Significance
Life provides the best demonstration of complex and adaptive nonequilibrium system, which executes biological functions through nonequilibrium structure transformation. Creating lifelike materials to achieve part of or full biological functions is a grand challenge. Traditional memories from hard and dry materials are static, with no ability of forgetting; here we propose to use soft and wet hydrogels to achieve dynamic memories with spontaneous but learning-strength-dependent forgetting ability by using nonequilibrium structure transformation, in analogy to the human brain. We believe the concept of dynamic memorizing–forgetting behavior proposed here inspires further research on developing lifelike materials based on the nonequilibrium process of soft matter.
Keywords: dynamic memory, spontaneous forgetting, hydrogel, frustrated structure, nonequilibrium process
Abstract
The memory of our brain, stored in soft matter, is dynamic, and it forgets spontaneously to filter unimportant information. By contrast, the existing manmade memory, made from hard materials, is static, and it does not forget without external stimuli. Here we propose a principle for developing dynamic memory from soft hydrogels with temperature-sensitive dynamic bonds. The memorizing–forgetting behavior is achieved based on fast water uptake and slow water release upon thermal stimulus, as well as thermal-history-dependent transparency change of these gels. The forgetting time is proportional to the thermal learning time, in analogy to the behavior of brain. The memory is stable against temperature fluctuation and large stretching; moreover, the forgetting process is programmable. This principle may inspire future research on dynamic memory based on the nonequilibrium process of soft matter.
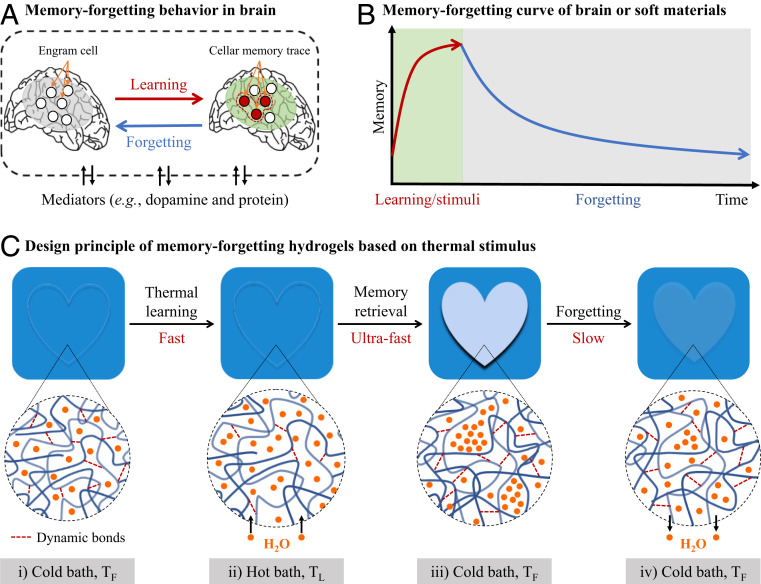
The memorizing–forgetting behavior in the human brain, stored in soft and wet matter, is a dynamic and nonequilibrium process (1–4). The acquisition of information, or learning, forms cellular memory traces in the brain, and the traces spontaneously decay over time (5, 6). It is considered that forgetting may be the default mode of the brain, which filters out noise and unimportant information to achieve high efficiency (4). In this dynamic process, certain mediators such as dopamine and proteins are essential in structuring cellular memory traces (Fig. 1A) (5). By contrast, the existing manmade memories, made from hard and dry materials, work in equilibrium manner, through a quick switch between two phases induced by external energy, such as electric (7) or magnetic energy (8). Different from the brains, no exchange of substances with externals occurs in manmade memories. Consequently, these memories can store information with accuracy and stability, but they are static, with no ability of spontaneous forgetting.
Fig. 1.
Conceptual scheme of dynamic memorizing–forgetting behavior from human to soft materials. (A) Memorizing–forgetting behavior in brain. Certain mediates such as dopamine and protein are involved in the process of forming memory through learning, and the memory decays gradually and spontaneously with time, exhibiting a dynamic forgetting. (B) Memorizing–forgetting curve of brain or soft materials. The memorizing–forgetting behavior of soft materials is based on its nonequilibrium process in response to stimuli. Stimuli-on induces quick formation of memory, which gradually decays with time after stimuli-off. (C) Design principle of dynamic memorizing–forgetting hydrogels based on thermal stimulus. We use a hydrogel containing abundant dynamic bonds. The gel has unique nonequilibrium features: It swells fast in a hot bath (learning temperature TL) and shrinks slowly in a cold bath (forgetting temperature TF). Moreover, the gel instantly turns from transparent to opaque (memory retravel) when being switched from the hot bath to the cold bath due to structure frustration. Consequently, the thermal history of the gel can be memorized and detected by its opaque appearance. With the shrinking progress toward the equilibrium, the gel gradually recovers to transparent, exhibiting a spontaneous and slow forgetting of the thermal history. The forgetting time (tF) in the cold bath is correlated to the thermal learning time (tL) in the hot bath as tF/tL = Dsw/Dsh, where Dsw and Dsh are the cooperative diffusion coefficients corresponding to swelling and shrinking, respectively. The fast water-absorbing and slow-releasing kinetics of the gel, corresponding to Dsw/Dsh >> 1, result in the fast learning and slow forgetting behaviors.
Creating dynamic memory based on out-of-equilibrium process of soft matter, similar to the brains, is a grand challenge. So far, only several attempts were made along this line, mainly in solutions, utilizing sophisticated chemistry and experimental setup (9–12). In this work, we propose a very simple approach to develop dynamic memory that exhibits memorizing–forgetting behavior by utilizing hydrogels, in analogy to the human brain. We show that the hydrogels containing dynamic bonds can encode two-dimensional (2D) information through thermal learning and the information spontaneously forgets at a forgetting time that is proportional to the learning strength (learning time and temperature). The principle of memorizing–forgetting is based on asymmetric water absorption and release of dynamic bond hydrogels in response to thermal stimulus.
Design Principle.
The cognitive view divides the memory process of brain into three stages: encoding, storage, and retrieval (13), while the nature of forgetting is the decay of encoded memory trace (4). To achieve dynamic memorizing–forgetting behavior, our strategy is utilizing asymmetric and spontaneous structure transformation process of soft materials. When a soft material in equilibrium state is applied with a stimulus (learning), the change of thermodynamic environment results in the formation of an excited state (encoding information). When the stimulus is removed, the excited state returns to the initial equilibrium structure (forgetting by decaying of stored information, Fig. 1B). To apply such a general stimuli-responsive process for dynamic memorizing–forgetting, we require a strongly asymmetric but correlated kinetics between encoding and forgetting. That is, a relatively faster kinetics to form the excited structure (encoding or learning time) and a slower kinetics to recover to the original structure (storage or forgetting time); the recovery time (forgetting time) needs to be dependent on stimulus strength (learning strength). Moreover, a mechanism is necessary to facilely retrieve the stored information that decays with time.
Our idea is to use thermal swelling hydrogels to construct soft and dynamic memory. Hydrogels have similar soft and wet nature as well as permeability to small molecules as biological tissues (14), and these materials have been used to mimic muscle growth (15) and associative learning (9). The most common feature specific to hydrogels is the reversible swelling and shrinking in response to various stimuli, such as pH and temperature (16–18). To demonstrate this principle, we consider the swelling and shrinking kinetics of a hydrogel of thickness d in response to thermal stimulus. We use two water baths of different temperatures, TL and TF, for thermal learning and forgetting, respectively, where TL > TF. The gel is initially equilibrated in the cold bath; then it is moved to the hot bath for a thermal learning time tL; finally, it is moved back to the cold bath. Hereafter we denote this thermal learning process as TL–tL–TF. When placed in the hot bath, the gel swells by absorbing water, and it shrinks when being moved back to the cold bath by releasing water. The kinetics of swelling and shrinking are governed by the cooperative diffusion coefficients of hydrogel, Dsw and Dsh, respectively. The time for gel to reach swelling equilibrium in the hot bath is τe ∼ d2/4Dsw (19, 20). When thermal learning time tL is shorter than τe, the gel does not reach swelling equilibrium, and water absorption only occurs in the surface layers of sample with thickness dsw (< d/2). Therefore, only these layers shrink at cooling. Under such a condition, the time for swelling in the hot bath tL and shrinking in the cold bath tF are correlated through dsw by tL ∼ and tF ∼ . The water-releasing time in the cold bath can be considered as the forgetting time tF of sample with thermal learning. Accordingly, the ratio of forgetting time to learning time is
| [1] |
Eq. 1 indicates that, if a hydrogel has a strong asymmetric swelling/shrinking kinetics by thermal stimulus (Dsw/Dsh >> 1), it might show a memorizing–forgetting behavior, with a forgetting time (shrinking time) proportional to but much longer than the thermal learning time (swelling time) (tF/tL >> 1). The ratio tF/tL can be considered as the learning efficiency. In principle, Eq. 1 only holds before reaching the learning saturation time (tL < τe). When the learning time tL is longer than τe, the memory saturates and the longest forgetting time is about d2/4Dsh, independent of the learning time. The saturated learning time and longest forgetting time can be tuned by sample thickness d.
To achieve the strongly asymmetric water absorption–release kinetics in response to thermal stimulus, we adopt hydrogels containing physical interactions, like hydrogen bonds and Coulombic interactions (21–24). Different from the common chemical gels that do not contain physical bonds and show no observable size and appearance change with temperature (SI Appendix, Fig. S1), many of these physical gels swell at heating and shrink at cooling, and the shrinking kinetics is slower than the swelling kinetics (25, 26). As the thermal conduction rate is significantly faster than the water diffusion rate, abrupt cooling induces a large-scale frustrated structure in these physical gels, which often appears as an immediate transparent-to-opaque change in the gels by cooling (27). That is, the gel is transparent when being equilibrated in the cold bath or being suddenly put in the hot bath (Fig. 1 C, i and ii), but immediately turns opaque when being placed back to the cold bath owing to the large-scale frustrated structure formation (Fig. 1 C, iii). The turbidity of the opaque state decays with time in accompany with water release (Fig. 1 C, iv), and finally recovers to transparent when reaching the equilibrium again. Thus, the thermal-stimulus encoded information can be retrieved from the opaque state of gels, and the forgetting time corresponds to the time recovers to transparent. The learning temperature TL and forgetting temperature TF can be arbitrarily chosen, independent of the specific chemical structure of hydrogels.
Asymmetric Water Absorption–Release Kinetics of Polyampholyte Hydrogels.
As a model system, we adopted a hydrogel composed of polyampholytes to verify this principle. Polyampholytes carry opposite charges on the same polymer chain and form hydrogels (PA gels) containing ∼45 wt % water by inter- and intrachain ionic bonding. The PA gels are viscoelastic, exhibiting high toughness, self-healing, and fatigue resistance (22, 28). The advantage of using PA gels is that the size change with temperature is small while the turbidity change caused by sudden cooling is distinct.
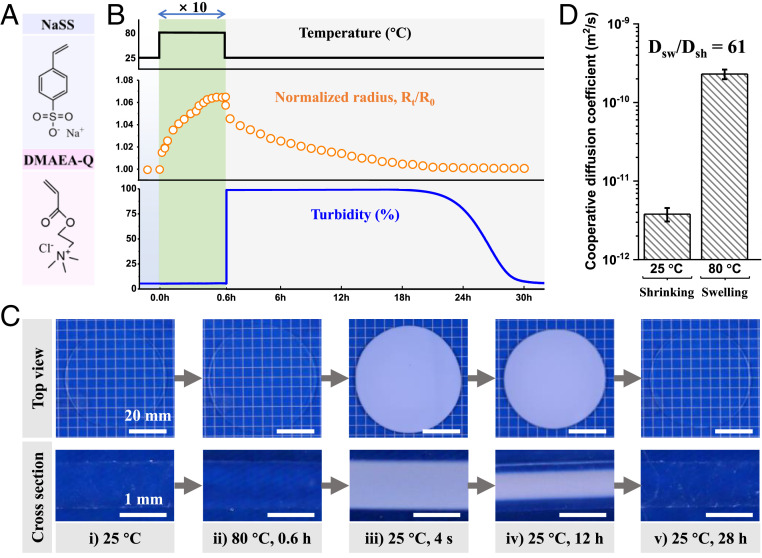
We first show the thermal-induced swelling/shrinking behavior and turbidity change of a PA gel made from sodium p-styrenesulphonate (NaSS) and methyl chloride quarternized N,N-dimethylamino ethylacrylate (DMAEA-Q, Fig. 2A). When the gel, of 25-mm radius and 1.15-mm thickness, is moved from a 25 °C bath to an 80 °C bath, it swells gradually and reaches equilibrium after 0.6 h, showing an increase of only 7% in radius and 17% in weight (Fig. 2B). By quenching to 25 °C, the gel gradually recovers to its original size after a prolonged time by releasing water (Fig. 2B). The gel is transparent in the cold bath (TF = 25 °C, Fig. 2 C, i), and it retains the transparency when being moved to the hot bath (TL = 80 °C, Fig. 2 C, ii). However, when the gel is removed back to the cold bath, the entire sample turns turbid within 4 s (Fig. 2 C, iii and Movie S1). The transmittance is measured to quantify the turbidity (1 - transmittance) of the sample. The turbidity spontaneously decays and the gel becomes transparent gradually with releasing water, starting from the surface to the center (Fig. 2 C, iv and v). The recoveries of sample size and transparency in the cold bath are strongly coupled (Fig. 2B and Movie S2).
Fig. 2.
Asymmetric water absorption–release kinetics and frustrated structure formation in PA gels by thermal stimulus. (A) Chemical structures of monomers for PA gels. (B) Time profiles of temperature-induced radius and turbidity (1 - transmittance) changes of PA gel. To aid the visual, the timescale in the hot bath is enlarged by 10 times. (C) Optical images of the sample at different states: (i) in the cold bath (TF = 25 °C, equilibrium temperature), (ii) in the hot bath (TL = 80 °C, tL = 0.6 h), (iii) after a hot-to-cold jump (80–25 °C for 4 s), (iv) partial recovery in the cold bath (25 °C for 12 h), and (v) full recovery in the cold bath (25 °C for 28 h). The gel thickness at 25 °C is about 1.15 mm and tL = 0.6 h is sufficient for the gel to reach swelling equilibrium at TL = 80 °C. (D) Cooperative diffusion coefficients of PA gel for shrinking at 25 °C (Dsh) and swelling at 80 °C (Dsw).
The shrinking time required in the cold bath (tF = 28 h) is much longer than the swelling time in the hot bath (tL = 0.6 h). The relatively fast swelling and slow shrinking indicate the strong asymmetric diffusion kinetics of water. Indeed, the cooperative diffusion coefficient for swelling in the 80 °C bath (Dsw) is measured as 2.3 × 10−10 m2/s, while that for shrinking in the 25 °C bath (Dsh) is 3.8 × 10−12 m2/s (SI Appendix, Figs. S2–S5). Therefore, for a temperature difference ΔT = 55 °C, the ratio Dsw/Dsh of the PA gel is as high as 61 (Fig. 2D). The slow shrinking process may be caused by the formation of more bound water, skin layer, or structure frustration in the turbid gel. Differential scanning calorimetry (DSC) results show that the content of bound water is almost the same for the transparent gel and the turbid gel (SI Appendix, Fig. S6). The cut cross-section of sample totally turns to turbid quickly when being cooled (Fig. 2 C, iii), excluding the possibility of the formation of skin layer on the surface of the gel. Therefore, the frustrated structure caused by sudden cooling, as seen by the instant transparent to opaque change of gel at cooling, should be responsible for the slow shrinking kinetics.
The formation of frustrated structure can be rationalized with the following picture. When the sample is heated to TL, the number of associated ionic bonds in the gel decreases and the osmotic pressure of gel increases, which leads to the swelling of gel. By a sudden cooling to TF, the number of associated bonds increases quickly (the average association time of ionic bonds is several microseconds at 25 °C) (29), while the absorbed water at TL cannot be expelled out of gel instantly, as the water diffusion is much slower than the heat conduction (SI Appendix). Consequently, water is trapped between the aggregated polymers to form the transient frustrated structure, leading to a slow shrinking process. The asymmetry of swelling/shrinking behavior changes with the density of ionic bonds, i.e., the ionic bond strength (SI Appendix, Fig. S7), further supporting the above analysis. Scanning electron microscope (SEM) observation reveals that the frustrated structure has a characteristic length around 300 nm (SI Appendix, Fig. S8), which results in the opaque appearance of gel. The diffusion time for local water aggregation with such a length scale is in the order of tens of microseconds, much shorter than the thermal conduction time of several seconds through the entire sample (SI Appendix, Fig. S9), which results in the instant turbidity change by sudden cooling. It is surprising that the formation of such a prominent frustrated structure is caused by a considerably small amount (17 wt %) of extra water in the sample.
Indeed, when the water absorption is prohibited during heating, for example, by sealing the sample with a plastic bag, the gel retains the transparency by sudden cooling from TL to TF, indicating no frustrated structure formation. As the water absorption is driven by osmotic pressure of polymer, it can also be prohibited by imposing a pressure on the sample. For the PA gel used in this work, a nominal stress of 0.10 MPa is sufficient to prohibit water absorption (SI Appendix, Fig. S10). This property permits us to control the learning regions in PA gels with a relatively sharp spatial resolution.
Thermal Learning and Memorizing–Forgetting Behaviors of PA Gels.
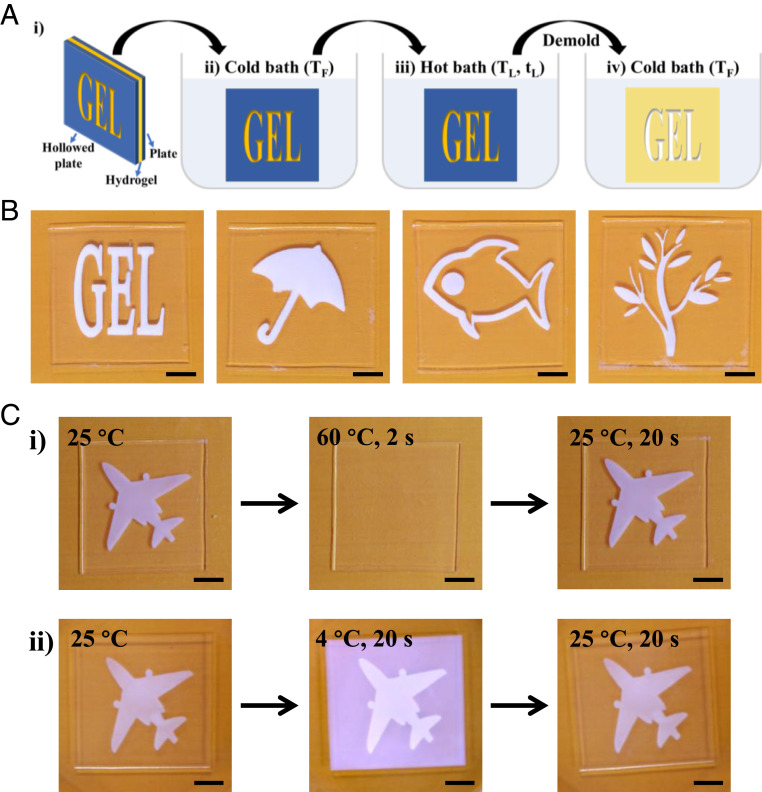
Based on the asymmetric water absorption–release kinetics and the thermal-history-dependent turbidity change of the PA gel, we can construct dynamic memory devices. To spatially control the thermal learning process, we use a mask with a hollowed-out pattern featuring 2D information to be memorized. The gel preequilibrated in the cold bath at TF is sandwiched between two plates with a slight compression, and one of the plates is the mask with the hollowed-out pattern. Then, the sample is immersed in the hot bath for thermal learning at TL. During thermal learning, the gel can only absorb water in the hollowed region, which memorizes the 2D information of the mask. After that, the sample is moved to the cold bath and taken out from the plates to retrieve the memorized information (Fig. 3A).
Fig. 3.
Thermal learning and memory of PA gels. (A) Thermal learning process TL–tL–TF. (i) A PA gel is sandwiched between two plates with a slight compression and one plate is a mask having a hollowed-out area featuring the memorized information; (ii) the sandwiched sample is put into a cold bath of temperature TF for equilibrium; (iii) the sample is then moved into a hot bath of temperature TL for a time tL for learning information, during which the hollow area absorbs water; (iv) after thermal learning, the two mold plates are removed and the gel is placed into the cold bath to retrieve the memorized information. (B) Various memorized information through different masks with the same 80 °C–5 min–25 °C learning process. The photos are captured after jumping temperature from 80 to 25 °C for 1 min. (C) Results to demonstrate the memory nonvolatility against high- and low-temperature perturbation. (i) High-temperature perturbation. The gel is first performed a 60 °C–10 min–25 °C learning process to memorize an airplane pattern. Then, the gel is put into a 60 °C bath and the pattern disappears within 2 s. After putting the gel back to the 25 °C bath, the airplane pattern remerges again. (ii) Low-temperature perturbation. The gel is performed a 60 °C–3 min–25 °C learning process. Then, it is put into a 4 °C bath for 20 s and the surrounding region becomes opaque. After putting back into the 25 °C bath, the surrounding noise disappears, and the airplane pattern remerges clearly again. (Scale bars, 1 cm.)
Fig. 3B shows a series of memorized information obtained through an 80 °C–5 min–25 °C learning process by using different masks, such as a word “GEL,” patterns of “umbrella,” “fish,” and “twig.” The memorized information is highly stretchable, and the stretched information is fully recoverable after releasing load (Movie S3), benefiting from the high stretchability and self-recovery of PA gels (22). As the sample becomes opaque whenever experiences a sudden cooling, independent of the specific temperature and structure of materials (30), the choice of TL and TF is arbitrary, provided the difference between TL and TF is sufficiently large (SI Appendix, Fig. S11). For instance, a 3 °C temperature jump, from TL = 28 °C to TF = 25 °C, is enough to induce the turbidity change for memorizing and retrieving information (SI Appendix, Fig. S11).
The memorized information in PA gels does not lose when the environmental temperature temporarily fluctuates (Fig. 3C), which is similar to the nonvolatility of memory materials in computers (7). Fig. 3 C, i exhibits an example of memory nonvolatility at a high temperature. A PA gel is initially performed with a 60 °C–10 min–25 °C learning process to memorize an airplane pattern; then, it was moved to the 60 °C bath. Within 2 s, the airplane pattern disappears and the entire gel becomes fully transparent. After moved back to the 25 °C bath, interestingly, the airplane pattern emerges again. This process can be repeated several times (Movie S4). Here we should mention that the time for the fluctuation treatment of the gel in the 60 °C bath must be very short to have negligible water absorption. Otherwise, the gel would absorb considerable amount of water and the entire gel would turn to turbid after moving back to the 25 °C bath. Similarly, Fig. 3 C, ii exhibits an example of memory nonvolatility at a low temperature. The airplane pattern is memorized upon 60 °C–3 min–25 °C learning process; then, the gel is moved to a 4 °C bath for 20 s. The entire gel becomes turbid, which interferes with the airplane pattern. However, after moved back into the 25 °C bath, this airplane pattern reappears clearly as the rest parts of gel become transparent.
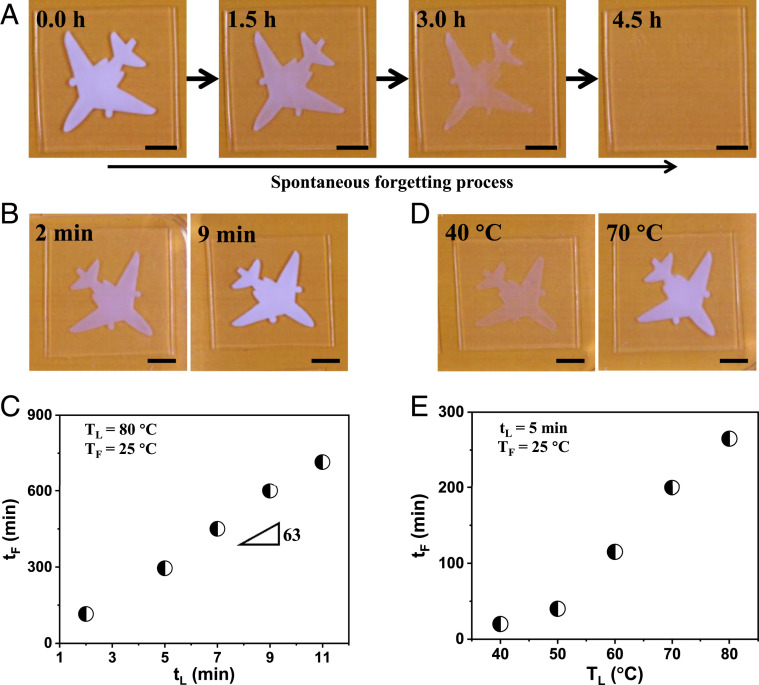
The memorized information decays spontaneously, without any external stimuli. Fig. 4A shows an example of the forgetting process of PA gel after experiencing an 80 °C–5 min–25 °C learning process. The memorized airplane pattern decays and finally disappears within 4.5 h at 25 °C, and the entire gel becomes fully transparent (Movie S5). The forgetting time that the memorized information completely disappears depends on the learning strength, that is, the learning time and learning temperature. Fig. 4 B and C show the effect of learning time tL on forgetting time tF. TL and TF are fixed at 80 and 25 °C, respectively, and tL is changed from 2 to 11 min. For tL = 2 min, the initial memorized airplane pattern is translucent, indicating a relatively weak memory. By contrast, the initial memorized airplane pattern for tL = 9 min is opaque, suggesting a relatively strong memory. The forgetting time increases almost linearly with learning time, from 115 to 715 min by increasing learning time from 2 to 11 min. The measured ratio of forgetting time to learning time, tF/tL, keeps almost constant value of 63, independent of learning time tL (tL < τe = 14 min, SI Appendix, Fig. S12). This is reasonably consistent with the ratio Dsw/Dsh = 61 (Fig. 2D), confirming the validity of Eq. 1. Fig. 4 D and E show that the forgetting time increases with the learning temperature, for fixed tL = 5 min and TF = 25 °C. tF/tL increases with the increase of TL for a fixed TF, due to the increase of temperature difference. These results show that learning with longer tL or learning at higher TL and retrieving at lower TF leads to a stronger memory and vice versa. This behavior is similar to the memory in the human brain, where the memory strength or forgetting time increases with learning time (31) or/and the magnitude of emotional stimulus (32).
Fig. 4.
Spontaneous forgetting process of PA gels. (A) Example to show spontaneous forgetting process of a gel experienced an 80 °C–5 min–25 °C learning process to memorize an airplane pattern. The elapsed time after cooling in 25 °C bath is shown in the photos. (B and C) Effect of thermal learning time tL on memory strength (B) and forgetting time tF (C) for the same TL and TF. The time in the images is the learning time tL. (D and E) Effect of thermal learning temperature TL on memory strength (D) and forgetting time tF (E) for the same tL and TF. The temperature in the images is the learning temperature TL. In all experiments, tL is shorter than the equilibrium swelling time of sample and the forgetting time tF is determined by the recovery time from opaque to transparent. (Scale bars, 1 cm.)
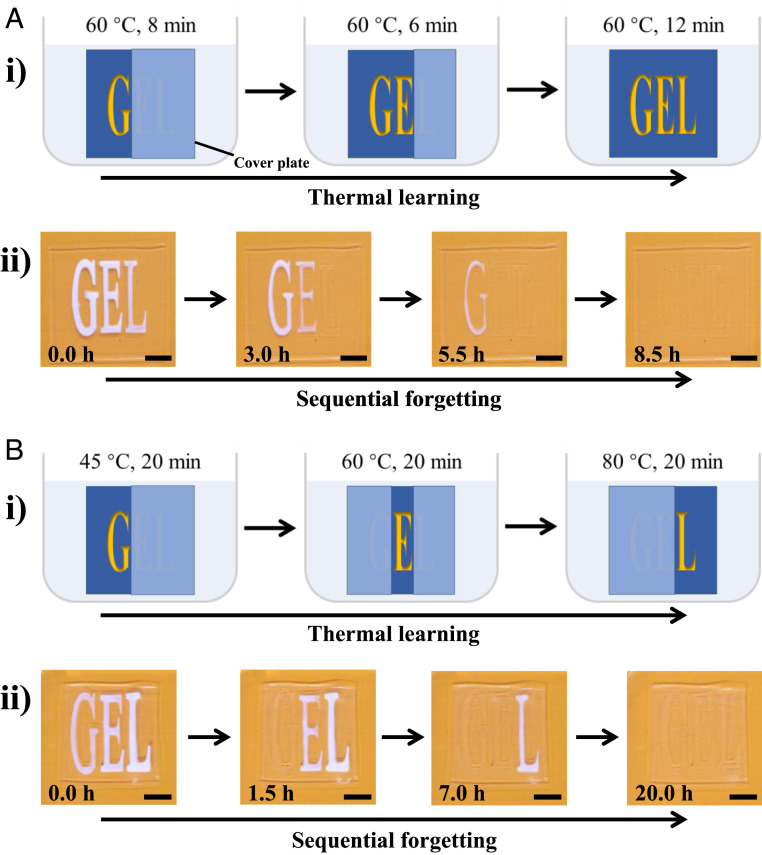
The dependence of memory on learning time and learning temperature allows us to program the memorizing–forgetting process in PA gels. Fig. 5 gives two typical examples to demonstrate sequential forgetting by programing learning time and learning temperature. We set TL = 60 °C and TF = 25 °C, and endow the learning time of letters “G,” “E,” and “L” for 26, 18, and 12 min, respectively. This is achieved by supplying water for different time during thermal learning (Fig. 5 A, i): The hollowed-out areas of E and L are initially covered by a plate, and only the letter G can contact with water at TL = 60 °C for 8 min; then, both G and E are allowed to contact with water at TL = 60 °C for 6 min; finally, all three letters G, E, and L are allowed to contact with water at TL = 60 °C for 12 min. Fig. 5 A, ii shows the sequential forgetting process at TF = 25 °C: The letter L disappears after about 3.0 h; then, E disappears after about 5.5 h; finally, L disappears after about 8.5 h (Movie S6). Using the same method, we set the learning temperature for G, E, and L as 45, 60, and 80 °C, respectively, while maintaining tL = 20 min and TF = 25 °C (Fig. 5 B, i), and a sequential forgetting behavior is also achieved (Fig. 5 B, ii).
Fig. 5.
Programmable forgetting process of PA gels. By using additional plates to selectively cover the hollowed-out area for prohibiting water uptake, the gels show programmable forgetting behaviors. (A) Sequential forgetting process by tuning the thermal learning time. (i) The learning times for G, E, and L are 26, 18, and 12 min, respectively. (ii) The optical images of gel during spontaneous sequential forgetting in a 25 °C bath. (B) Sequential forgetting process by tuning the thermal learning temperature. (i) The learning temperatures for G, E, and L are 45, 60, and 80 °C, respectively. (ii) The optical images of gel during spontaneous sequential forgetting in a 25 °C bath. (Scale bars, 1 cm.)
The memorizing–forgetting behavior also allows us to control the drug release from the gel (SI Appendix, Fig. S12). We put two pieces of PA gels with the same size into a Rhodamine B (RB) solution to reach equilibrium. Then one gel undergoes an 80 °C–2 h–25 °C learning process while the other does not. After that we put them into pure water to observe the release process of RB. The gel with thermal learning has a significantly longer RB release process than that of the gel without thermal learning even at the same release temperature, benefiting from the formation of frustrated structure in thermal learning.
The principle proposed in this work for constructing dynamic memorizing–forgetting behavior is universal, as it is applicable to many other hydrogels containing dynamic bonds. Indeed, we have confirmed that PA gels made from two other cationic and anionic monomer combinations also show similar dynamic memorizing–forgetting behavior (SI Appendix, Fig. S13). Furthermore, such behavior is not limited to gels with ionic bonds; gels with other dynamic bonds, such as hydrogen bonds, also show dynamic memorizing–forgetting behavior (SI Appendix, Fig. S13).
The thermal-response behavior of our gels containing dynamic bonds is distinctly different from conventional thermal-sensitive gels with upper or lower critical solution temperature (30, 33, 34) in which the transparent-to-opaque transition only occurs at the critical temperature specific to the materials. These gels, also showing an asymmetric swelling/shrinking behavior (26, 35), in principle, can act as dynamic memory but only working at their critical temperatures. Moreover, the large volume change (tens or hundreds of times in volume) at the critical temperature (36, 37) brings difficulty to construct memory devices.
Conclusion
In summary, we successfully realized dynamic memorizing–forgetting behavior in analogy to that in the human brain by utilizing hydrogels containing dynamic bonds. The behavior is based on the strongly asymmetric swelling/shrinking kinetics of hydrogels due to the formation of frustrated structure at sudden cooling. The distinct turbidity change of these hydrogels provides a facile mechanism to retrieve the memorized information. Moreover, the small size change with temperature results in the easy construction of memory devices, and the high compliance of materials imparts high stretchability of the memory. This work opens an opportunity to develop an intelligent device with brainlike memorizing–forgetting behavior. The dynamic and learning-strength-dependent turbidity change as well as the asymmetric swelling/shrinking process may also find applications in smart display and controllable drug release. More importantly, the concept of using soft materials to achieve dynamic memorizing–forgetting behavior is expected to inspire further research on developing lifelike materials based on the nonequilibrium process of soft matter.
Methods
Synthesis of PA Hydrogels.
PA gels were synthesized by one-step random radical copolymerization of anionic and cationic monomers at charge-balance point in a concentrated aqueous solution, following our previous work (22). The prescribed amounts of cationic monomer, anionic monomer, ultraviolet (UV) initiator, and chemical cross-linker were dissolved in deionized water; then, the solution was injected into a reaction cell consisting of a pair of glass plates with a spacer of varying thickness values, and the reaction cell was irradiated with UV lights (∼365 nm) for 10 h under argon atmosphere. Subsequently, the as-prepared gels in a platelet shape were immersed in a large amount of pure water for 1 mo to remove the counterions. The gel at equilibrium state had a water content of around 45 wt %.
Transmittance Measurement.
The light transmittance of the gel was characterized using a Shimadzu UV spectrophotometer (UV-1800) at a wavelength of 550 nm. The gel was put into a cell filled with water when performing this measurement.
All details associated with sample preparations, DSC, and SEM measurements are available in SI Appendix.
Supplementary Material
Acknowledgments
We thank Prof. Michael Rubinstein for helpful discussion. C.Y. thanks Japanese Ministry of Education, Culture, Sports, Science and Technology for providing scholarship, and Zhijian Yu for calculating the heat transfer. This research was supported by Japan Society for the Promotion of Science KAKENHI (Grants JP17H06144, JP17H06376, and JP19K23617). The Institute for Chemical Reaction Design and Discovery was established by World Premier International Research Initiative, Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2006842117/-/DCSupplemental.
Data Availability.
All data are included in the main text and SI Appendix.
References
- 1.Gorgoraptis N., Catalao R. F. G., Bays P. M., Husain M., Dynamic updating of working memory resources for visual objects. J. Neurosci. 31, 8502–8511 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nader K., Memory traces unbound. Trends Neurosci. 26, 65–72 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Wickelgren W. A., Single-trace fragility theory of memory dynamics. Mem. Cognit. 2, 775–780 (1974). [DOI] [PubMed] [Google Scholar]
- 4.Hardt O., Nader K., Nadel L., Decay happens: The role of active forgetting in memory. Trends Cogn. Sci. 17, 111–120 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Davis R. L., Zhong Y., The biology of forgetting—A perspective. Neuron 95, 490–503 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonegawa S., Pignatelli M., Roy D. S., Ryan T. J., Memory engram storage and retrieval. Curr. Opin. Neurobiol. 35, 101–109 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Scott J. F., Paz de Araujo C. A., Ferroelectric memories. Science 246, 1400–1405 (1989). [DOI] [PubMed] [Google Scholar]
- 8.Parkin S. S. P., Hayashi M., Thomas L., Magnetic domain-wall racetrack memory. Science 320, 190–194 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Zhang H., Zeng H., Priimagi A., Ikkala O., Programmable responsive hydrogels inspired by classical conditioning algorithm. Nat. Commun. 10, 3267 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacVittie K., Halámek J., Privman V., Katz E., A bioinspired associative memory system based on enzymatic cascades. Chem. Commun. (Camb.) 49, 6962–6964 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Kundu P. K. et al., Light-controlled self-assembly of non-photoresponsive nanoparticles. Nat. Chem. 7, 646–652 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Mohammed J. S., Murphy W. L., Bioinspired design of dynamic materials. Adv. Mater. 21, 2361–2374 (2009). [Google Scholar]
- 13.Halgren E., Smith M. E., Cognitive evoked potentials as modulatory processes in human memory formation and retrieval. Hum. Neurobiol. 6, 129–139 (1987). [PubMed] [Google Scholar]
- 14.Lee K. Y., Mooney D. J., Hydrogels for tissue engineering. Chem. Rev. 101, 1869–1879 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Matsuda T., Kawakami R., Namba R., Nakajima T., Gong J. P., Mechanoresponsive self-growing hydrogels inspired by muscle training. Science 363, 504–508 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Osada Y., Gong J., Soft and wet materials: Polymer gels. Adv. Mater. 10, 827–837 (1998). [Google Scholar]
- 17.Tanaka T. et al., Phase transitions in ionic gels. Phys. Rev. Lett. 45, 1636–1639 (1980). [Google Scholar]
- 18.Ward M. A., Georgiou T. K., Thermoresponsive polymers for biomedical applications. Polymers 3, 1215–1242 (2011). [Google Scholar]
- 19.Peters A., Candau S. J., Kinetics of swelling of spherical and cylindrical gels. Macromolecules 21, 2278–2282 (1988). [Google Scholar]
- 20.Tanaka T., Fillmore D. J., Kinetics of swelling of gels. J. Chem. Phys. 70, 1214–1218 (1979). [Google Scholar]
- 21.Dai X. et al., A mechanically strong, highly stable, thermoplastic, and self-healable supramolecular polymer hydrogel. Adv. Mater. 27, 3566–3571 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Sun T. L. et al., Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 12, 932–937 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Sun J. Y. et al., Highly stretchable and tough hydrogels. Nature 489, 133–136 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui K. et al., Multiscale energy dissipation mechanism in tough and self-healing hydrogels. Phys. Rev. Lett. 121, 185501 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Zrinyi M., Wolfram E., Experimental study of phase separation phenomena in swollen polyvinyl acetate and polystyrene gels near the critical solution temperature. J. Colloid Interface Sci. 90, 34–43 (1982). [Google Scholar]
- 26.Zrinyi M., Rosta J., Horkay F., Studies on the swelling and shrinking kinetics of chemically cross-linked disk-shaped poly(vinyl acetate) gels. Macromolecules 26, 3097–3102 (1993). [Google Scholar]
- 27.Ihsan A. B. et al., Self-healing behaviors of tough polyampholyte hydrogels. Macromolecules 49, 4245–4252 (2016). [Google Scholar]
- 28.Li X. et al., Mesoscale bicontinuous networks in self-healing hydrogels delay fatigue fracture. Proc. Natl. Acad. Sci. U.S.A. 117, 7606–7612 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy C. K. et al., Self-adjustable adhesion of polyampholyte hydrogels. Adv. Mater. 27, 7344–7348 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Seuring J., Agarwal S., Polymers with upper critical solution temperature in aqueous solution. Macromol. Rapid Commun. 33, 1898–1920 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Craik F. I. M., Lockhart R. S., Levels of processing: A framework for memory research. J. Verbal Learn. Verbal Behav. 11, 671–684 (1972). [Google Scholar]
- 32.Roozendaal B., McGaugh J. L., Memory modulation. Behav. Neurosci. 125, 797–824 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Z. et al., Polymer gels with engineered environmentally responsive surface patterns. Nature 393, 149–152 (1998). [Google Scholar]
- 34.Roy D., Brooks W. L. A., Sumerlin B. S., New directions in thermoresponsive polymers. Chem. Soc. Rev. 42, 7214–7243 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Tajima H., Yoshida Y., Yamagiwa K., Experimental study of swelling and shrinking kinetics of spherical poly(N,N-diethylacrylamide) gel with continuous phase transition. Polymer 52, 732–738 (2011). [Google Scholar]
- 36.Xu Y. et al., Development of visible-light responsive and mechanically enhanced “smart” UCST interpenetrating network hydrogels. Soft Matter 14, 151–160 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Erol O. et al., Transformer hydrogels: A review. Adv. Mater. Technol. 4, 1900043 (2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the main text and SI Appendix.