Abstract
Hepatic fibrosis is a significant complication in adult Fontan patients suggesting development as a function of time since the surgery. Children with Fontan circulation are not routinely assessed for development of liver disease. We aimed to evaluate the effectiveness of serologic biomarkers and acoustic radiation force impulse (ARFI) elastography to detect liver disease in pediatric Fontan patients. Patients ≥ 1 year after Fontan operation prospectively had hepatic US with acoustic radiation force impulse and laboratory testing. Clinical cardiac data (echocardiograms, cardiac catheterizations) were reviewed. Statistical analysis was performed using Pearson’s correlation coefficient, Wilcoxon rank-sum test and Kruskal-Wallis test. Forty patients were enrolled with median age of 11 years and median time since Fontan of 6.5 years. Platelet count negatively correlated with years since Fontan (p < 0.000). Thrombocytopenia was noted in 15% of patients with the lowest platelet count of 78 K/cu mm, in a patient >10 years from the Fontan (DORV) operation. Alanine transaminase (ALT, p = 0.034) and aspartate aminotransferase (AST, p = 0.009) were higher in patients with Extracardiac Conduit Fontan and not in other Fontan operations. Heterogeneous echotexture on liver ultrasound correlated with years since Fontan (p = 0.022), however all acoustic radiation force impulse values were elevated (> 1.34 m/s) and did not correlate with age, years since Fontan, labs or imaging. FibroSure values did not correlate with years since Fontan. This suggests that ARFI may be elevated due to passive hepatic congestion, limiting its value in this patient population. Additional testing is necessary to identify reliable noninvasive screening modalities for hepatic fibrosis in Fontan patients. Our study is the largest pediatric study to evaluate ARFI in patients after the Fontan operation and showed increased shear wave speed for all patients with no correlation with time since palliation. Decreasing platelet count may indicate the development of liver fibrosis.
Keywords: Fontan, Acoustic Radiation Force Impulse (ARFI), congenital heart disease, hepatic fibrosis, congestive hepatopathy, elastography
INTRODUCTION
The Fontan operation was first described in 1971 by Drs. Fontan and Baudet for tricuspid atresia (1). The original Fontan procedure including the indications and surgical technique has been revised over the past 40 years (2). Single ventricle (SV) congenital heart disease (CHD) occurs in approximately 5 out of 100,000 live births. The modern Fontan procedure has become the standard of care for SV CHD patients. The Fontan operation diverts hepatic and inferior vena cava blood from the right atrium to the pulmonary arteries resulting in decreased preload reserve, lower resting cardiac output, elevated systemic vascular resistance, abnormal ventricular-vascular coupling, and elevated central venous pressure (3–6). Though the short-term operative outcomes have improved over the last 4 decades, the long-term complications of the Fontan operation and its effects on end-organ function are becoming increasingly recognized. The liver is at substantial long-term risk in patients with Fontan physiology with hepatic fibrosis now recognized as a significant complication in adult patients (7, 8). Increased hepatic venous pressures and lower oxygen delivery secondary to diminished cardiac output across the stages of palliation are proposed etiologies for liver disease in this population (9, 10) but the precise pathophysiologic mechanism is unclear. Hepatic venous blood flow is abnormal in Fontan patients (11) and is heavily influenced by the liver acting as a reservoir to increase pulmonary blood flow with inspiration (6). Children and adolescents status-post Fontan may develop hepatic fibrosis and cirrhosis (3, 5, 12) but the timeframe for this progression and the stratification of patient risk factors remains unclear.
The gold standard for diagnosing and staging liver fibrosis is a liver biopsy. Hepatic disease in Fontan patients is characterized by centrilobular fibrosis surrounding the central vein and extending along the sinusoids with little inflammatory activity (7, 13). Sinusoidal fibrosis occurs within the space of Disse in a pericellular manner along with marked sinusoidal dilatation due to increased central venous pressure. The extent of fibrosis is postulated to relate to an increase in hepatic venous pressure, low cardiac index, and decreased ventricular function (10). In order to minimize the risks associated with liver biopsy noninvasive methods are being explored to replace or help select appropriate patients for biopsy (14). Ultrasound elastography is a noninvasive modality used to assess hepatic fibrosis by measuring tissue stiffness. There are 4 techniques currently available including shear wave elastography (SWE, SuperSonic Imagine, Aix-en-Provence, France), transient elastography (TE, FibroScan, Echosens, Place D’Italie, France) magnetic resonance (MR) elastography (MR Touch, GE Healthcare, United States) and acoustic radiation force impulse (ARFI, Siemens-ACUSON, Erlangen, Germany) imaging (15). ARFI is a ultrasound-based technique integrated into a conventional ultrasound machine and used to measure liver stiffness through measurement of wave propagation speed or shear wave speed (SWS) in a region of interest (ROI) (16, 17). It evaluates deep tissue stiffness by generating qualitative and quantitative velocity values measured in meters per second (m/s) through an acoustic pulse generated by the ultrasound probe (17–19). The amplitude of the SWS is inversely proportional to the tissue elasticity or stiffness, thus the propagation speed increases with worsening fibrosis (18, 19). The usefulness of ARFI imaging has been recently described in adult Fontan patients but no studies have been reported in the pediatric Fontan population (20).
Currently, children with SV physiology are not routinely assessed for the development of liver disease, in part due to absence of proven, low-risk screening methods. Therefore, hepatic fibrosis in Fontan survivors is often not recognized until it is in advanced stages. We aimed to evaluate ARFI, routine ultrasound, and other biochemical tests in a cross-section of patients with SV physiology greater than 1 year status-post Fontan operation. Because the incidence of liver fibrosis increases with patient age, we hypothesized that markers of hepatic fibrosis would correlate with years since Fontan. We also evaluated aberrations in Fontan physiology as possible risk factors for hepatic fibrosis.
METHODS
This prospective, single-center, cross-sectional, observational study was approved by the Institutional Review Board at Nationwide Children’s Hospital. It was funded by an institutional grant from the Center for Cardiovascular & Pulmonary Research and The Heart Center. Dr. Sylvia Ofei was also supported by the NIH/NHLBI T32 training grant in Congenital and Acquired Heart Disease.
The study was performed from November 2014 through April 2015. Eligible patients had SV physiology and were at least one year status-post Fontan operation and clinically well as outpatients. Patients were recruited through mailed invitations and during routine follow-up appointments in the Cardiology clinic. Written informed consent was obtained from parents, guardians or patients 18 years-of-age and older. Written assent was obtained from all patients 9–17 years of age. Patients were excluded if they had history of infectious hepatitis, Wilson’s disease, autoimmune hepatitis, alpha-1 antitrypsin deficiency, non-alcoholic fatty liver disease (NAFLD), metabolic liver disease or Fontan revision. All patients had same day abdominal ultrasound, Doppler ultrasound, and hepatic ARFI study. Serum laboratory tests were obtained within 1 week of the radiologic evaluation. Medical records were reviewed for relevant demographic, surgical, anatomic and clinical patient information. Echocardiographic and cardiac catheterization data within 1 year of the ultrasound study were extracted from the medical records and reviewed by a single investigator (CP and KT).
The laboratory tests were performed as part of standard clinical practice. The tests included albumin, alkaline phosphatase, conjugated bilirubin, platelet count and non-alcoholic steatohepatitis (NASH) FibroSure (LabCorp, Burlington, NC, USA). NASH FibroSure is a patented biomarker panel of serologic markers (haptoglobin, apolipoprotein A-1, total bilirubin, ϒ-glutamyltransferase (GGT), alanine transaminase (ALT), aspartate aminotransferase (AST), alpha-2 macroglobulin, total cholesterol, serum glucose, and triglycerides) used in conjunction with the weight and height of the patient to generate a score that has been shown to correlate with liver fibrosis in adult patients with NASH. The score is converted into fibrosis stages according to the METAVIR scoring system for fibrosis on liver biopsy. The diagnostic utility of FibroSure has been validated in chronic viral hepatitis (HCV FibroSure), advanced fibrosis in NAFLD and alcoholic liver disease (21). The panel is not validated for use in the pediatric population and a fibrosis score is not available for patients younger than 14 years of age; the score was obtained for all patients 14 years of age or greater.
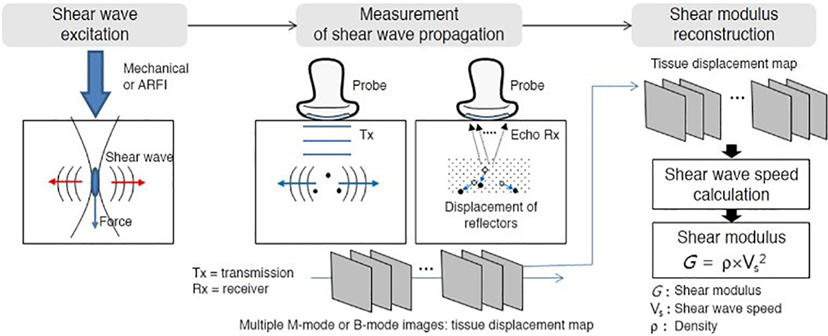
Standard 2-dimensional Doppler and ARFI studies were performed utilizing Siemens Virtual Touch Quantification software. Grayscale and duplex abdominal ultrasound was performed per standard protocol at Nationwide Children’s Hospital. Sonographers trained in the shear wave imaging technique performed the exams, which were read by two radiologists experienced in Doppler abdominal ultrasound and elastography technique and interpretation. For ARFI imaging acquisition, the right hepatic lobe was imaged, as diagramed in Figure 1 (22). The patient was placed in the supine position with arms maximally abducted. If possible, breath-holding for shear wave speed acquisition was performed. The liver was visualized through the intercostal or subcostal space, with longitudinal probe placement preferred. ARFI measurements were obtained at a minimum of 1.5 to 2 cm deep to the liver capsule, at an area of homogeneous echotexture devoid of large vessels or other liver structures. Ten successive acquisitions of SWS were obtained at varying locations, expressed as m/s. The probe was removed from the patient between successive measures. The following data points were collected from the grayscale and duplex portions of the exam: common bile duct diameter (mm), spleen length (cm), kidney lengths (cm), IVC diameter (mm), portal vein velocity (cm/s), main hepatic artery velocity (cm/s), hepatic artery resistive index (number), and hepatic vein diameter (mm). For the elastography portion of the exam, the highest and lowest of the 10 velocity values were dropped, and 8 SWS values were reported (m/s) from which the mean value was calculated. Studies in healthy children, children with liver disease of non-cardiac etiology and adults suggest a mean ARFI of <1.34 predicts an absence of fibrosis on biopsy.
Figure 1:
Acoustic radiation force impulse (ARFI) schematic and process of how the study is performed and calculated.
Echocardiograms were reviewed by a single investigator to assess ventricular morphology, ventricular function, and degree of atrioventricular valve regurgitation. Fontan type was characterized by technique and cardiac catheterization was included in the clinical review only when done within 12 months of the liver testing.
Statistical analysis was performed with SAS 9.3 (SAS Institute Inc., Cary, NC, USA). Data not normally distributed were subjected to non-parametric testing and associations were determined by Pearson’s correlation coefficient, Wilcoxon rank-sum test and Kruskal-Wallis test. Pearson’s chi-square test was used for associations between categorical variables. Categorical variables are presented as n (%) and descriptive statistics including mean (SD), median, minimum and maximum values were used to describe continuous variables. P values of ≤ 0.05 were considered statistically significance.
RESULTS
Two hundred sixty three patients were approached with recruitment of 48 patients. Forty patients were successfully studied. Baseline characteristics are summarized in Table 1. Summary of serum biomarkers and imaging results based on years from Fontan are summarized in Table 2.
Table 1:
Baseline Characteristics of the Subjects
| Median (range) or Number (%) | |
|---|---|
| Characteristics | |
| Demographic | |
| Age at enrollment (years) | 11 (3–34) |
| Male | 30 (75%) |
| Caucasian | 32 (80%) |
| BMI | 17.16 (12.77–35.36) |
| Age at Fontan operation (years) | 2 (1–16) |
| Years from Fontan (years) | 6.50 (1–28) |
| Primary congenital defect | |
| HLHS | 16 (40%) |
| TA | 7 (17.5%) |
| DORV | 10 (25%) |
| Other | 7 (17.5%) |
| Systemic ventricle anatomy | |
| Morphologically left | 14 (35%) |
| Morphologically right | 21 (52.5%) |
| Morphologically both | 5 (12.5%) |
| Fontan Surgery | |
| Extracardiac conduit | 10 (25%) |
| Extracardiac pericardial | 15 (37.5%) |
| Lateral tunnel | 15 (37.5%) |
BMI: body mass index; DORV: double outlet right ventricle; HLHS: hypoplastic left heart syndrome; TA: tricuspid atresia
Table 2:
Serum biomarkers, abdominal ultrasound and ARFI results based on years from Fontan.
| Serum biomarkers | Abdominal ultrasound | ARFI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Years from Fontan | ALT (U/L) Median (range) | AST (U/L) Median (range) | Pit (K/cu mm) Median (range) | APRI | Heterogeneous echotexture Number (%) | Irregular liver margins Number (%) | Mean SWS m/s (Std dev) | |||
| 1–5 | 33 (20–62) | 45 (29–97) | 253 (177–342) | 0.356 | 1 (6.3%) | 1 (6.3%) | 2.058 (0.236) | |||
| 5–10 | 32.5 (20–46) | 39.5 (26–54) | 233.5 (165–366) | 0.338 | 2 (22.2%) | 0 (0%) | 2.083 (0.341) | |||
| > 10 | 32 (17–54) | 28 (18–51) | 157.5 (78–222) | 0.356 | 7 (50%) | 4 (28.6%) | 2.196 (0.706) | |||
| p value | p = 0.934 | p = 0.001 | p <0.000 | p = 0.022 | p = 0.059 | p = 0.996 | ||||
ALT: Alanine transaminase; APRI: AST/platelet index; ARFI: acoustic radiation force impulse; AST: aspartate aminotransferase; Plt: platelet; SWS: shear wave speed
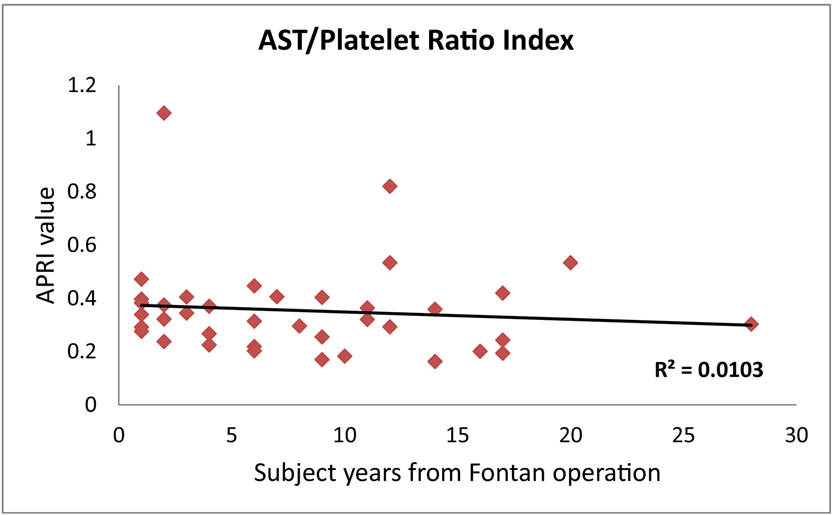
Platelet count negatively correlated with age at study (p < 0.001) and years from Fontan (p < 0.000, Table 2). Mean platelet count was 253 K/cu mm for study patients 1–5 years from their Fontan operation and decreased to 158 K/cu mm for patients > 10 years from their Fontan. The lowest platelet count was 177 K/cu mm for 1–5 years from the Fontan operation and 78 K/cu mm for >10 years from Fontan. Overall, 6 patients (15%) of patients had thrombocytopenia. Eight of forty patients and 5/40 patients had ALT and AST levels outside the normal range at our institution respectively. Alanine transaminase (ALT) and aspartate aminotransferase (AST) were higher but often not abnormal in patients with Extracardiac Conduit Fontan compared to those with Extracardiac Pericardial and Lateral Tunnel; p = 0.034 and p = 0.009, respectively. APRI is a noninvasive tool used to evaluate liver fibrosis which has been validated primarily in hepatitis C (23, 24) where APRI values < 0.5 are correlated with the absence of significant fibrosis, >0.5 indicates some liver damage, >0.7 correlates with hepatic fibrosis and >1.0 correlates with cirrhosis (23, 24). APRI was greater than 0.5 in 4 of 39 patients (11.1%, Figure 2) but did not correlate with years from Fontan, lab values, platelet counts or ARFI results. Calculated FibroSure scores were available on 13 patients and did not correlate with years since Fontan, lab values, platelet counts or ARFI results (Table 3).
Figure 2:
Calculated AST to platelet ratio index (APRI) in correlation with years from Fontan. Threshold is 0.5. Any APRI value < 0.5 rules out significant fibrosis, > 0.5 is indicative of some liver damage, > 0.7 is significant for hepatic fibrosis and > 1.0 is an indicator of cirrhosis.
Table 3:
FibroSure results with patient characteristics (ordered based on fibrosis severity)
| Fibrosis stage | Fibrosis score | SWS (m/s) | Age (years) | Years from Fontan | Cardiac anatomy | Fontan type | ALT/AST (IU/L) | Platelet count(K/cu mm) | AST/Platelet ratio (APRI) |
|---|---|---|---|---|---|---|---|---|---|
| F0- No fibrosis | <0.21 | 2.1 | 22 | 20 | HLHS | Lateral tunnel | 32/31 | 116 | 0.534 |
| F0-F1 | 0.21–0.27 | 2.03 | 14 | 10 | HLHS | Lateral tunnel | 25/31 | 346 | 0.183 |
| F0-F1 | 0.21–0.27 | 1.83 | 16 | 14 | HLHS | Lateral tunnel | 20/24 | 222 | 0.163 |
| F1- Portal fibrosis | 0.27–0.31 | 4.1 | 19 | 17 | Other | Lateral tunnel | 28/25 | 119 | 0.42 |
| F1- Portal fibrosis | 0.27–0.31 | 2.0 | 21 | 17 | TA | Lateral tunnel | 17/18 | 186 | 0.194 |
| F1- Portal fibrosis | 0.27–0.31 | 1.43 | 15 | 12 | HLHS | Lateral tunnel | 21/22 | 150 | 0.293 |
| F1- Portal fibrosis | 0.27–0.31 | 1.96 | 14 | 11 | Other | Extracardiac conduit | 30/30 | 165 | 0.364 |
| F1- Portal fibrosis | 0.27–0.31 | 3 | 14 | 12 | DORV | Lateral tunnel | 33/32 | 78 | 0.821 |
| F1-F2 | 0.31–0.48 | 2.6 | 32 | 28 | TA | Extracardiac conduit | 42/27 | 178 | 0.303 |
| F3-Bridging fibrosis many septa | 0.58–0.72 | 2.11 | 14 | 12 | DORV | Extracardiac conduit | 54/51 | 191 | 0.534 |
| F3-Bridging fibrosis many septa | 0.58–0.72 | 1.46 | 17 | 14 | TA | Lateral tunnel | 25/35 | 195 | 0.359 |
| F3-Bridging fibrosis many septa | 0.58–0.72 | 2.42 | 34 | 17 | DORV | Extracardiac conduit | 42/28 | 216 | 0.243 |
| F3-Bridging fibrosis many septa | 0.58–0.72 | 1.6 | 19 | 16 | HLHS | Lateral tunnel | 32/28 | 138 | 0.201 |
ALT: alanine transaminase; AST: aspartate aminotransferase; DORV: double outlet right ventricle; HLHS: hypoplastic left heart syndrome; SWS: shear wave speed; TA: tricuspid atresia
Heterogeneous liver echotexture on hepatic US suggestive of hepatocellular disease, was reported more frequently in older patients, p < 0.003. The frequency of finding heterogeneous liver echotexture and irregular liver margins was correlated with increased years since Fontan (Table 2). Hepatomegaly and splenomegaly were not associated with years of Fontan, p = 0.772 and 0.189 respectively. There was no association between primary cardiac anatomy and heterogeneous liver echotexture, irregular liver margins, hepatomegaly and splenomegaly. Patients with a systemic left ventricular anatomy were more likely to have irregular liver margins compared to those with a systemic right ventricular anatomy, p = 0.026. There was no association between ventricular anatomy, type of Fontan and ultrasound findings of common bile duct diameter, echotexture and hepatosplenomegaly.
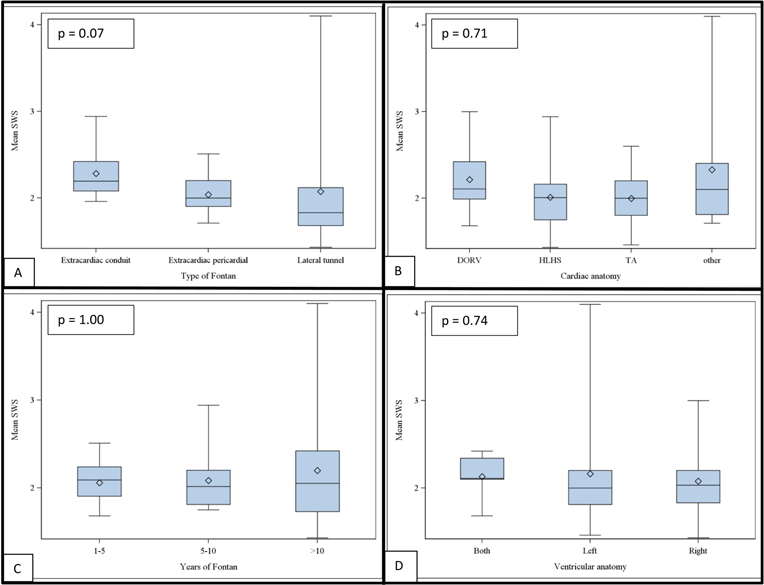
ARFI mean score was elevated using a cut-off of 1.34 m/s in all patients and there was no association between years since Fontan (Table 2), type of primary cardiac anatomy, right or left ventricular anatomy or the type of Fontan. Mean ARFI SWS is presented in Figure 3.
Figure 3:
Boxplots illustrating the lack of correlation (Pearson’s chi-squared) between acoustic radiation force impulse (ARFI) mean shear wave speed (SWS) and (A) type of Fontan operation, p = 0.07; (B) primary cardiac anatomy, p = 0.71; (C) years of Fontan operation, p = 1.00; (D) ventricular anatomy, p = 0.74. DORV: double outlet right ventricle; HLHS: hypoplastic left heart syndrome; TA: tricuspid atresia; (Boxes represent upper and lower quartiles and horizontal line within the boxes represents the median value).
Transthoracic echocardiograms and cardiac catheterization data was reported in patients with available data. Fourteen patients had Fontan pressures documented, 23 patients had left and right pulmonary artery pressures documented and all patients had documented ventricular function. Of the 14 patients with documented Fontan pressures, 4 had pressures >15 mmHg. Three of the 4 had HLHS anatomy. Association between cardiac anatomy and Fontan pressure was not statistically significant with p = 0.090. There was no correlation with years from the Fontan operation, hepatic ultrasound, ARFI or serologic data.
DISCUSSION
Fontan palliation is the culmination of an intensive rerouting of a SV patient’s blood flow to create a single systemic ventricular chamber, ameliorate the cyanosis induced by the mixing of systemic deoxygenated and pulmonary oxygenated blood by creating a pathway for passive pulmonary blood flow, and reconstruct an unobstructed systemic outflow tract. As survival after the Fontan procedure has improved, cardiologists and hepatologists now recognize liver fibrosis and cirrhosis as an important long-term complication of the palliative strategy (25) and predictive of mortality (26, 27). Patients included in this study demonstrated acceptable Fontan physiology with abnormal ventricular function documented in 10% of enrolled patients. Thus the pediatric population studied demonstrated both low ventricular diastolic pressures and low pulmonary vascular resistances optimal for a well-functioning Fontan circulation (8). Such patients could be considered normal risk for the development of liver dysfunction at the time they were enrolled. Although the population demonstrated good ventricular function, Fontan pressure measurements were only obtained in 35% of the study population and thus limited appropriate association and significance between hemodynamic data and serologic markers and radiologic markers due to the small sample size. The lack of cardiac catheterization data is primarily due to our center not pursuing cardiac catheterizations on a routine basis on children with Fontan palliation who were doing clinically well in the outpatient setting.
This study demonstrates the difficulty in early identification of liver disease in the pediatric population with SV physiology post a Fontan operation with no correlation found between time since Fontan with transaminase values, APRI, NASH FibroSure, or ARFI. We identified a correlation between time since Fontan and an abnormal appearing liver on hepatic ultrasonography; unfortunately, this may be a late finding. There were no associations between primary cardiac anatomy and heterogeneous hepatic echotexture, irregular liver margins, and hepatosplenomegaly. We also did not find any significant association between ultrasound findings and the type of Fontan or time since the Fontan operation. One subject, >10 years after Fontan operation (Extracardiac Conduit) was reported to have hepatic nodularity. A greater number of patients with a Lateral Tunnel had heterogeneous echotexture (35.71%) and coarsened hepatic echotexture, however, they were not statistically significant. Reduced platelet count with increasing time since the Fontan operation was the only significant finding. Most of these platelet counts were within the normal range suggesting that careful attention to trends in the platelet count may able to identify developing hepatic fibrosis.
Thrombocytopenia is associated with complications in chronic liver disease (28, 29) including splenic sequestration, bone marrow suppression and decreased thrombopoietin as proposed etiologies (28). In our study, chronological age and longer duration of Fontan physiology negatively correlated with platelet levels. Thrombocytopenia can be seen in 15–25% of adult Fontan patients and can inhibit routine care including diagnostic and therapeutic procedures (28, 30). Our study which incorporates only pediatric patients had similar findings to those reported in adult Fontan literature (28, 30). Decreased platelet count does not seem to be associated with an enlarged spleen as it is in many other forms of progressive hepatic fibrosis with the concomitant development of portal hypertension.
Serum transaminases are common screening tests for liver disease, however, in the Fontan population they have not been shown to be useful in screening for hepatic fibrosis. Transaminases are released into the circulation in response to direct hepatocellular injury, usually from inflammation. Laboratory finding of hepatocellular injury and cholestasis were not significantly deranged in our patients, consistent with previously reported findings (8, 31, 32). An adequate evaluation of the NASH FibroSure test will require a larger cohort of patients over the age of 14 year, since the FibroSure test has not been validated for use in the pediatric population but is routinely used in adult patients for screening for liver fibrosis.
Both ultrasound and MRI elastography have been used to assess hepatic fibrosis by measuring tissue stiffness in the Fontan population (18, 33–36). Transient elastography, although most commonly used is limited by obesity (37). MR elastography has a high success rate among obese patients however it is inhibited by inflammation, passive congestion and is cost prohibitive (37). We chose to examine the population with ARFI elastography because of the ease of implementation in children. Based on previously published cut-off values, all of our patients had an abnormal ARFI with the lowest mean SWS 1.46 m/s. Melero-Ferrer et al found linkage between ARFI elastography and abnormal blood liver test, however, our serological abnormalities did not correlate with ARFI elastography (20). Friedrich-Rust et al reported correlation between fibrosis stage using transient elastography and the time interval since Fontan operation with significant increase in liver fibrosis at 5 years from Fontan (38).
Passive hepatic congestion has been shown to increase SWS in an adult study by Shim et al. This likely explains why none of our patients had normal ARFI values and raises the question of the reliability of ARFI to predict progression to fibrosis in the Fontan population. The role of hepatic congestion vs fibrosis in contributing to elevated SWS remains unclear in our population. The concept of cumulative liver congestion creating abnormal ultrasound and shear wave elastography prior to Fontan palliation in the bidirectional Glenn population was investigated by Kutty et al (39) who reported increased hepatic stiffness in children with Glenn physiology compared with healthy controls that correlated with right atrial pressure and increased following Fontan. Additional studies have demonstrated abnormal echotexture in SV patients prior to Fontan palliation (40). The inability to discriminate between findings of congestion of the liver and fibrosis by SWS substantially limits the utility of this test in early assessment of liver disease in Fontan patients. Substantially elevated APRI scores in 2 patients that were two years from Fontan palliation suggests that liver injury may precede the Fontan palliation. Earlier evaluation of children prior to beginning the staged SV palliation pathway may provide a better assessment of the progression of hepatic congestion to liver fibrosis and eventually cirrhosis and allow clinicians to modify additional risk factors for these patients.
LIMITATIONS
Although this is the largest pediatric cross-sectional observational study evaluating the ARFI as a tool for assessment, the sample size was small and limited the statistical power when patients were aggregated into different groups for statistical analysis. We did not include a control group which likely affected the statistical validity of our findings. The ARFI score and FibroSure have not been validated specifically in pediatric Fontan patients. Also our laboratory and imaging findings were not confirmed with liver biopsy, the gold standard for assessing liver fibrosis.
CONCLUSION
The usefulness of ARFI elastography to noninvasively evaluate hepatic fibrosis in this patient population is poor. Our study is the largest pediatric study to evaluate ARFI in patients after the Fontan operation and showed increased SWS for all patients with no correlation with time since palliation. Decreasing platelet count in the absence of an enlarged spleen may indicate the development of liver fibrosis in this population. Increasing years from Fontan operation was evident with abnormal hepatic echotexture on abdominal US. Further studies are needed to adequately establish a noninvasive screening modality for Fontan patients in order to perform conservative surveillance in these patients due to the contribution of liver disease to adverse outcomes in this population.
HIGHLIGHTS.
Hepatic fibrosis is a significant complication in single ventricle Fontan patients
ARFI elastography may be used to detect Fontan related liver disease
Platelet count negatively correlates with age and longer duration Fontan
Serological lab abnormalities did not correlate with ARFI elastography results
Passive hepatic congestion may increase elastography score, without hepatic fibrosis
ACKNOWLEDGMENTS
Study was performed using research funding from The Center for Cardiovascular & Pulmonary Research and The Heart Center at Nationwide Children’s Hospital (Grant # 20053514). Dr. Sylvia Ofei was a trainee under the National Institutes of Health T32 Training Grant in Congenital and Acquired Heart Disease sponsored by the Research Institute at Nationwide Children’s Hospital and the Davis Heart and Lung research Institute at The Ohio State University (Grant: 5 T32 HL 98039–5).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST: None
REFERENCES
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26(3):240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choussat A, Fontan F, Besse P, Vallot F, Chauve A, Bricuad H. Selection criteria for Fontan procedure In: Anderson F, Shinebourne E, editors. Pediatric cardiology. White Plains: Churchill Livingstone; 1978. p. 599–66. [Google Scholar]
- 3.Rychik J, Veldtman G, Rand E, et al. The precarious state of the liver after a Fontan operation: summary of a multidisciplinary symposium. Pediatric cardiology. 2012;33(7):1001–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu FM, Jonas MM, Opotowsky AR, et al. Portal and centrilobular hepatic fibrosis in Fontan circulation and clinical outcomes. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34(7):883–91. doi: 10.1016/j.healun.2015.01.993. [DOI] [PubMed] [Google Scholar]
- 5.Greenway SC, Crossland DS, Hudson M, et al. Fontan-associated liver disease: Implications for heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2016;35(1):26–33. doi: 10.1016/j.healun.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Redington A The physiology of the Fontan circulation. Progress in Pediatric Cardiology. 2006;22(2):179–86. doi: doi: 10.1016/j.ppedcard.2006.07.007. [DOI] [Google Scholar]
- 7.Schwartz MC, Sullivan L, Cohen MS, et al. Hepatic pathology may develop before the Fontan operation in children with functional single ventricle: An autopsy study. J Thorac Cardiov Sur. 2012;143(4):904–9. doi: DOI 10.1016/j.jtcvs.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 8.Wu FM, Ukomadu C, Odze RD, Valente AM, Mayer JE Jr., Earing MG. Liver disease in the patient with Fontan circulation. Congenital heart disease. 2011;6(3):190–201. doi: 10.1111/j.1747-0803.2011.00504.x. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz MC, Sullivan LM, Glatz AC, et al. Portal and sinusoidal fibrosis are common on liver biopsy after Fontan surgery. Pediatric cardiology. 2013;34(1):135–42. doi: 10.1007/s00246-012-0402-9. [DOI] [PubMed] [Google Scholar]
- 10.Asrani SK, Asrani NS, Freese DK, et al. Congenital heart disease and the liver. Hepatology. 2012;56(3):1160–9. doi: 10.1002/hep.25692. [DOI] [PubMed] [Google Scholar]
- 11.Hsia TY, Khambadkone S, Redington AN, Migliavacca F, Deanfield JE, de Leval MR. Effects of respiration and gravity on infradiaphragmatic venous flow in normal and Fontan patients. Circulation. 2000;102(19 Suppl 3):III148–53. [DOI] [PubMed] [Google Scholar]
- 12.Elder RW, McCabe NM, Veledar E, et al. Risk factors for major adverse events late after Fontan palliation. Congenital heart disease. 2015;10(2):159–68. doi: 10.1111/chd.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiesewetter CH, Sheron N, Vettukattill JJ, et al. Hepatic changes in the failing Fontan circulation. Heart. 2007;93(5):579–84. doi: 10.1136/hrt.2006.094516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furukawa T, Akimoto K, Ohtsuki M, et al. Non-invasive assessment of liver fibrosis in patients after the Fontan operation. Pediatrics international : official journal of the Japan Pediatric Society. 2011;53(6):980–4. doi: 10.1111/j.1442-200X.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 15.Kutty SS, Peng Q, Danford DA, et al. Increased hepatic stiffness as consequence of high hepatic afterload in the Fontan circulation: a vascular Doppler and elastography study. Hepatology. 2014;59(1):251–60. doi: 10.1002/hep.26631. [DOI] [PubMed] [Google Scholar]
- 16.Canas T, Macia A, Munoz-Codoceo RA, et al. Hepatic and Splenic Acoustic Radiation Force Impulse Shear Wave Velocity Elastography in Children with Liver Disease Associated with Cystic Fibrosis. BioMed research international. 2015;2015:517369. doi: 10.1155/2015/517369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Onofrio M, Crosara S, De Robertis R, et al. Acoustic radiation force impulse of the liver. World journal of gastroenterology : WJG. 2013;19(30):4841–9. doi: 10.3748/wjg.v19.i30.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yap WW, Kirke R, Yoshida EM, Owen D, Harris AC. Non-invasive assessment of liver fibrosis using ARFI with pathological correlation, a prospective study. Annals of hepatology. 2013;12(4):608–15. [PubMed] [Google Scholar]
- 19.Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver international : official journal of the International Association for the Study of the Liver. 2013;33(8):1138–47. doi: 10.1111/liv.12240. [DOI] [PubMed] [Google Scholar]
- 20.Melero-Ferrer JL, Osa-Saez A, Buendia-Fuentes F, et al. Fontan Circulation in Adult Patients: Acoustic Radiation Force Impulse Elastography as a Useful Tool for Liver Assessment. World journal for pediatric & congenital heart surgery. 2014;5(3):365–71. doi: 10.1177/2150135114530172. [DOI] [PubMed] [Google Scholar]
- 21.Ratziu V, Massard J, Charlotte F, et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC gastroenterology. 2006;6:6. doi: 10.1186/1471-230X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong WK, Lim HK, Lee HK, Jo JM, Kim Y. Principles and clinical application of ultrasound elastography for diffuse liver disease. Ultrasonography. 2014;33(3):149–60. doi: 10.14366/usg.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cardenas E, Sanchez-Avila F, Vargas-Vorackova F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Annals of hepatology. 2008;7(4):350–7. [PubMed] [Google Scholar]
- 24.Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53(3):726–36. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 25.Stanton RE, Lurie PR, Lindesmith GG, Meyer BW. The fontan procedure for tricuspid atresia. Circulation. 1981;64(2 Pt 2):II140–6. [PubMed] [Google Scholar]
- 26.Assenza G, Graham D, Landzberg M, et a. MELD-XI score and cardiac mortality or transplantation in patients after Fontan surgery. Heart. 2013;99:491–6. [DOI] [PubMed] [Google Scholar]
- 27.Elder R, McCabe N, Hebson C, et a. Features of portal hypertension are associated with major adverse events in Fontan patients: the VAST study. Int J Cardiol. 2013;168:3764–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. Journal of hepatology. 2008;48(6):1000–7. doi: 10.1016/j.jhep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz MC, Glatz AC, Daniels K, et al. Hepatic Abnormalities Are Present Before and Early After the Fontan Operation. The Annals of thoracic surgery. 2015;100(6):2298–304. doi: 10.1016/j.athoracsur.2015.06.071. [DOI] [PubMed] [Google Scholar]
- 30.Deal BJ, Jacobs ML. Management of the failing Fontan circulation. Heart. 2012;98(14):1098–104. doi: 10.1136/heartjnl-2011-301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ofei SY, Gariepy C, Hanje J, Sisk T, Daniels CJ, Zaidi AN. Liver fibrosis in adults with Fontan palliation: Do common screening studies predict disease severity? Int J Cardiol. 2015;181:174–5. doi: 10.1016/j.ijcard.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 32.Bradley E, Hendrickson B, Daniels C. Fontan Liver Disease: Review of an Emerging Epidemic and Management Options. Current treatment options in cardiovascular medicine. 2015;17(11):51. doi: 10.1007/s11936-015-0412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallihan DB, Podberesky DJ, Marino BS, Sticka JS, Serai S. Relationship of MR elastography determined liver stiffness with cardiac function after Fontan palliation. J Magn Reson Imaging. 2014;40(6):1328–35. doi: 10.1002/jmri.24496. [DOI] [PubMed] [Google Scholar]
- 34.Wallihan DB, Podberesky DJ. Hepatic pathology after Fontan palliation: spectrum of imaging findings. Pediatric radiology. 2013;43(3):330–8. doi: 10.1007/s00247-012-2531-y. [DOI] [PubMed] [Google Scholar]
- 35.Wolff D, van Melle JP, Dijkstra H, et al. The Fontan circulation and the liver: A magnetic resonance diffusion-weighted imaging study. Int J Cardiol. 2016;202:595–600. doi: 10.1016/j.ijcard.2015.09.088. [DOI] [PubMed] [Google Scholar]
- 36.Wu FM, Opotowsky AR, Raza R, et al. Transient elastography may identify Fontan patients with unfavorable hemodynamics and advanced hepatic fibrosis. Congenital heart disease. 2014;9(5):438–47. doi: 10.1111/chd.12159. [DOI] [PubMed] [Google Scholar]
- 37.Tapper EB, Lok AS. Use of Liver Imaging and Biopsy in Clinical Practice. N Engl J Med. 2017;377(8):756–68. doi: 10.1056/NEJMra1610570. [DOI] [PubMed] [Google Scholar]
- 38.Friedrich-Rust M, Koch C, Rentzsch A, et al. Noninvasive assessment of liver fibrosis in patients with Fontan circulation using transient elastography and biochemical fibrosis markers. The Journal of thoracic and cardiovascular surgery. 2008;135(3):560–7. doi: 10.1016/j.jtcvs.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 39.Kutty S, Zhang M, Danford D, et al. Hepatic stiffness in the bidirectional cavopulmonary circulation: The Liver Adult-Pediatric-Congenital-Heart-Disease Dysfunction Study group. The Journal of thoracic and cardiovascular surgery. 2016:678–84. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz M, Glatz A, Daniels K, et al. Hepatic Abnormalities Are Present Before and Early After the Fontan Operation. The Annals of thoracic surgery. 2015;100:2298–304. [DOI] [PubMed] [Google Scholar]