Abstract
Several new discoveries over the past decade have shown that metabolic syndrome, a cluster of metabolic disorders, including increased visceral obesity, hyperglycemia, hypertension, dyslipidemia and low HDL-cholesterol, is commonly associated with skeletal muscle insulin resistance. More recently, non-alcoholic fatty liver disease (NAFLD) was recognized as an additional condition that is strongly associated with features of metabolic syndrome. While the pathogenesis of skeletal muscle insulin resistance and fatty liver is multifactorial, the role of dysregulated redox signaling has been clearly demonstrated in the regulation of skeletal muscle insulin resistance and NAFLD. In this review, we aim to provide recent updates on redox regulation with respect to (a) pro-oxidant enzymes (e.g. NAPDH oxidase and xanthine oxidase); (b) mitochondrial dysfunction; (c) endoplasmic reticulum (ER) stress; (d) iron metabolism derangements; and (e) gut-skeletal muscle or gut-liver connection in the development of skeletal muscle insulin resistance and NAFLD. Furthermore, we discuss promising new therapeutic strategies targeting redox regulation currently under investigation for the treatment of skeletal muscle insulin resistance and NAFLD.
Keywords: Redox stress, skeletal muscle insulin resistance, non-alcoholic fatty liver disease, mitochondrial dysfunction, ER stress, iron metabolism derangements, gut-skeletal muscle axis, gut-liver axis
Introduction
Metabolic syndrome is a cluster of metabolic disorders, which includes increased visceral obesity, hyperglycemia, dyslipidemia, increased blood pressure and decreased high-density lipoprotein (HDL)-cholesterol. In the last decade, skeletal muscle insulin resistance has been considered to be the major pathological condition associated with features of metabolic syndrome and the subsequent development of type 2 diabetes along with the accompanying complications. More recently, non-alcoholic fatty liver disease (NAFLD) was recognized as an additional condition that is closely associated with features of metabolic syndrome. While the pathogenesis of skeletal muscle insulin resistance and fatty liver is multifactorial, redox stress resulting from overproduction of reactive oxygen species (ROS) and reactive nitrogen species (RNS) is rapidly gaining wide recognition for its role in the regulation of metabolic syndrome and the subsequent development of type 2 diabetes, together with the associated cardiovascular diseases. In this review, we discuss recent advances in the involvement of dysregulated redox regulation in the pathophysiology of skeletal muscle insulin resistance and NAFLD, with a focus on pro-oxidant and antioxidant enzymes, mitochondrial dysfunction, endoplasmic reticulum (ER) stress, aberrant iron metabolism and dysbiosis of gut microbiota. We also highlight potential therapeutic targets and provide insights into the role of antioxidants and drugs currently under investigation for the treatment of skeletal muscle insulin resistance and NAFLD. This information is timely and important because it enables awareness of the mediation of redox regulation in two major metabolic regulatory organs for glucose and lipid metabolism, and paves avenue to the development of potential therapeutic interventions.
Redox regulation of skeletal muscle insulin resistance
Skeletal muscle tissue is a primary tissue responsible for 70-90% of total body insulin-stimulated glucose uptake and oxidative metabolism. It is well established that skeletal muscle insulin resistance and impaired glucose metabolism, both due in part to reduced insulin action and glucose uptake, play a central role in the whole-body insulin resistance, as well as in the subsequent development of metabolic syndrome, type 2 diabetes, NAFLD and the associated cardiovascular diseases. Pathophysiological mechanisms responsible for dysregulated redox signaling, including excessive ROS production, mitochondrial dysfunction, ER stress, iron metabolism derangements and gut-skeletal muscle axis, have been implicated in skeletal muscle insulin resistance (Figure 1). In recent years, NADPH oxidases (Nox) have emerged as the initial and primary source of ROS in skeletal muscle cells [1, 2]. It has been shown that Nox1, Nox2, Nox4, DUOX1 and DUOX2 are expressed in various subcellular compartments of skeletal muscle cells [2]. While Nox2-derived ROS mediates insulin signaling, skeletal muscle glucose transport, calcium release and muscle differentiation under physiological conditions, angiotensin II-induced over-production of Nox2-generated ROS impairs insulin signaling in muscle cells [3]. An increase in Nox2 and other Nox subunits was found in skeletal muscle in a mouse model of diet-induced insulin resistance [1]. In addition, Nox inhibitor apocynin was found to attenuate skeletal muscle insulin resistance in mice with heart failure after myocardial infarction [4]. Most recently, it has been reported that Nox2-generated ROS contributes to skeletal muscle insulin resistance in mice fed a high-fat diet (HFD) and that deficiency of Nox2 restores skeletal muscle insulin sensitivity by improving glucose uptake in skeletal muscle cells, suggesting a direct role for Nox2 in skeletal muscle insulin resistance [5]. These findings may enable the use of specific inhibitors, such as GSK2795039 and NOx2ds-tat, in the treatment of skeletal muscle insulin resistance and metabolic syndrome.
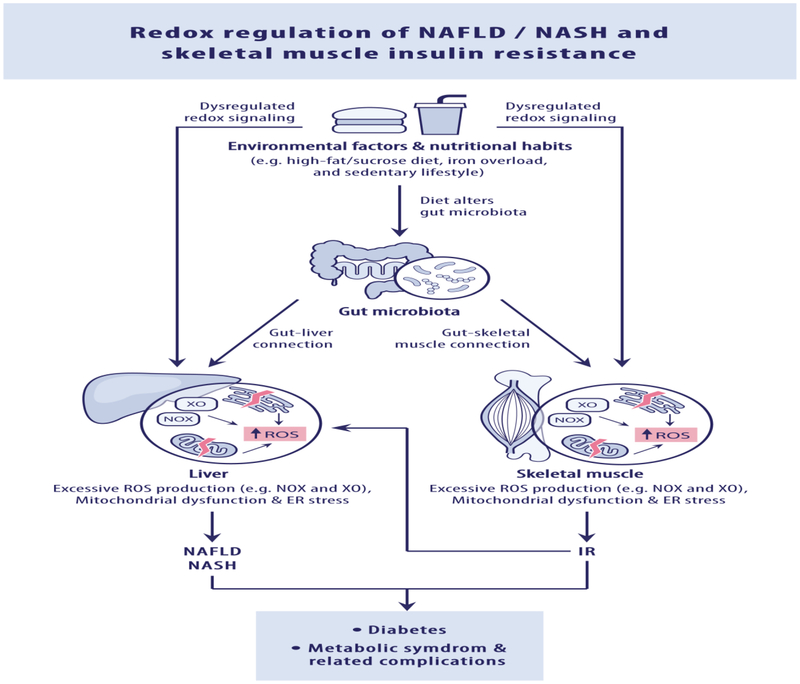
Figure 1: Redox regulation of skeletal muscle insulin resistance and NAFLD/NASH.
Pathophysiological mechanisms responsible for dysregulated redox signaling, including increased pro-oxidant enzymes (e.g. NAPDH oxidase and xanthine oxidase), mitochondrial dysfunction, ER stress, iron metabolism derangements and gut-skeletal muscle or gut-liver connection, in the development of skeletal muscle insulin resistance and non-alcoholic fatty liver disease (NAFLD)/ nonalcoholic steatohepatitis (NASH).
Despite being recognized as major sources of ROS in skeletal muscle, available evidence regarding xanthine oxidase (XO), mitochondrial ROS and phospholipase A2 in the context of skeletal muscle insulin resistance is limited to a small number of studies in recent years. An increase in XO activity has been shown in skeletal muscle of diabetic mice and in patients with diabetes [6, 7]. It has also been found that treatment with XO inhibitor oxypurinol reduces oxidative stress and improves mitochondrial function in mouse models of diabetes induced by streptozotocin and diet [6]. Most recently, a new highly potent XO inhibitor, febuxostat, has been reported to improve skeletal muscle insulin resistance in HFD-treated rats [8]. Nevertheless, it has been shown that high ROS production by XO increases insulin sensitivity, glucose uptake and mitochondrial oxidative activity in skeletal muscle of senescence-accelerated mice, and that a treatment with N-acetylcysteine reverses these phenotypes [9]. Thus, the answer to the question of whether inhibition of XO has benefits in skeletal muscle insulin resistance, however, remains elusive.
While the role of mitochondrial ROS and dysfunction in skeletal muscle insulin resistance remain controversial (for a more detailed recent review, see ref [10]), excessive mitochondrial ROS and mitochondrial dysfunction have been observed in skeletal muscle of patients with type 2 diabetes and in skeletal muscle of mice fed a HFD [1, 11]. Most recently, a mechanism involving at least in part nucleotide-binding oligomerization domain protein-2 (NOD2)-mediated mitochondrial ROS generation has been linked to induction of insulin resistance in skeletal muscle cells [12]. Excessive mitochondrial ROS production and mitochondrial dysfunction have been associated with obesity and profound skeletal muscle insulin resistance in mice which specifically lack estrogen receptor alpha (ERα) in skeletal muscle [13]. Additionally, decreased levels of mitochondrial deacetylase, sirtuin-3 (SIRT3), have been shown to play an important role in skeletal muscle insulin resistance via mitochondrial ROS production [14]. In accord with this observation, decreased levels of SIRT3 in skeletal muscle have been associated with the development of metabolic syndrome-associated pulmonary hypertension. Agents that increase SIRT3 activation in skeletal muscle, such as nitrite and metformin, improved insulin sensitivity and reduced pulmonary pressures in rats with metabolic syndrome-associated pulmonary hypertension. It is worth noting that nitrite-mediated SIRT3 activation in this study required ROS generation [15].
Additionally, mitochondrial inefficiency and increased mitochondrial ROS production induced by hypoxia in skeletal muscle have been shown to improve glucose disposal independent of weight loss or improvement in insulin responsiveness [16]. Interestingly, using DJ-1-deficient mice fed a HFD, a recent study has revealed a novel mechanism by which ROS-induced mitochondrial uncoupling promotes energy expenditure in the skeletal muscle and leads to protection of skeletal muscle insulin resistance and glucose intolerance, presenting an appealing therapeutic potential of mild mitochondrial uncoupling for the treatment of type 2 diabetes together with related metabolic disorders from the skeletal muscle perspective [17].
The literature is currently scarce on the topics related to the influence of ER stress, iron metabolism derangements and gut microbiota in skeletal muscle insulin resistance. ER stress-induced insulin resistance in skeletal muscle has been shown in women with gestational diabetes and/or maternal obesity, and it was found that activation of AMPK protected against the observed phenotypes [18, 19]. Additionally, deletion of tribbles 3 (TRB3), a pseudokinase that is upregulated during the ER stress, has been shown to improve skeletal muscle insulin resistance in HFD-treated mice [20], suggesting inhibition of skeletal muscle TRB3 expression as a new therapeutic strategy for managing insulin resistance. It has also been reported that iron overload mediated insulin resistance in human skeletal muscle cells and iron deprivation enhanced insulin receptor and glucose transporter 4 (GLUT4) transcription, along with increasing protein oxidation levels in skeletal muscle of rats [21, 22]. Human data indicate that gut microbes and high levels of gut oxidative stress are related to a predisposition for diabetic complications [23]. While the gut microbes are restricted to the gut, it has been shown that the extracellular vesicles secreted by the gut microbes induce insulin resistance by impairing glucose metabolism in skeletal muscle of mice fed a HFD [24]. This provides a new insight into pathogenesis of type 2 diabetes and its related complications in the context of the gut-muscle crosstalk.
Redox regulation of NAFLD
NAFLD is defined as the presence of cytoplasmic lipid droplets in more than 5% of hepatocytes in the absence of excessive alcohol consumption (14 drinks/week for men and 7 drinks/week for women). It is one of the most prevalent chronic liver diseases and has posed a significant threat in public health. The worldwide prevalence ranges from 20% to 30%, with nearly 100 million individuals affected in the US [25]. NAFLD is associated with increased risk for diabetes, cardiovascular disease, chronic kidney disease, cirrhosis, hepatocellular carcinoma and both all-cause and liver mortality [26]. Nonalcoholic Steatohepatitis (NASH), a severe form of NAFLD, is characterized by hepatic steatosis with the concurrent histological manifestation of lobular inflammation and ballooning degeneration. NASH is associated with higher risk of advanced liver disease and has become the leading cause of liver transplantation in the US.
The pathophysiology of NAFLD is complex. NAFLD and NASH are strongly associated with obesity, insulin resistance and dyslipidemia, along with chronic systemic oxidative stress [27]. A previous study has suggested a “multiple parallel-hit model” as the main mechanism for the pathogenesis of NAFLD [28]. In this model, multiple conditions have synergistically influence on individuals who have genetic predisposition for NAFLD. Insulin resistance, a key factor, promotes hepatic de novo lipogenesis, adipose tissue dysfunction and fatty acid export impairment, which lead to the accumulation of fat in the liver [29, 30]. The deposition of triglyceride and other lipid metabolites renders the tissue sensitive to the insults of oxidative stress and other lipotoxic mechanisms. Additionally, excessive ROS production that increase redox stress, which in turn triggers mitochondrial dysfunction, ER stress, inflammation and the inability of hepatocytes to synthesize endogenous antioxidant, has been clearly demonstrated in the regulation of NAFLD. Other factors such as altered iron metabolism, high levels of gut oxidative stress, genetic predisposition and epigenetic alterations also play a pivotal role in regulating the fat content and inflammatory state of liver (Figures 1 and 2).
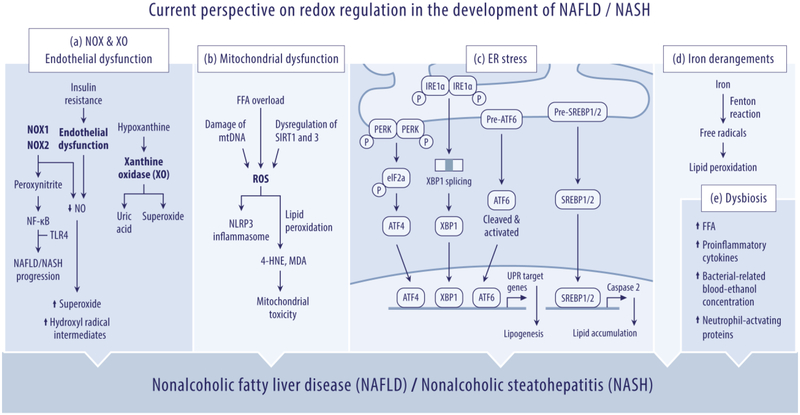
Figure 2: Current perspective on redox regulation in the development of NAFLD/NASH.
Current view on the conditions of the “multiple parallel-hit model” that synergistically influences the pathogenesis of NAFLD/NASH with respect to (a) NOX, XO and endothelial dysfunction; (b) mitochondrial dysfunction; (c) endoplasmic reticulum (ER) stress; (d) iron metabolism derangements; and (e) dysbiosis of gut microbiota.
Multiple new studies in the recent years have demonstrated evidence that Nox and XO are the predominant intracellular source of ROS in the liver. It has been shown that Nox1 and Nox2 are increased in the liver of NASH patients and that levels of serum alanine aminotransferase (ALT), a marker of NAFLD, are decreased in Nox1 and Nox2 KO mice fed a high fat/cholesterol diet or a HFD [31-33], suggesting a key role for Nox1 and Nox2 in NAFLD and NASH. It has also been reported that generation of peroxynitrite by ROS derived from Nox1 and Nox2 can trigger hepatocellular injury and Kupffer cell activation in mouse models of NAFLD [31, 33]. Nox-mediated peroxynitrite was also found to up-regulate NF-κB activity and lead to NASH progression via recruiting TLR4 into lipid rafts [34]. Moreover, an increase in XO activity has been shown in rat model of NAFLD induced by HFD [35]. Administration of XO inhibitor, febuxostat, suppresses the development of NASH induced by trans-fatty acid-contained HFD [36]. These findings may enable the use of specific inhibitors of Nox1, Nox2, XO, as well as dark chocolate, which has been shown to downregulate Nox2 in patients with NASH [37], in the treatment of NAFLD and NASH.
Mitochondria are one of the key players in the hepatic lipid homeostasis. Beta-oxidation of fatty acid in mitochondria is a major process for energy production. Mitochondrial dysfunction could be categorized as primary and acquired. Primary mitochondrial dysfunction includes damage of mitochondrial DNA and dysregulation of sirtuins, a group of NAD(+)-dependent deacetylases [38]. In addition to the known role of SIRT1 in NAFLD, decreased levels of SIRT3 have recently been shown in livers from mice with diet-induced NAFLD [39]. It has also been found that overexpression of SIRT3 prevents diet-mediated NAFLD through ERK-CREB-Bnip3-regulated mitophagy, suggesting a potential strategy of SIRT3-targeting therapy in the treatment of NAFLD [39]. Acquired mitochondrial dysfunction can be triggered by overload of free fatty acids (FFAs), which causes an increased permeability of inner membrane of mitochondria and impairment of ATP synthesis process [40]. Both primary and acquired mitochondrial dysfunction block the fatty acid β-oxidation and promote the production of ROS. Excessive ROS instigate lipid peroxidation, a process in which lipids containing carbon-carbon double bond(s) are attacked by free radicals, with subsequent formation of carbonyl-containing molecules such as 4-hydroxy-2-nonenal (4-HNE) and malondialdehyde (MDA) [41], 4-HNE and MDA are highly reactive aldehydes and are toxic to mitochondria and activators of hepatic stellate cells, a predominant player in liver fibrogenesis. In addition, mitochondrial ROS trigger the activation of NLRP3 inflammasome [42], which is related to liver inflammation and fibrosis in mouse model of NASH [43].
ER has a central role in protein and lipid biosynthesis. The accumulation of saturated fatty acids undermines the ER homeostasis and triggers the ER stress, which is associated with NAFLD in many aspects. In addition to inducing lipogenesis, decreasing liver insulin sensitivity and activating nuclear factor erythroid 2-related factor 2 (Nrf2) (for a more detailed recent review, see ref [44]), a recent study has revealed a new mechanism by which ER stress causes lipogenesis and NASH development via caspase-2 activation of sterol regulatory element-binding protein 1 and 2 (SREBP1/2) [45]. Ablation of caspase-2 or pharmacological intervention with caspase-2 was found to prevent diet-induced NASH progression in ER stress-prone mice, thus presenting a new opportunity for the inhibition of caspase-2 in prevention or treatment of ER stress-driven fatty liver disease. Furthermore, in order to restore ER homeostasis, unfolded protein response (UPR) is activated. UPR is energy consuming and could further aggravate the dysfunctional status of mitochondria. Mechanistically, recent studies have shown that various pathways of UPR signaling network, including PERK/eIF2α/ATF4, IRE1α–XBP1 and activating transcription factor 6 (ATF6), regulate the lipid metabolism and play a role in lipid accumulation [46-48] (Figure 2). A detrimental role of activating transcription factor 3 (ATF3) induced by ER stress or excessive ROS has also been shown to be associated with the development of hepatic steatosis and type 2 diabetes in Zucker diabetic fatty rats and in patients with NAFLD [49], Given that in vivo ATF3 silencing was found to reduce ER stress-mediated hepatic steatosis and glucose intolerance, ATF3-targeting may be a potential strategy for prevention and management of NAFLD and type 2 diabetes.
The literature is currently scarce on the influence of iron metabolism derangements and gut microbiota in the development of NAFLD. Iron catalyzes the production of free radicals through Fenton reaction, which results in oxidative stress and lipid peroxidation. An increase in serum ferritin levels has been shown in patients with NAFLD [50]. It has also been reported that iron overload induced oxidative stress and promoted liver steatosis in rats fed an iron-rich diet, suggesting iron depletion might be beneficial for the treatment of NAFLD [51]. Nevertheless, a recent meta-analysis study demonstrated that iron depletion by phlebotomy does not improve insulin resistance, serum ALT levels and liver histology in patients with NAFLD [52]. Thus, further investigations are required to determine clearly the relationship between iron overload/depletion and NAFLD. High levels of gut oxidative stress and inflammasome-mediated dysbiosis have been linked recently to the progression of NAFLD [53, 54]. It has been shown that microbiomes rich in ethanol-producing Escherichia may be responsible for elevated blood-ethanol concentration and increased oxidative stress in patients with NASH [55]. It has also been shown that dysbiosis diminished the fermentation of some carbohydrates to produce short-chain fatty acids and increased the production of free fatty acid [56]. This study also emphasized the upregulation of proinflammatory cytokines under dysbiosis status, pinpointing the role of gut microbiota in the development of NAFLD. Conversely, microbiota may abate the protection against obesity brought by fasting induced adipose factor (FIAF), a lipoprotein lipase inhibitor (LPL). Therefore, more studies will be needed to decipher the underlying mechanisms and potential therapeutic targets.
Summary and future perspectives
Unraveling the role of redox stress in the regulation of skeletal muscle insulin resistance and NAFLD has provided new insights in the development of future treatments. In addition to emerging therapeutic approaches/targets discussed above, several experimental and clinical studies have demonstrated that the use of vitamins C, D, E and glutathione (GSH) ameliorates oxidative stress and improves NAFLD and skeletal muscle insulin sensitivity [57-61]. Targeting oxidative stress and mitochondrial dysfunction with the treatment of nitrate and nitrite has also been reported to improve hepatosteatosis, ageing-related liver degeneration and skeletal muscle insulin resistance in experimental studies [62-65]. Moreover, activation of proliferator-activated receptor-gamma (PPAR-γ) by pioglitazone was reported to attenuate oxidative stress and to improve NAFLD and skeletal muscle insulin resistance [66-68]. Therapeutic agents with antioxidative activity, such as apoptosis signal-regulating kinase 1 (ASK1) inhibitor and vascular adhesion protein-1 (VAP-1) inhibitor, are now being evaluated in patients with NASH. Along with recent developments in skeletal muscle insulin resistance and NAFLD, future studies related to redox regulation and mechanisms are still needed to enhance further our understanding of the pathogenesis of skeletal muscle insulin resistance and NAFLD and to provide potential translational options for the treatment of these metabolic disorders and the associated diseases.
Highlights.
Multiple dysregulated redox signaling pathways synergistically influence the development of skeletal muscle insulin resistance and NAFLD.
Targeting of the dysregulated redox signaling networks as a promising new strategy in the treatment of skeletal muscle insulin resistance and NAFLD.
Acknowledgments:
The authors thank Dr. Sergei Snovida for helpful comments on the manuscript and Elfy Chiang for assistance and production of the figures. The writing of this review is supported by National Heart, Lung, And Blood Institute (R01HL142638) and American Heart Association (17SDG33400233).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared.
Declarations of interest: none.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- [1].Bonnard C, Durand A, Peyrol S et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 2008; 118:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ferreira LF, Laitano O. Regulation of NADPH oxidases in skeletal muscle. Free Radic Biol Med 2016; 98:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wei Y, Sowers JR, Nistala R et al. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem 2006; 281:35137–35146. [DOI] [PubMed] [Google Scholar]
- [4].Ohta Y, Kinugawa S, Matsushima S et al. Oxidative stress impairs insulin signal in skeletal muscle and causes insulin resistance in postinfarct heart failure. American journal of physiology. Heart and circulatory physiology 2011; 300:H1637–1644. [DOI] [PubMed] [Google Scholar]
- [5] •.Souto Padron de Figueiredo A, Salmon AB, Bruno F et al. Nox2 mediates skeletal muscle insulin resistance induced by a high fat diet. J Biol Chem 2015; 290:13427–13439.Figueiredo et al. examine the relationship between Nox2 and skeletal muscle insulin resistance in mice fed a high-fat diet (HFD). The authors found that Nox2-generated ROS contributes to skeletal muscle insulin resistance and that deficiency of Nox2 restores skeletal muscle insulin sensitivity by improving glucose uptake in skeletal muscle cells.
- [6].Bravard A, Bonnard C, Durand A et al. Inhibition of xanthine oxidase reduces hyperglycemia-induced oxidative stress and improves mitochondrial alterations in skeletal muscle of diabetic mice. Am J Physiol Endocrinol Metab 2011; 300:E581–591. [DOI] [PubMed] [Google Scholar]
- [7].Miric DJ, Kisic BM, Filipovic-Danic S et al. Xanthine Oxidase Activity in Type 2 Diabetes Mellitus Patients with and without Diabetic Peripheral Neuropathy. J Diabetes Res 2016; 2016:4370490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].MORIYA CT, SATOH H, WATADA H. Febuxostat Improves Insulin Resistance in the Skeletal Muscle In Vitro and In Vivo. Diabetes 2018; 67:1927-P. [Google Scholar]
- [9].Barquissau V, Capel F, Dardevet D et al. Reactive oxygen species enhance mitochondrial function, insulin sensitivity and glucose uptake in skeletal muscle of senescence accelerated prone mice SAMP8. Free Radic Biol Med 2017; 113:267–279. [DOI] [PubMed] [Google Scholar]
- [10].Di Meo S, Iossa S, Venditti P. Skeletal muscle insulin resistance: role of mitochondria and other ROS sources. The Journal of endocrinology 2017; 233:R15–R42. [DOI] [PubMed] [Google Scholar]
- [11].Szendroedi J, Schmid AI, Meyerspeer M et al. Impaired mitochondrial function and insulin resistance of skeletal muscle in mitochondrial diabetes. Diabetes Care 2009; 32:677–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maurya CK, Arha D, Rai AK et al. NOD2 activation induces oxidative stress contributing to mitochondrial dysfunction and insulin resistance in skeletal muscle cells. Free Radic Biol Med 2015; 89:158–169. [DOI] [PubMed] [Google Scholar]
- [13].Ribas V, Drew BG, Zhou Z et al. Skeletal muscle action of estrogen receptor alpha is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med 2016; 8:334ra354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jing E, Emanuelli B, Hirschey MD et al. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proceedings of the National Academy of Sciences of the United States of America 2011; 108:14608–14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lai YC, Tabima DM, Dube JJ et al. SIRT3-AMP-Activated Protein Kinase Activation by Nitrite and Metformin Improves Hyperglycemia and Normalizes Pulmonary Hypertension Associated With Heart Failure With Preserved Ejection Fraction. Circulation 2016; 133:717–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ioja S, Singamsetty S, Corey C et al. Nocturnal Hypoxia Improves Glucose Disposal, Decreases Mitochondrial Efficiency, and Increases Reactive Oxygen Species in the Muscle and Liver of C57BL/6J Mice Independent of Weight Change. Oxid Med Cell Longev 2018; 2018:9649608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17] ••.Shi SY, Lu SY, Sivasubramaniyam T et al. DJ-1 links muscle ROS production with metabolic reprogramming and systemic energy homeostasis in mice. Nat Commun 2015; 6:7415.Shi et al. reveal a novel mechanism by which ROS-induced mitochondrial uncoupling promotes energy expenditure in the skeletal muscle and leads to protection of skeletal muscle insulin resistance and glucose intolerance in DJ-1-deficient mice fed a HFD. This study suggests that promoting mitochondrial uncoupling may be a potential strategy for the treatment of obesity-associated metabolic disorders from the skeletal muscle perspective.
- [18].Liong S, Lappas M. Endoplasmic reticulum stress regulates inflammation and insulin resistance in skeletal muscle from pregnant women. Mol Cell Endocrinol 2016; 425:11–25. [DOI] [PubMed] [Google Scholar]
- [19].Saktiawati AM, Sturkenboom MG, Stienstra Y et al. Impact of food on the pharmacokinetics of first-line anti-TB drugs in treatment-naive TB patients: a randomized cross-over trial. J Antimicrob Chemother 2016; 71:703–710. [DOI] [PubMed] [Google Scholar]
- [20].Koh HJ, Toyoda T, Didesch MM et al. Tribbles 3 mediates endoplasmic reticulum stress-induced insulin resistance in skeletal muscle. Nat Commun 2013; 4:1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cui R, Choi SE, Kim TH et al. Iron overload by transferrin receptor protein 1 regulation plays an important role in palmitate-induced insulin resistance in human skeletal muscle cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2018:fj201800448R. [DOI] [PubMed] [Google Scholar]
- [22].Mehdad A, Campos NA, Arruda SF, Siqueira EM. Iron Deprivation May Enhance Insulin Receptor and Glut4 Transcription in Skeletal Muscle of Adult Rats. J Nutr Health Aging 2015; 19:846–854. [DOI] [PubMed] [Google Scholar]
- [23].Qin J, Li Y, Cai Z et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012; 490:55–60. [DOI] [PubMed] [Google Scholar]
- [24].Choi Y, Kwon Y, Kim DK et al. Gut microbe-derived extracellular vesicles induce insulin resistance, thereby impairing glucose metabolism in skeletal muscle. Scientific reports 2015; 5:15878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Younossi Z, Anstee QM, Marietti M et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15:11–20. [DOI] [PubMed] [Google Scholar]
- [26].Zeb I, Li D, Budoff MJ et al. Nonalcoholic Fatty Liver Disease and Incident Cardiac Events: The Multi-Ethnic Study of Atherosclerosis. Journal of the American College of Cardiology 2016; 67:1965–1966. [DOI] [PubMed] [Google Scholar]
- [27].Del Ben M, Baratta F, Polimeni L, Angelico F. Non-alcoholic fatty liver disease and cardiovascular disease: epidemiological, clinical and pathophysiological evidences. Intern Emerg Med 2012; 7 Suppl 3:S291–296. [DOI] [PubMed] [Google Scholar]
- [28].Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016; 65:1038–1048. [DOI] [PubMed] [Google Scholar]
- [29].Flannery C, Dufour S, Rabol R et al. Skeletal muscle insulin resistance promotes increased hepatic de novo lipogenesis, hyperlipidemia, and hepatic steatosis in the elderly. Diabetes 2012; 61:2711–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schwarz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr 2003; 77:43–50. [DOI] [PubMed] [Google Scholar]
- [31].Matsumoto M, Zhang J, Zhang X et al. The NOX1 isoform of NADPH oxidase is involved in dysfunction of liver sinusoids in nonalcoholic fatty liver disease. Free Radic Biol Med 2018; 115:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Garcia-Ruiz I, Solis-Munoz P, Fernandez-Moreira D et al. NADPH oxidase is implicated in the pathogenesis of oxidative phosphorylation dysfunction in mice fed a high-fat diet. Scientific reports 2016; 6:23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chatterjee S, Ganini D, Tokar EJ et al. Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental non-alcoholic steatohepatitis. J Hepatol 2013; 58:778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Das S, Alhasson F, Dattaroy D et al. NADPH Oxidase-Derived Peroxynitrite Drives Inflammation in Mice and Human Nonalcoholic Steatohepatitis via TLR4-Lipid Raft Recruitment. Am J Pathol 2015; 185:1944–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang B, Yang RN, Zhu YR et al. Involvement of xanthine oxidase and paraoxonase 1 in the process of oxidative stress in nonalcoholic fatty liver disease. Mol Med Rep 2017; 15:387–395. [DOI] [PubMed] [Google Scholar]
- [36].Nakatsu Y, Seno Y, Kushiyama A et al. The xanthine oxidase inhibitor febuxostat suppresses development of nonalcoholic steatohepatitis in a rodent model. Am J Physiol Gastrointest Liver Physiol 2015; 309:G42–51. [DOI] [PubMed] [Google Scholar]
- [37].Loffredo L, Del Ben M, Perri L et al. Effects of dark chocolate on NOX-2-generated oxidative stress in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2016; 44:279–286. [DOI] [PubMed] [Google Scholar]
- [38].Ding RB, Bao J, Deng CX. Emerging roles of SIRT1 in fatty liver diseases. Int J Biol Sci 2017; 13:852–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li R, Xin T, Li D et al. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: The role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol 2018; 18:229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Grattagliano I, de Bari O, Bernardo TC et al. Role of mitochondria in nonalcoholic fatty liver disease--from origin to propagation. Clin Biochem 2012; 45:610–618. [DOI] [PubMed] [Google Scholar]
- [41].Ucar F, Sezer S, Erdogan S et al. The relationship between oxidative stress and nonalcoholic fatty liver disease: Its effects on the development of nonalcoholic steatohepatitis. Redox Rep 2013; 18:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Heid ME, Keyel PA, Kamga C et al. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J Immunol 2013; 191:5230–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43] •.Mridha AR, Wree A, Robertson AAB et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 2017; 66:1037–1046.Heid et al. examine the role of inflammasome (NLRP3) activation in the development of NASH. The authors found that treatment with MCC950, an NLRP3 selective inhibitor, improved NAFLD pathology and fibrosis in obese diabetic mice. This study highlights targeting of NLRP3 as a logical direction towards pharmacotherapy of NASH.
- [44].Zhang XQ, Xu CF, Yu CH et al. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol 2014; 20:1768–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45] ••.Kim JY, Garcia-Carbonell R, Yamachika S et al. ER Stress Drives Lipogenesis and Steatohepatitis via Caspase-2 Activation of S1P. Cell 2018; 175:133–145 ell5.Kim et al. reveal a new mechanism by which ER stress causes lipogenesis and NASH development via caspase-2 activation of sterol regulatory element-binding protein 1 and 2 (SREBP1/2). The athours found that caspase-2 inhibition offers a specific and effective strategy for prevention or treatment of stress-driven fatty liver diseases.
- [46].Wang S, Chen Z, Lam V et al. IRE1alpha-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metab 2012; 16:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Xiao G, Zhang T, Yu S et al. ATF4 protein deficiency protects against high fructose-induced hypertriglyceridemia in mice. J Biol Chem 2013; 288:25350–25361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yamamoto K, Takahara K, Oyadomari S et al. Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol Biol Cell 2010; 21:2975–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kim JY, Park KJ, Hwang JY et al. Activating transcription factor 3 is a target molecule linking hepatic steatosis to impaired glucose homeostasis. J Hepatol 2017; 67:349–359. [DOI] [PubMed] [Google Scholar]
- [50].Hagstrom H, Nasr P, Bottai M et al. Elevated serum ferritin is associated with increased mortality in non-alcoholic fatty liver disease after 16 years of follow-up. Liver Int 2016; 36:1688–1695. [DOI] [PubMed] [Google Scholar]
- [51].Valenzuela R, Rincon-Cervera MA, Echeverria F et al. Iron-induced pro-oxidant and pro-lipogenic responses in relation to impaired synthesis and accretion of long-chain polyunsaturated fatty acids in rat hepatic and extrahepatic tissues. Nutrition 2018; 45:49–58. [DOI] [PubMed] [Google Scholar]
- [52].Murali AR, Gupta A, Brown K. Systematic review and meta-analysis to determine the impact of iron depletion in dysmetabolic iron overload syndrome and non-alcoholic fatty liver disease. Hepatol Res 2018; 48:E30–E41. [DOI] [PubMed] [Google Scholar]
- [53] •.Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol 2018; 68:280–295.This recent review provides the overview of the modifications to the microbiota by lipotoxic agents in NAFLD/NASH pathogenesis. It also summarises the literature addressing the cellular and molecular players involved in the cross-talk between the gut and the liver in the development of NAFLD/NASH.
- [54].Henao-Mejia J, Elinav E, Jin C et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012; 482:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhu L, Baker SS, Gill C et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 2013; 57:601–609. [DOI] [PubMed] [Google Scholar]
- [56].Rodriguez-Carrio J, Salazar N, Margolles A et al. Free Fatty Acids Profiles Are Related to Gut Microbiota Signatures and Short-Chain Fatty Acids. Front Immunol 2017; 8:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sanyal AJ, Chalasani N, Kowdley KV et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010; 362:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mason SA, Della Gatta PA, Snow RJ et al. Ascorbic acid supplementation improves skeletal muscle oxidative stress and insulin sensitivity in people with type 2 diabetes: Findings of a randomized controlled study. Free Radic Biol Med 2016; 93:227–238. [DOI] [PubMed] [Google Scholar]
- [59].Honda Y, Kessoku T, Sumida Y et al. Efficacy of glutathione for the treatment of nonalcoholic fatty liver disease: an open-label, single-arm, multicenter, pilot study. BMC Gastroenterol 2017; 17:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhu CG, Liu YX, Wang H et al. Active form of vitamin D ameliorates non-alcoholic fatty liver disease by alleviating oxidative stress in a high-fat diet rat model. Endocr J 2017; 64:663–673. [DOI] [PubMed] [Google Scholar]
- [61].Benetti E, Mastrocola R, Chiazza F et al. Effects of vitamin D on insulin resistance and myosteatosis in diet-induced obese mice. PloS one 2018; 13:e0189707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ohtake K, Ehara N, Chiba H et al. Dietary nitrite reverses features of postmenopausal metabolic syndrome induced by high-fat diet and ovariectomy in mice. Am J Physiol Endocrinol Metab 2017; 312:E300–E308. [DOI] [PubMed] [Google Scholar]
- [63].Jiang H, Torregrossa AC, Potts A et al. Dietary nitrite improves insulin signaling through GLUT4 translocation. Free Radic Biol Med 2014; 67:51–57. [DOI] [PubMed] [Google Scholar]
- [64].Ohtake K, Nakano G, Ehara N et al. Dietary nitrite supplementation improves insulin resistance in type 2 diabetic KKA(y) mice. Nitric Oxide 2015; 44:31–38. [DOI] [PubMed] [Google Scholar]
- [65].Wang H, Hu L, Li L et al. Inorganic nitrate alleviates the senescence-related decline in liver function. Sci China Life Sci 2018; 61:24–34. [DOI] [PubMed] [Google Scholar]
- [66].van der Veen JN, Lingrell S, Gao X et al. Pioglitazone attenuates hepatic inflammation and fibrosis in phosphatidylethanolamine N-methyltransferase-deficient mice. Am J Physiol Gastrointest Liver Physiol 2016; 310:G526–538. [DOI] [PubMed] [Google Scholar]
- [67].Shang J, Previs SF, Conarello S et al. Phenotyping of adipose, liver, and skeletal muscle insulin resistance and response to pioglitazone in spontaneously obese rhesus monkeys. Am J Physiol Endocrinol Metab 2017; 312:E235–E243. [DOI] [PubMed] [Google Scholar]
- [68].Boettcher E, Csako G, Pucino F et al. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2012; 35:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]