metastasis they may render surgery futile, and to provide appropriate material to optimize neo-adjuvant therapeutic regimens that may be considered.
As with other countries worldwide, Canada must implement national guidelines based on its own particular resources and health-care programs. The Canadian Society of EUS and other societies need to develop a coordinated approach to the suspicious pancreas mass; one which should include EUS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Canadian Cancer Statistics. 2017. [Last accessed on 2019 Feb 10]. Available from: https://wwwcancerca/en/cancer-information/cancer-type/pancreatic/prognosis-and-survival/survival-statistics .
- 2.Hart AR, Kennedy H, Harvey I. Pancreatic cancer: A review of the evidence on causation. Clin Gastroenterol Hepatol. 2008;6:275–82. doi: 10.1016/j.cgh.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 3.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694–705. doi: 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197–209. doi: 10.1016/j.bpg.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Shaib YH, Davila JA, El-Serag HB. The epidemiology of pancreatic cancer in the United States: Changes below the surface. Aliment Pharmacol Ther. 2006;24:87–94. doi: 10.1111/j.1365-2036.2006.02961.x. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer. 2007;120:1993–8. doi: 10.1002/ijc.22535. [DOI] [PubMed] [Google Scholar]
- 7.Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch Physiol Biochem. 2008;114:63–70. doi: 10.1080/13813450801954451. [DOI] [PubMed] [Google Scholar]
- 8.NCCN Guidelines. Pancreatic Adenocarcinoma Version 2.2018. 2018 [Google Scholar]
- 9.Glazer ES, Rashid OM, Klapman JB, et al. Endoscopic ultrasonography complements computed tomography in predicting portal or superior mesenteric vein resection in patients with borderline resectable pancreatic carcinoma. Pancreatology. 2017;17:130–4. doi: 10.1016/j.pan.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Giovinazzo F, Turri G, Katz MH, et al. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br J Surg. 2016;103:179–91. doi: 10.1002/bjs.9969. [DOI] [PubMed] [Google Scholar]
- 11.Du T, Bill KA, Ford J, et al. The diagnosis and staging of pancreatic cancer: A comparison of endoscopic ultrasound and computed tomography with pancreas protocol. Am J Surg. 2018;215:472–5. doi: 10.1016/j.amjsurg.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Alberghina N, Sánchez-Montes C, Tuñón C, et al. Endoscopic ultrasonography can avoid unnecessary laparotomies in patients with pancreatic adenocarcinoma and undetected peritoneal carcinomatosis. Pancreatology. 2017;17:858–64. doi: 10.1016/j.pan.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 13.DeWitt J, LeBlanc J, McHenry L, et al. Endoscopic ultrasound-guided fine-needle aspiration of ascites. Clin Gastroenterol Hepatol. 2007;5:609–15. doi: 10.1016/j.cgh.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Morris-Stiff G, Escofet X, Barry JD, et al. Selective use of endoscopic ultrasound in the evaluation of carcinomas of the pancreatic head. Dig Surg. 2011;28:373–8. doi: 10.1159/000334546. [DOI] [PubMed] [Google Scholar]
- 15.DeWitt J, LeBlanc J, McHenry L, et al. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: A large single-center experience. Am J Gastroenterol. 2003;98:1976–81. doi: 10.1111/j.1572-0241.2003.07638.x. [DOI] [PubMed] [Google Scholar]
- 16.Rustagi T, Gleeson FC, Chari ST, et al. Remote malignant intravascular thrombi: EUS-guided FNA diagnosis and impact on cancer staging. Gastrointest Endosc. 2017;86:150–5. doi: 10.1016/j.gie.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Eloubeidi MA, Decker GA, Chandrasekhara V, et al. ASGE Standards of Practice Committee. The role of endoscopy in the evaluation and management of patients with solid pancreatic neoplasia. Gastrointest Endosc. 2016;83:17–28. doi: 10.1016/j.gie.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(5):v56–68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi K, Okusaka T, Shimizu K, et al. Clinical Practice Guidelines for Pancreatic Cancer 2016 From the Japan Pancreas Society: A synopsis. Pancreas. 2017;46:595–604. doi: 10.1097/MPA.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 20.Tamburrino D, Riviere D, Yaghoobi M, et al. Diagnostic accuracy of different imaging modalities following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev. 2016;9:CD011515. doi: 10.1002/14651858.CD011515.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad NA, Kochman ML, Lewis JD, et al. Endosonography is superior to angiography in the preoperative assessment of vascular involvement among patients with pancreatic carcinoma. J Clin Gastroenterol. 2001;32:54–8. doi: 10.1097/00004836-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Ardengh JC, de Paulo GA, Ferrari AP. Pancreatic carcinomas smaller than 3.0 cm: Endosonography (EUS) in diagnosis, staging and prediction of resectability. HPB (Oxford) 2003;5:226–30. doi: 10.1080/13651820310001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haseeb A, Taylor LJ, Adler DG. Comparing endoscopic ultrasound-guided core biopsies of solid pancreatic and extrapancreatic lesions: A large single-operator experience with a new fine-needle biopsy needle. Ann Gastroenterol. 2018;31:742–6. doi: 10.20524/aog.2018.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerritsen A, Molenaar IQ, Bollen TL, et al. Preoperative characteristics of patients with presumed pancreatic cancer but ultimately benign disease: A multicenter series of 344 pancreatoduodenectomies. Ann Surg Oncol. 2014;21:3999–4006. doi: 10.1245/s10434-014-3810-7. [DOI] [PubMed] [Google Scholar]
- 25.van Heerde MJ, Biermann K, Zondervan PE, et al. Prevalence of autoimmune pancreatitis and other benign disorders in pancreatoduodenectomy for presumed malignancy of the pancreatic head. Dig Dis Sci. 2012;57:2458–65. doi: 10.1007/s10620-012-2191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kessler RE, Falkenstein DB, Clemett AR, et al. Indications, clinical value and complications of endoscopic retrograde cholangiopancreatography. Surg Gynecol Obstet. 1976;142:865–70. [PubMed] [Google Scholar]
- 27.Navaneethan U, Njei B, Lourdusamy V, et al. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: A systematic review and meta-analysis. Gastrointest Endosc. 2015;81:168–76. doi: 10.1016/j.gie.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsi MA, Deepinder F, Lopez R, et al. Factors affecting the yield of brush cytology for the diagnosis of pancreatic and biliary cancers. Pancreas. 2011;40:52–4. doi: 10.1097/MPA.0b013e3181f3aa96. [DOI] [PubMed] [Google Scholar]
- 29.de Vries AB, van der Heide F, Ter Steege RW, et al. Limited diagnostic accuracy and clinical impact of single-operator peroral cholangioscopy for indeterminate biliary strictures. Endoscopy. 2020;52:107–14. doi: 10.1055/a-1061-7067. [DOI] [PubMed] [Google Scholar]
- 30.DeWitt J, Misra VL, Leblanc JK, et al. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc. 2006;64:325–33. doi: 10.1016/j.gie.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 31.Khashab MA, Fockens P, Al-Haddad MA. Utility of EUS in patients with indeterminate biliary strictures and suspected extrahepatic cholangiocarcinoma (with videos) Gastrointest Endosc. 2012;76:1024–33. doi: 10.1016/j.gie.2012.04.451. [DOI] [PubMed] [Google Scholar]
- 32.Lee YN, Moon JH, Kim HK, et al. Core biopsy needle verses standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: A randomized parallel-group study. Endoscopy. 2014;46:1056–62. doi: 10.1055/s-0034-1377558. [DOI] [PubMed] [Google Scholar]
- 33.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 34.De Moura DT, Moura EG, Bernardo WM, et al. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: Systematic review and meta-analysis. Endosc Ultrasound. 2018;7:10–9. doi: 10.4103/2303-9027.193597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weilert F, Bhat YM, Binmoeller KF, et al. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: Results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014;80:97–104. doi: 10.1016/j.gie.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 36.Moura DT, de Moura EG, Matuguma SE, et al. EUS-FNA versus ERCP for tissue diagnosis of suspect malignant biliary strictures: A prospective comparative study. Endosc Int Open. 2018;6:E769–77. doi: 10.1055/s-0043-123186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JJ, Walia S, Lee SH, et al. Lower yield of endoscopic ultrasound-guided fine-needle aspiration in patients with pancreatic head mass with a biliary stent. Dig Dis Sci. 2015;60:543–9. doi: 10.1007/s10620-014-3367-0. [DOI] [PubMed] [Google Scholar]
- 38.Antonini F, Fuccio L, Giorgini S, et al. Biliary plastic stent does not influence the accuracy of endoscopic ultrasound-guided sampling of pancreatic head masses performed with core biopsy needles. Dig Liver Dis. 2017;49:898–902. doi: 10.1016/j.dld.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Aslanian HR, Estrada JD, Rossi F, et al. Endoscopic ultrasound and endoscopic retrograde cholangiopancreatography for obstructing pancreas head masses: Combined or separate procedures? J Clin Gastroenterol. 2011;45:711–3. doi: 10.1097/MCG.0b013e3182045923. [DOI] [PubMed] [Google Scholar]
- 40.Gooiker GA, van Gijn W, Wouters MW, et al. Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg. 2011;98:485–94. doi: 10.1002/bjs.7413. [DOI] [PubMed] [Google Scholar]
- 41.Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: A consensus statement by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2014;155:977–88. doi: 10.1016/j.surg.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC staging manual and the future of TMN. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Y, Su Y, Chen Y, Li Z. Refining the American Joint Committee on cancer staging scheme for resectable pancreatic ductal adenocarcinoma using recursive partitioning analysis. J Cancer. 2017;8:2765–73. doi: 10.7150/jca.19515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vauthey JN, Dixon E. AHPBA/SSO/SSAT Consensus Conference on Resectable and Borderline Resectable Pancreatic Cancer: Rationale and overview of the conference. Ann Surg Oncol. 2009;16:1725–6. doi: 10.1245/s10434-009-0409-5. [DOI] [PubMed] [Google Scholar]
- 46.National Cancer Institute. Cancer of the Pancreas – Cancer Stat Facts Surveillance, Epidemiology and End Results Program. 2015 [Google Scholar]
- 47.Sadot E, Doussot A, O’Reilly EM, et al. FOLFIRINOX induction therapy for stage 3 pancreatic adenocarcinoma. Ann Surg Oncol. 2015;22:3512–21. doi: 10.1245/s10434-015-4647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rombouts SJ, Walma MS, Vogel JA, et al. Systematic review of resection rates and clinical outcomes after FOLFIRINOX-based treatment in patients with locally advanced pancreatic cancer. Ann Surg Oncol. 2016;23:4352–60. doi: 10.1245/s10434-016-5373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Portales F, Gagniard B, Thesenas S, et al. Feasability and impact on resectability of FOLFIRINOX in locally-advanced and borderline pancreatic cancer. Am Soc Clin Oncol Annu Meet. 2016;34(l):e15708. [Google Scholar]
- 50.Driedger MR, Dixon E, Mohamed R, et al. The diagnostic pathway for solid pancreatic neoplasms: Are we applying too many tests? J Surg Res. 2015;199:39–43. doi: 10.1016/j.jss.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 51.Iordache S, Albulescu DM, Săftoiu A. The borderline resectable/locally advanced pancreatic ductal adenocarcinoma: EUS oriented. Endosc Ultrasound. 2017;6:S83–6. doi: 10.4103/eus.eus_68_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th ed. New York NY: Springer; 2010. Exocrine and endocrine pancreas; pp. 241–6. [Google Scholar]
- 53.Fong ZV, Alvino DM, Castillo CF, et al. Reappraisal of staging laparoscopy for patients with pancreatic adenocarcinoma: A contemporary analysis of 1001 patients. Ann Surg Oncol. 2017;24:3203–11. doi: 10.1245/s10434-017-5973-5. [DOI] [PubMed] [Google Scholar]
- 54.Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: Results from a single institution over three decades. Ann Surg Oncol. 2012;19:169–75. doi: 10.1245/s10434-011-1900-3. [DOI] [PubMed] [Google Scholar]
- 55.Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 56.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–24. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 57.Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372–7. doi: 10.1097/SLA.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 58.Barenboim A, Lahat G, Geva R, et al. Neoadjuvant FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer: An intention to treat analysis. Eur J Surg Oncol. 2018;44:1619–23. doi: 10.1016/j.ejso.2018.07.057. [DOI] [PubMed] [Google Scholar]
- 59.Abou-Khalil J, Rocha FG. Surgical strategies and novel therapies for locally advanced pancreatic cancer. J Surg Oncol. 2017;116:16–24. doi: 10.1002/jso.24654. [DOI] [PubMed] [Google Scholar]
- 60.van Tienhoven G, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): A randomized, controlled, multicenter phase III trial. J Clin Oncol. 2020:JCO1902274. doi: 10.1200/JCO.19.02274. doi: 10.1200/JCO.19.02274. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitano M, Yoshida T, Itonaga M, et al. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol. 2019;54:19–32. doi: 10.1007/s00535-018-1519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]