Abstract
Background
Chronic idiopathic constipation (CIC) is a common gastrointestinal disorder in community settings. Limited information exists on its treatment with the prosecretory agents linaclotide and lubiprostone. This retrospective cohort study investigated real-world pharmacotherapy patterns of linaclotide and lubiprostone.
Methods
Patients (≥18 years) with CIC who received linaclotide or lubiprostone between January 2013 and December 2015 were identified in a United States health insurance claims database. Follow-up was from the date of the earliest claim for either drug to the end of continuous enrolment or switch to the alternative agent. Patterns of pharmacotherapy, evidence of irritable bowel syndrome (IBS), and concomitant use of selective serotonin reuptake inhibitors were examined using the International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification codes and National Drug Codes.
Results
In total, 43,164 and 17,743 patients with CIC received linaclotide and lubiprostone, respectively (~80% women, mean age ~47 years). Approximately 40% of subjects (linaclotide: 40.1%; lubiprostone: 37.6%) had evidence of IBS. Over a mean follow-up of 17 months, mean (standard deviation) treatment duration in patients without IBS was 6.6 (7.9) months for linaclotide and 4.5 (6.5) months for lubiprostone. Treatment episodes >180 days were more common with linaclotide (36.1%) than with lubiprostone (23.2%). At 12 months, Kaplan–Meier estimates of switching from lubiprostone to linaclotide and from linaclotide to lubiprostone were 13.4 and 5.6%, respectively. The number of patients receiving serotonin reuptake inhibitors was unchanged with treatment (~22%).
Conclusions
Most patients with CIC receive linaclotide or lubiprostone for <6 months; few remain on therapy for >1 year. Additional research is warranted to understand the potential reason(s) for early discontinuation.
Keywords: chloride channel agonists, constipation, guanylate cyclase-cagonists, lubiprostone, practice patterns
Introduction
Chronic idiopathic constipation (CIC) is a common gastrointestinal disorder characterized by difficult, infrequent, or incomplete bowel movements; the disorder has a significant impact on health-related quality of life.1,2 CIC principally affects adult women,3 with an estimated prevalence of approximately 10–15% in the United States.3 The disease process and clinical symptoms manifest as a result of a disordered gut–brain interaction.4 To meet the Rome IV criteria for CIC, an individual must have experienced at least two of the following symptoms in 25% of defecations in the last 3 months (and have fulfilled insufficient criteria for a diagnosis of irritable bowel syndrome [IBS] with constipation [IBS-C]): hard or lumpy stools; straining with defecation; a sensation of incomplete evacuation; a sensation of anorectal obstruction or blockage; use of maneuvers to assist defecation; and fewer than three bowel movements per week.1 There is significant crossover between the diagnostic criteria for IBS-C and CIC; however, IBS-C is characterized by abdominal pain related to defecation.1 IBS-C is not typically diagnosed in the absence of abdominal pain.1
Conventional treatment options for CIC include lifestyle changes, such as regular physical activity, increased fiber and water intake, dietary modifications, and use of over-the-counter laxatives.5 Prescription therapies are the mainstay of pharmacological treatment for patients who do not respond to lifestyle or dietary modifications over an extended period. Several classes of prescription therapies are available, including stool softeners, stimulant laxatives, and prosecretory agents.6 The prosecretory agents lubiprostone (a chloride-channel activator), linaclotide and plecanatide (guanylate cyclase-C agonists), and prucalopride (a selective 5-hydroxytryptamine [serotonin] type 4 receptor agonist) were approved by the US Food and Drug Administration in 2006, 2012, 2017, and 2018, respectively, for the treatment of CIC in adults.7–10 Linaclotide, lubiprostone, and plecanatide are also indicated for the treatment of IBS-C7–9 and have demonstrated efficacy in randomized controlled trials in reducing abdominal pain and/or discomfort associated with IBS-C.11–13
Abdominal pain in CIC and IBS-C has been attributed to malfunction of the gut–brain axis and is often managed ineffectively by conventional pain medications.14 Thus, other classes of drugs, including anxiolytics and antidepressants, have been used to alleviate pain associated with these disorders, with some success.14–16 Selective serotonin reuptake inhibitors (SSRIs) are of particular interest in this context, given that serotonin mediates several functions in the gut, including bowel motility, and serotoninergic neurotransmission may be abnormal in patients with functional gastrointestinal disorders such as CIC and IBS-C.
Until now, limited information has been available on patterns of pharmacotherapy in patients with CIC in real-world clinical practice in the United States. The objective of this retrospective cohort study was to examine patterns of use of linaclotide and lubiprostone, primarily focusing on duration of treatment and switching between agents, in patients with CIC, with and without IBS, using a large US health insurance claims database. Plecanatide and prucalopride were not approved for use in the United States at the time the study was initiated and therefore were not included in the analysis.
Methods
Study design and data source
Data were obtained from the IQVIA Real-World Data Adjudicated Claims database (formerly IMS PharMetrics Plus) from the United States, which contains information from adjudicated healthcare claims from retail pharmacies, medical providers, and healthcare facilities as well as data on age, sex, and dates of coverage for approximately 100 million covered lives.17 This study evaluated de-identified claims database records and was compliant with the Health Insurance Portability and Accountability Act of 1996 (‘HIPAA-compliant’). This study did not meet the definition of research in human subjects and therefore no patient consent or institutional review board approval was required.
Each retail pharmacy claim includes a National Drug Code (NDC) for the dispensed medication, the dispensing date, the quantity of medication dispensed, and the number of therapy-days supplied. Healthcare facility and medical provider claims include dates of service and diagnosis codes based on the International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification (ICD-9-CM or ICD-10-CM, respectively).
Study population
The study population consisted of all patients aged 18 years or older with evidence of receipt of linaclotide or lubiprostone between January 1, 2013, and December 31, 2015, and no evidence of receipt of either drug prior to January 1, 2013. All study subjects also had to have had at least one healthcare encounter with a diagnosis of a functional digestive disorder, not elsewhere classified, at any time prior to their earliest date of receipt of either study drug (index date). Continuous medical and pharmacy coverage in the 12 months preceding their index date was also required. Patients with less than 12 months of continuous medical and pharmacy coverage prior to their index date or with missing or invalid data in the ‘days supplied’ field on pharmacy claims for linaclotide or lubiprostone were excluded from the study.
Study subjects were identified based on healthcare encounters with an ICD-9-CM diagnosis code of 564.0X (excluding 564.02) or an ICD-10-CM diagnosis code of K59.0X (excluding K59.02; Supplementary Table 1; available at: https://www.drugsincontext.com/wp-content/uploads/2020/08/dic.2020-5-10-Suppl.pdf ). Owing to the absence of a diagnosis code specific to CIC in the ICD-9-CM coding system, the broader set of codes for chronic constipation was used. Receipt of linaclotide or lubiprostone was identified using NDCs on retail pharmacy claims. Patients with evidence of receipt of both drugs during the study period were assigned two index dates, corresponding to the earliest date of receipt of each drug. Follow-up began on the index date and continued until the end of continuous enrolment or the date of a switch from one study drug to the other, whichever came first.
Study measures
The study outcomes were the patterns of use of linaclotide and lubiprostone, including switching from one study drug to the other (i.e. from linaclotide to lubiprostone, if linaclotide was the first agent received, or from lubiprostone to linaclotide, if lubiprostone was the first agent received) as well as adherence to therapy with each agent.
The duration of treatment episodes was defined as the number of days from the index date to the date of the final pharmacy claim for the drug received on the index date, the date of first receipt of the other study drug, or loss to follow-up (whichever came first), plus the number of therapy-days supplied on the final pharmacy claim. The total number of therapy-days supplied was defined as the sum of all therapy-days supplied on pharmacy claims from the index date to the date of the final pharmacy claim. If the end date of therapy with one study drug overlapped the date of initiation of therapy with the other study drug, this was accounted for by truncating the treatment episode of the first drug at the date of initial receipt of the second drug.
Evidence of IBS was defined as any healthcare encounter with an ICD-9-CM or ICD-10-CM diagnosis code for IBS (Supplementary Table 1).
Adherence to therapy was assessed using the proportion of days covered (PDC), defined as the ratio of the number of days of study drug supplied during a treatment episode to the total number of calendar days between the start and end of that episode.
The use of SSRIs and other selected agents commonly employed in conjunction with linaclotide and lubiprostone for the treatment of constipation was also examined during the pre-index and follow-up periods (Supplementary Table 2). Receipt of these drugs was identified using NDCs on retail pharmacy claims. All patients were assessed for evidence of a depressive disorder based on ICD-9-CM or ICD-10-CM diagnosis codes (Supplementary Table 1).
Data analyses
Analyses were primarily descriptive in nature. For continuous variables, means, standard deviations (SDs), medians, and interquartile ranges were reported. Kaplan–Meier methods were used to estimate the rates of switching from one study drug to the other at 12 months.
Analyses of patterns of use of linaclotide and lubiprostone were conducted for all patients who met study inclusion criteria, with additional analyses performed for subgroups with and without evidence of IBS (identified by ICD-9-CM or ICD-10-CM diagnosis codes; Supplementary Table 1). Primary analyses focused on patients with CIC who had no evidence of IBS.
Results
Trends in receipt of linaclotide and lubiprostone
The IQVIA Real-World Data Adjudicated Claims database contains pharmacy and healthcare claims information for approximately 100 million covered lives from 2007 to present in the United States; approximately 47 million individuals annually have both pharmacy and medical coverage.17 From 2012 to 2015, the number of covered lives with CIC increased from 761,066 to 875,251. The proportion of all covered lives with CIC receiving linaclotide increased from 0.02% (n=130) in 2012 to 3.5% (n=30,268) in 2015, while the proportion of patients receiving lubiprostone declined slightly over the same period (from 2.2% [n=16,736] in 2012 to 1.5% [n=12,886] in 2015).
Patterns of use of linaclotide and lubiprostone
Between January 1, 2013, and December 31, 2015, 43,164 and 17,743 patients with CIC received linaclotide and lubiprostone, respectively, and met the study criteria. The mean (SD) age in the two cohorts was 47.1 (13.5) years for linaclotide and 47.5 (14.2) years for lubiprostone; the majority of patients were women (86.1 and 83.0%, respectively; Supplementary Table 3). A total of 3970 patients received both drugs during the study period and therefore contributed data to both cohorts.
Patients with CIC and without evidence of IBS
Over half of all patients with CIC who met study inclusion criteria had no evidence of IBS (59.9% [n=25,843] of those who began treatment with linaclotide and 62.5% [n=11,081] of those who began treatment with lubiprostone). The demographic characteristics of these patients are shown in Table 1. Evidence of prior receipt of the other study drug was similar in both cohorts, with 8.0% (n=2063) of patients in the linaclotide group having previously received lubiprostone and 9.4% (n=1042) of those in the lubiprostone group having previously received linaclotide during the 12-month period preceding the index date.
Table 1.
Baseline characteristics of patients with CIC but no evidence of IBSa.
| Characteristic | Linaclotide (N=25,843) | Lubiprostone (N=11,081) |
|---|---|---|
| Mean (SD) age, years | 47.9 (13.5) | 48.7 (14.2) |
| Women, n (%) | 21,643 (83.8) | 8785 (79.3) |
| Plan type, n (%) | ||
| Commercial | 16,010 (62.0) | 6720 (60.6) |
| State Children’s Health Insurance Program | 5 (<0.1) | 3 (<0.1) |
| Medicare Risk/Medicare Advantage | 266 (1.0) | 206 (1.9) |
| Self-insured | 9562 (37.0) | 4152 (37.5) |
| Region, n (%) | ||
| North-east | 4368 (16.9) | 2023 (18.3) |
| South | 14,305 (55.4) | 5745 (51.9) |
| Midwest | 5333 (20.6) | 2388 (21.6) |
| West | 1728 (6.7) | 824 (7.4) |
| Other | 109 (0.4) | 101 (0.9) |
| Use of CIC-related drugs during pre-index period, n (%) | ||
| Linaclotide | – | 1042 (9.4) |
| Lubiprostone | 2063 (8.0) | – |
| Lactulose | 1317 (5.1) | 627 (5.7) |
| MiraLAX | 8 (<0.1) | 5 (<0.1) |
| Any of the above | 3185 (12.3) | 1580 (14.3) |
| Use of an SSRIb, n (%) | 5763 (22.3) | 2455 (22.2) |
| Mean (SD) follow-up, days | 504.5 (318.9) | 520.6 (341.7) |
Includes patients who began treatment with linaclotide or lubiprostone from January 1, 2013, to December 31, 2015.
Includes any of the following: citalopram, escitalopram, fluoxetine, paroxetine, and sertraline.
CIC, chronic idiopathic constipation; IBS, irritable bowel syndrome; SD, standard deviation; SSRI, selective serotonin reuptake inhibitor.
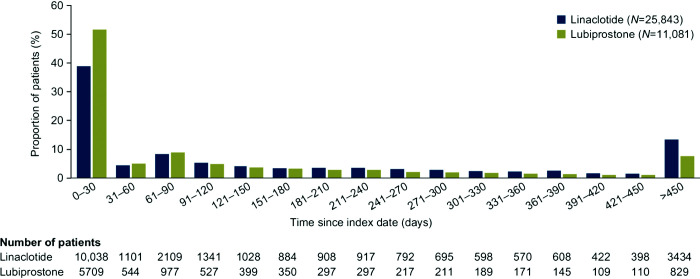
The mean (SD) duration of follow-up was similar between the two cohorts: 16.8 (10.6) months among patients with CIC beginning treatment with linaclotide and 17.4 (11.4) months among those beginning treatment with lubiprostone. The mean (SD) duration of treatment episodes in the linaclotide cohort was 6.6 (7.9) months, and the corresponding value in the lubiprostone cohort was 4.5 (6.5) months. Of those patients beginning treatment with linaclotide, 38.8% (n=10,038) remained on treatment for 0–30 days, whereas for lubiprostone, the corresponding proportion was 51.5% (n=5709) (Figure 1). For linaclotide and lubiprostone, 36.1% (n=9342) and 23.2% (n=2575) of treatment episodes, respectively, were longer than 180 days; the corresponding values for treatment episodes lasting longer than 360 days were 18.8% (n=4862) and 10.8% (n=1193).
Figure 1. Distribution of patients with CIC and without evidence of IBS by duration of treatment.
CIC, chronic idiopathic constipation; IBS, irritable bowel syndrome.
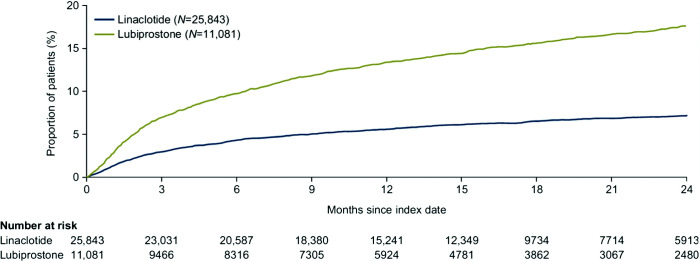
The mean (SD) PDC during treatment episodes was high in both cohorts: 83.0% (25.0%) for linaclotide and 87.0% (22.0%) for lubiprostone. PDC was at least 90% for more than 60% of treatment episodes in both cohorts. Treatment switching by month 12 was more common from lubiprostone to linaclotide than from linaclotide to lubiprostone (13.4 and 5.6%, respectively; Figure 2).
Figure 2. Time to therapy switching in patients with CIC and without evidence of IBS.
CIC, chronic idiopathic constipation; IBS, irritable bowel syndrome.
The duration of treatment episodes and rates of switching from one study drug to the other were similar across the full study cohort (Supplementary Figures 1 and 2).
SSRI use
Fewer than 25% of patients with CIC and without evidence of IBS received SSRIs during the pre-index period (22.3% [n=5763] with linaclotide and 22.2% [n=2455] with lubiprostone). During the pre-index period, escitalopram was the most commonly received SSRI (6.0% of patients in both groups with no evidence of IBS). In the follow-up period, 23.7% (n=6120) and 23.6% (n=2611) of patients in the linaclotide and lubiprostone cohort, respectively, received SSRIs. Of the 6120 patients in the linaclotide cohort who received SSRIs during the follow-up period, 4224 (69.0%) had also received SSRIs in the pre-index period. In the lubiprostone group, of the 2611 patients who were given SSRIs in the follow-up period, 1742 (66.7%) had also received SSRIs in the pre-index period. Approximately 60% of individuals with evidence of receipt of SSRIs during the pre-index and/or follow-up periods (linaclotide, 4731 of 7659 individuals; lubiprostone, 2165 of 3324 individuals) also had a diagnosis of a depressive disorder.
Discussion
This retrospective cohort study examined real-world patterns of pharmacotherapy in patients with CIC initiating treatment with linaclotide or lubiprostone, using a United States health insurance claims database. Key study measures included trends in receipt of, adherence to, and switching between study drugs.
The use of linaclotide among patients with CIC increased between the years 2012 and 2015, while the proportion of individuals receiving lubiprostone declined slightly over the same period. Approximately 60% of study participants in both cohorts had no evidence of IBS. The mean duration of treatment in patients with CIC and without evidence of IBS was 6.6 months for linaclotide and 4.5 months for lubiprostone. Kaplan–Meier estimates of the proportion of patients switching by month 12 from lubiprostone to linaclotide and from linaclotide to lubiprostone were 13.4 and 5.6%, respectively. Early discontinuation was common with both study drugs.
We found no clear differences in the duration of treatment episodes or the frequency of therapy switching between our full study cohort and the subgroup of patients with CIC without evidence of IBS. This contrasts with the findings of Suresh et al., who observed that the presence of IBS may be associated with a higher rate of treatment discontinuation for both linaclotide and lubiprostone (hazard ratio: 1.4; 95% confidence interval: 1.1–1.6; p=0.001).18
The proportion of patients with CIC and without evidence of IBS who received either study drug on only one occasion was relatively high for both drugs, whereas the percentage of individuals who continued taking either drug for at least 360 days was low. Previous studies have demonstrated that diarrhea is a common adverse event occurring after linaclotide and lubiprostone therapy and may result in early discontinuation of treatment.19,20 Additional research is needed to assess the reasons for therapy discontinuation with these drugs. It is also important to note that CIC is an illness with symptoms that wax and wane over time; therefore, treatment discontinuation and re-initiation over time may be expected.21
Our results show that a large proportion of patients in both cohorts (approximately 67%) who were receiving SSRIs before CIC treatment remained on them after initiation of CIC treatment. This suggests that treatment with linaclotide and lubiprostone has no effect on the use of SSRIs.
The limitations of the study include those inherent to database studies. The accuracy of using NDCs and ICD-9-CM/ICD-10-CM codes to identify CIC and IBS is unknown. The likelihood of incorrectly identifying patients is increased by the substantial overlap in symptoms between the two diseases. Furthermore, the absence of an ICD-9-CM code specific to CIC means that codes relating to chronic constipation in general were used – this may have resulted in the identification of patients with chronic constipation with a known cause as opposed to CIC. Similarly, the ICD code for IBS does not differentiate between IBS and IBS-C. Finally, this study was carried out using a United States healthcare claims database; therefore, whether findings from this analysis are applicable in other geographical settings requires further research.
Conclusion
In conclusion, although treatment duration was relatively short in approximately half of patients who initiated therapy with either linaclotide or lubiprostone, a small subgroup of patients remained on therapy for an extended period of time. Findings were largely consistent between the full cohort and the group of patients who had no evidence of IBS. Additional research is needed to determine why some patients discontinue therapy and others do not.
Acknowledgements
The authors wish to thank Margaret Gerbasi, a former employee of Policy Analysis Inc., for her contribution to the study. Medical writing support was provided by Maxine Cox, BSc, of PharmaGenesis London, London, UK.
Footnotes
Contributions: AN is the guarantor for the submission. All authors contributed to the conception and design of the study, analysis or interpretation of the data, preparation of the manuscript, and critical review of the manuscript. All authors have read and approved the final version of the manuscript, including the authorship list. Medical writing support was provided by Maxine Cox, BSc, of PharmaGenesis London, London, UK.
Disclosure and potential conflicts of interest: AN is an employee of Shire, a member of the Takeda group of companies, and is a stockholder of Takeda. RB and GO are employees of Policy Analysis Inc., which received financial support from Shire, a member of the Takeda group of companies, for their participation in the design of the study, the analyses of the data, and the interpretation of findings. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2020/07/dic.2020-5-10-COI.pdf
Funding declaration: Funding support for this study was provided by Shire Human Genetic Therapies Inc., a member of the Takeda group of companies, Indianapolis, IN, USA. Funding for medical writing support was provided by Shire Human Genetic Therapies Inc., a member of the Takeda group of companies, Indianapolis, IN, USA.
Correct attribution: Copyright © 2020 Nag A, Bornheimer R, Oster G. https://doi.org/10.7573/dic.2020-5-10. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Peer review comments to author: 8 June 2020
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393–1407. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 2.Belsey J, Greenfield S, Candy D, Geraint M. Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther. 2010;31(9):938–949. doi: 10.1111/j.1365-2036.2010.04273.x. [DOI] [PubMed] [Google Scholar]
- 3.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(9):1582–1591. doi: 10.1038/ajg.2011.164. [DOI] [PubMed] [Google Scholar]
- 4.Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150(6):1257–1261. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 5.Sharma A, Rao S. Constipation: pathophysiology and current therapeutic approaches. Handb Exp Pharmacol. 2017;239:59–74. doi: 10.1007/164_2016_111. [DOI] [PubMed] [Google Scholar]
- 6.Tack J, Muller-Lissner S. Treatment of chronic constipation: current pharmacologic approaches and future directions. Clin Gastroenterol Hepatol. 2009;7(5):502–508. doi: 10.1016/j.cgh.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 7.US FDA. Lubiprostone product information. [Accessed October 10, 2018]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021908s010lbl.pdf.
- 8.US FDA. [Accessed October 10, 2018];Linaclotide product information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202811s010lbl.pdf. [Google Scholar]
- 9.US FDA. [Accessed December 17, 2018];Plecanatide product information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208745s001lbl.pdf. [Google Scholar]
- 10.US FDA. [Accessed March 5, 2019];Prucalopride product information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210166s000lbl.pdf. [Google Scholar]
- 11.Rao SS, Quigley EM, Shiff SJ, et al. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol. 2014;12(4):616–623. doi: 10.1016/j.cgh.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Brenner DM, Fogel R, Dorn SD, et al. Efficacy, safety, and tolerability of plecanatide in patients with irritable bowel syndrome with constipation: results of two phase 3 randomized clinical trials. Am J Gastroenterol. 2018;113(5):735–745. doi: 10.1038/s41395-018-0026-7. [DOI] [PubMed] [Google Scholar]
- 13.Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome – results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29(3):329–341. doi: 10.1111/j.1365-2036.2008.03881.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Ilham SJ, Feng B. Pharmacological approach for managing pain in irritable bowel syndrome: a review article. Anesth Pain Med. 2017;7(2):e42747. doi: 10.5812/aapm.42747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108(1):65–72. doi: 10.1016/s0002-9343(99)00299-5. [DOI] [PubMed] [Google Scholar]
- 16.Tack J, Broekaert D, Fischler B, Van Oudenhove L, Gevers AM, Janssens J. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut. 2006;55(8):1095–1103. doi: 10.1136/gut.2005.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.IQVIA Real-World Data Adjudicated Claims: USA [QuintilesIMS PharMetrics Plus] 2017. [Accessed January 10, 2019]. https://tri.uams.edu/ims-lifelink/
- 18.Suresh SJ, Stidham RW, Chey WD, Shah ED. Evaluating why and when patients discontinue chronic therapy for IBS with constipation and chronic constipation: a real-world experience. Digestive Disease Week; 2–5 June 2018; Washington, DC, USA. [Google Scholar]
- 19.Lembo AJ, Johanson JF, Parkman HP, Rao SS, Miner PBJ, Ueno R. Long-term safety and effectiveness of lubiprostone, a chloride channel (ClC-2) activator, in patients with chronic idiopathic constipation. Dig Dis Sci. 2011;56(9):2639–2645. doi: 10.1007/s10620-011-1801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107(11):1702–1712. doi: 10.1038/ajg.2012.254. [DOI] [PubMed] [Google Scholar]
- 21.Halder SL, Locke GRr, Schleck CD, Zinsmeister AR, Melton L, Jr, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology. 2007;133(3):799–807. doi: 10.1053/j.gastro.2007.06.010. [DOI] [PubMed] [Google Scholar]