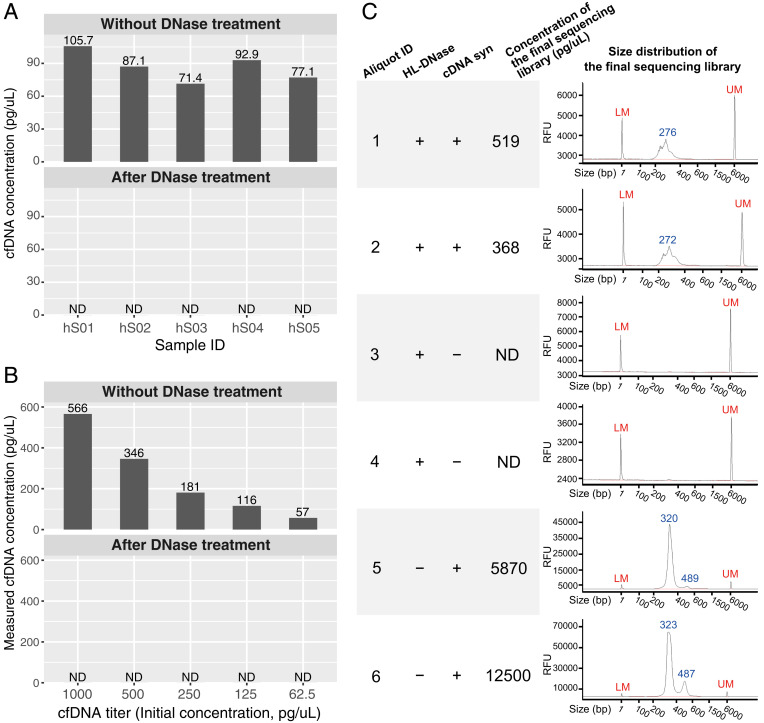
To evaluate any possible DNA contamination in Small Input Liquid Volume Extracellular RNA Sequencing (SILVER-seq), we carried out three types of tests. First, we tested the effectiveness of SILVER-seq’s DNase treatment step. Without DNase treatment, five of the five tested serum samples exhibited detectable cell-free DNA (cfDNA; Fig. 1A). After SILVER-seq’s DNase treatment step, none of the serum samples exhibited any detectable cfDNA (Fig. 1A), suggesting that SILVER-seq’s DNase treatment step thoroughly digested cfDNA in human serum samples.
Fig. 1.
DNA contamination tests. (A) Concentrations of cfDNA in five human serum samples (IDs: hS01 to hS05, columns) before and after SILVER-seq’s DNase treatment step. Each serum sample (column) was split into two 7-µL aliquots which were subjected to no treatment (Upper) or DNase treatment (Lower). ND, not detectable. The detection limit of Qubit’s high-sensitivity kit is 0.5 pg/µL. (B) Measured concentrations of cfDNA titers (columns) before (Upper) and after (Lower) SILVER-seq’s DNase treatment step. The detection limit of Qubit’s high-sensitivity kit is 0.5 pg/µL. (C) Size distributions of six sequencing libraries from six aliquots of the same serum sample (aliquot ID: 1 to 6), with two aliquots (1 and 2) following the intact SILVER-seq protocol (HL-DNase: +, cDNA syn: +), two aliquots (4 and 5) following the complete protocol except the cDNA syn step (HL-DNase: +, cDNA syn: −), and two aliquots (5 and 6) following the complete protocol except the DNA digestion step (HL-DNase: −, cDNA syn: +). The concentrations of the sequencing libraries (fourth column) were reported by Qubit’s high-sensitivity kit. The size distributions (fifth column) were reported by Fragment Analyzer. RFU, relative fluorescence unit; LM, lower marker; UM, upper marker.
Second, we tested whether SILVER-seq’s DNase treatment step can still thoroughly digest cfDNA with initial cfDNA concentrations higher than those of our serum samples. To this end, we retrieved cfDNA from large volumes of human sera and titrated the retrieved cfDNA into different concentrations (Fig. 1B). After SILVER-seq’s DNase treatment step, none of the titers exhibited detectable cfDNA (Fig. 1B). These data suggest that SILVER-seq’s DNase treatment step can thoroughly digest the cfDNA at concentrations higher than those of our tested human sera.
Third, we tested whether serum cfDNA can enter SILVER-seq’s sequencing library. To this end, we skipped the complementary DNA synthesis (cDNA syn) step while keeping every other step in the SILVER-seq protocol. We split a human serum sample into six aliquots. Two aliquots were subjected to the entire SILVER-seq procedure (HL-DNase: +, cDNA syn: +; Fig. 1C). The concentrations and the size distributions of the two final SILVER-seq sequencing libraries were consistent with those in the published SILVER-seq libraries (1). In contrast, the two aliquots with only the cDNA synthesis step skipped (and all other SILVER-seq steps remaining) did not yield any detectable product in the sequencing library (HL-DNase: +, cDNA syn: −; Fig. 1C). These data suggest that, unless RNA was converted to cDNA, there would be no DNA content in SILVER-seq’s sequencing library. The last two aliquots were subjected to the SILVER-seq protocol with the DNase step skipped. The resulting sequencing libraries exhibited larger peak sizes than those generated by the intact SILVER-seq protocol (HL-DNase: −, cDNA syn: +; Fig. 1C), confirming the size differences between circulating extracellular RNA (exRNA) and cfDNA.
We reasoned that a good exRNA measurement technique may be able to reveal a correlation between tissue-specific gene expression and the abundance of circulating exRNAs of these tissue-specific genes. Indeed, the brain expression levels of brain-specific genes correlated with the frequencies of detecting these genes in blood plasma by SILVER-seq. (2). Furthermore, expression changes of transposons in the brain correlated with exRNA changes of these transposons in blood plasma (2). It would be valuable to see whether the correlation found with SILVER-seq can be reproduced by other exRNA sequencing methods (3).
The prevalence of intergenic transcripts, dubbed “dark matter RNA,” was a major discovery of the pilot phase of the Encyclopedia of DNA Elements (ENCODE) project (4–8). Degradation and export of these intergenic transcripts are mediated by RNA exosomes [these are not exosome vehicles (9, 10)], and antibodies of RNA exosomes can be detected in patient sera (11). Therefore, the dark matter RNA in the extracellular space can be an exciting research frontier.
Footnotes
Competing interest statement: S.Z. is a board member and shareholder of Genemo Inc. Z.Z. and S.Z. are inventors of a patent application.
References
- 1.Zhou Z., et al. , Extracellular RNA in a single droplet of human serum reflects physiologic and disease states. Proc. Natl. Acad. Sci. U.S.A. 116, 19200–19208 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan Z., et al. , Presymptomatic increase of an extracellular RNA in blood plasma associates with the development of Alzheimer’s disease. Curr. Biol. 30, 1771–1782.e3 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Verwilt J., et al. , When DNA gets in the way: A cautionary note for DNA contamination in extracellular RNA-seq studies. Proc. Natl. Acad. Sci. U.S.A. 117, 18934–18936 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birney E. et al.; ENCODE Project Consortium; NISC Comparative Sequencing Program; Baylor College of Medicine Human Genome Sequencing Center; Washington University Genome Sequencing Center; Broad Institute; Children’s Hospital Oakland Research Institute , Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447, 799–816 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinstock G. M., ENCODE: More genomic empowerment. Genome Res. 17, 667–668 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Borel C., et al. , Mapping of small RNAs in the human ENCODE regions. Am. J. Hum. Genet. 82, 971–981 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Bakel H., Nislow C., Blencowe B. J., Hughes T. R., Most “dark matter” transcripts are associated with known genes. PLoS Biol. 8, e1000371 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapranov P., St Laurent G., Dark matter RNA: Existence, function, and controversy. Front. Genet. 3, 60 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preker P., et al. , RNA exosome depletion reveals transcription upstream of active human promoters. Science 322, 1851–1854 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Ogami K., Chen Y., Manley J. L., RNA surveillance by the nuclear RNA exosome: Mechanisms and significance. Noncoding RNA 4, 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanke K., et al. , Antibodies against PM/Scl-75 and PM/Scl-100 are independent markers for different subsets of systemic sclerosis patients. Arthritis Res. Ther. 11, R22 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]