Supplemental Digital Content is Available in the Text.
Distinct cerebrospinal fluid neuroinflammatory profiles may be associated with different objective characteristics of persistent pain in osteoarthritis patients undergoing total hip arthroplasty.
Keywords: Persistent pain, Neuroinflammation, Proinflammatory mediators, Cerebrospinal fluid, Quantitative sensory testing, Central sensitization, Osteoarthritis
Abstract
Neuroinflammation is implicated in the development and maintenance of persistent pain states, but there are limited data linking cerebrospinal fluid (CSF) inflammatory mediators with neurophysiological pain processes in humans. In a prospective observational study, CSF inflammatory mediators were compared between patients with osteoarthritis (OA) who were undergoing total hip arthroplasty due to disabling pain symptoms (n = 52) and pain-free comparison controls (n = 30). In OA patients only, detailed clinical examination and quantitative sensory testing were completed. Cerebrospinal fluid samples were analyzed for 10 proinflammatory mediators using Meso Scale Discovery platform. Compared to controls, OA patients had higher CSF levels of interleukin 8 (IL-8) (P = 0.002), intercellular adhesion molecule 1 (P = 0.007), and vascular cell adhesion molecule 1 (P = 0.006). Osteoarthritis patients with central sensitization possibly indicated by arm pressure pain detection threshold <250 kPa showed significantly higher CSF levels of Fms-related tyrosine kinase 1 (Flt-1) (P = 0.044) and interferon gamma-induced protein 10 (IP-10) (P = 0.024), as compared to subjects with PPDT above that threshold. In patients reporting pain numerical rating scale score ≥3/10 during peripheral venous cannulation, Flt-1 was elevated (P = 0.025), and in patients with punctate stimulus wind-up ratio ≥2, CSF monocyte chemoattractant protein 1 was higher (P = 0.011). Multiple logistic regression models showed that increased Flt-1 was associated with central sensitization, assessed by remote-site PPDT and peripheral venous cannulation pain, and monocyte chemoattractant protein-1 with temporal summation in the area of maximum pain. Multiple proinflammatory mediators measured in CSF are associated with persistent hip OA-related pain. Pain phenotype may be influenced by specific CSF neuroinflammatory profiles.
1. Introduction
Persistent pain and pain-related conditions are leading global causes of disability.32 In addition to the distress and suffering associated with pain itself, long-term pain impacts humans through negative mental health effects, sleep disturbance, and increased morbidity.28,67 Despite substantial research efforts, treatment options remain limited, only partially effective, and associated with side effects.29,84
Over the past 3 decades, neuroinflammation has emerged as a central feature of persistent pain states.34,46 Upon peripheral nerve injury or inflammation, spinal cord microglia and astrocytes are activated to produce proinflammatory cytokines, chemokines, and growth factors, which amplify central nervous system (CNS) immunoactivation.46,65 Central neuroimmune activation is well established in animal models of inflammation and neuropathic pain.34 For example, intrathecal (IT) administration of proinflammatory cytokines such as interleukin (IL) 1β, IL-8, monocyte chemoattractant protein 1 (MCP-1), and TNF-α induces allodynia and hyperalgesia, indicative of central sensitization (CS).2,63,73,83 Conversely, upregulation or administration of the anti-inflammatory cytokine IL-10, or IT treatment with an IL-4/IL-10 fusion protein, inhibits neuroinflammation in the dorsal root ganglia (DRG) and spinal cord, with subsequent decrease of nocifensive behavior.27,66 Knockout animals lacking IL-1β, IL-1 receptor 1, or MCP-1 demonstrate reduced or abrogated pain behavior in peripheral nerve injury models, and inhibition of IL-8 decreased pain in a rodent chronic low back pain model.1,20,54 Attenuation of neuroinflammation, through inhibition of microglial activation, targeting of specific microglial genes, or selective depletion of spinal microglia, relatively consistently reverses pain hypersensitivity.25,35
In contrast to preclinical findings that clearly indicate the importance of neuroimmune signaling for initiation and maintenance of persistent pain, studies in humans are limited. Emerging evidence from observational studies shows that proinflammatory mediators and glial activation may be important for modulation of pain perception in clinical persistent pain conditions, but numerous methodological considerations prevent firm conclusions.10,57 Moreover, research evaluating whether cerebrospinal fluid (CSF) inflammation is related to objective measures of pain, eg, quantitative sensory testing (QST), has remained largely absent. Investigation of links between CSF neuroinflammatory patterns and neurophysiological pain phenotypes, such as high levels of CS, may increase our understanding of pain mechanisms and guide identification of novel treatment targets.
In this study, we therefore prospectively examined associations between CSF proinflammatory mediators and advanced characteristics of pain, sleep, and neurophysiology in patients with disabling osteoarthritis (OA)-related pain undergoing total hip arthroplasty (THA). Specifically, to examine whether there are coherent changes in CSF inflammation associated with clinically relevant categorical objective measures of persistent pain, we predefined subgroups according to degree of sensitization. In addition, we extended these within-subjects analyses, and cross-sectionally evaluated differences in CSF concentrations of inflammatory mediators between OA subjects and well-characterized pain-free controls. Our main hypotheses were that CNS pain processes would be differentially regulated by inflammatory mechanisms such that persistent pain patients would have changes in CSF levels of inflammation, and that there would be a dose–response relationship in which increasing pain is associated with differential changes of CSF mediators.
2. Methods
2.1. Study design and overview
The current report presents data from a prospective, observational study termed neuroPSI (neuro pain sleep immunology). The study was conducted in the setting of a fast-track, state-of-the-art orthopedic surgical center (Department of Orthopedics, Hässleholm Hospital, Sweden), which has conducted the highest annual rate of THAs in Sweden since 2002. Preoperatively, a cross-sectional comparison of OA pain subjects and age- and sex-matched, pain-free healthy controls was conducted. In OA subjects only, repeated follow-up assessments after THA were conducted over the course of 6 months.
All procedures were approved by the Regional Ethics Committee (Dnr 2018/396 and Dnr 2008/290). Written informed consent was obtained from each study participant before study activities.
2.2. Participants
Patients scheduled to undergo fast-track THA were consecutively screened preoperatively through chart review and structured interview. Inclusion criteria were: age ≥18 years, persistent OA-related pain ≥12 months, average pain numerical rating scale (NRS) score ≥4 and/or movement-related pain score ≥4 after 5 minutes walking, and primary THA conducted under spinal anesthesia. Exclusion criteria were: acute illness (eg, infectious disease), malignancy, immunomodulating treatment, neurological disorder, severe psychiatric disorder (eg, severe major depression, bipolar disorder, and psychotic disorders), contraindications for lumbar puncture, American Society of Anesthesiology (ASA) physical status classification >3, substance abuse during the past 12 months, poor Swedish-language fluency, or inability to provide informed consent.
A total of 79 patients were screened for participation; 16 were deemed ineligible (low-pain OA n = 9, cognitive impairment n = 2, severe psychiatric comorbidity n = 1, systemic steroid treatment n = 1, acute infectious disease n = 1, leg ulcer n = 1, and non-Swedish speaking n = 1). Of the remaining 63 patients, 10 declined to participate, and for one patient anesthesia modality was changed from spinal anesthesia to general anesthesia on the day of surgery, resulting in 52 subjects being included. Study participants were included in September 2018 to November 2018, and follow-up assessments were concluded in May 2019. The subjects received no payment or other incentives for participating in the study.
The healthy controls consisted of 30 volunteers separately identified from a prior study on CSF inflammatory markers in Parkinson disease.36 The most important exclusion criteria for healthy control participants were: acute illness, contraindications to lumbar puncture, neurological disorder, cancer, substance or alcohol abuse, and inadequate Swedish-language fluency. In addition to demographic and clinical variables, information regarding pain and sleep status was obtained through a customized questionnaire. No control participant had a diagnosis associated with persistent pain. All control participants denied having experienced any significant acute pain for 7 days before enrollment, as confirmed by a negative response to the first question of the brief pain inventory short form (BPI-sf), modified to cover the past week.
2.3. Study procedures
2.3.1. Anesthesia, surgery, and perioperative care in the osteoarthritis patients
Spinal anesthesia was administered to all patients through IT injection of hyperbaric bupivacaine. The surgery was performed or supervised by 1 of the 5 consultant surgeons doing >50 THAs per year. A posterior approach was used in all cases and, according to the surgeon preference, patients had a cemented, hybrid, or uncemented THA. The stems used were a cemented Exeter V40 stem (Stryker Orthopaedics, Mahwah, NJ) or an uncemented Accolade II stem (Stryker Orthopaedics). The acetabular cups used were a cemented Exeter X3 RimFit socket (Stryker Orthopaedics) or an uncemented Trident Acetabular System (Stryker Orthopaedics).
Key elements of the perioperative care associated with fast-track THA at Hässleholm Hospital, aiming to reduce surgical stress and facilitate postoperative recovery, include early mobilization on the day of surgery and multimodal pain control (celecoxib 200 mg twice daily until postoperative day [POD] 3; paracetamol 1 g 3 times daily until POD7; and oxycodone 5 mg as needed).
2.3.2. Quantitative sensory testing in the osteoarthritis subjects
The QST protocol was based on protocols that have previously been validated for evaluation of somatosensory abnormalities in hip OA patients,50,51 and designed to comply with recommendations made by the German Research Network on Neuropathic Pain.74 All OA subjects had been given instructions to refrain from taking analgesic medications 24 hours before QST.
The QST was performed in a quiet, air-conditioned (20-24°C) room, approximately 60 minutes before surgery. All testing was conducted by one physician with extensive QST experience (M.F.B.). Throughout the QST, the participants were asked to keep their eyes closed to enable complete focus on the evoked sensations. To acclimate the participant to the different testing modalities, brief demonstrations of the procedures were performed on the nondominant forearm, before initiation of the QST protocol.
For all testing modalities, 3 body areas were examined: (1) the area of maximum pain on the OA-affected side, (2) the corresponding contralateral area, and (3) a proximal, volar area on the dominant forearm.
2.3.2.1. Pressure pain detection and pressure pain tolerance thresholds
To determine the pressure pain detection threshold (PPDT) and pressure pain tolerance threshold (PPTT), a digital algometer (SBMEDIC Electronics, Solna, Sweden) with a 1-cm2 probe area was applied to the skin at a constant rate of 20 kPa/second. The subject was instructed to indicate (verbally and/or through pushing a button) when the stimulus “first feels painful” (PPDT) or when the pain was no longer tolerable (PPTT). Three trials with a 30-second interstimulus interval were completed per area for the pressure pain detection; the PPDT was calculated as the average value. To assess PPTTs, only one trial was performed in each area. To avoid potential harm, pressure was not applied beyond 588 kPa.
2.3.2.2. Temporal summation
To evaluate CNS enhancement of mechanical pain caused by repeated painful stimulation (temporal summation, TS), a monofilament calibrated to deliver 588 mN of force (Aesthesio; DanMic Global, San Jose, CA) was used. After rating the pain response to a single punctate stimulus (0-100; 0 = no pain, 100 = worst pain imaginable), a sequence of 10 repeated stimuli (1 Hz) was administered within an area of 1 cm2; participants rated the peak pain experience during the sequence. The wind-up ratio (WUR) was calculated as: peak pain rating/pain rating of single stimulus. In addition, dynamic mechanical allodynia was assessed through light tactile stimulation with a cotton swab applied for 60 seconds (2 Hz) over a 2-cm2 area. Aftersensations (eg, paresthesias, numbness, and pain) lasting beyond 30 seconds after completion of the TS testing were recorded.
2.3.2.3. Conditioned pain modulation
The capacity of endogenous descending pain inhibitory systems was assessed through a QST paradigm comprising an occlusion cuff conditioning stimulus and a pressure pain test stimulus. After having assessed the PPDT, a 12-cm wide occlusion cuff was placed approximately 2 cm proximal to the cubital fossa on the dominant arm. The cuff was manually inflated and for each 10 to 15 mm Hg increase of pressure, the participant was asked to provide a pressure pain NRS grading 0 to 10 (0 = no pain, 10 = worst imaginable pain), until a pain NRS 5 was achieved. An arm occlusion cuff conditioning pain intensity level of 5/10 has previously been shown sufficient to induce a CPM effect.78,90 The pressure pain test stimulus was then applied to assess PPDT while the conditioning stimulus was maintained. During testing, if the conditioning stimulus produced an increasing pain level exceeding NRS 5, the cuff was deflated, and a 5-minute pause was implemented, after which the sequence could be repeated. The procedure was conducted in all 3 areas (area of maximum pain, contralateral site, and nondominant forearm). The CPM effect (%) for each area was calculated as: ((PPDTconditioning − PPDTbaseline)/PPDTbaseline) × 100.
2.3.2.4. Pain assessment during peripheral venous cannulation
The subjects were asked to rate the maximum pain intensity during peripheral venous cannulation (1.1 mm inner diameter; Becton Dickinson Venflon, Franklin Lakes, NJ) on a pain NRS 0 to 10. The cannulation site was standardized (back of the hand) and conducted by one physician (M.F.B.).
2.3.3. Cerebrospinal fluid collection
Before IT administration of local anesthetic, 10 mL of CSF was carefully aspirated using 2-mL syringes. The CSF was immediately transferred to 5-mL Protein LoBind Tubes (Eppendorf AG, Hamburg, Germany) and chilled for transport. To minimize circadian variation of biomarkers, all samples were collected between 8 am and 12 pm.
2.3.4. Questionnaires
To provide a comprehensive assessment of pain, sleep, psychopathology, health-related quality of life, OA-related symptoms, and physical activity, validated Swedish versions of the following questionnaires were completed preoperatively, on the day before surgery: BPI-sf,17 douleur neuropathique 4 (DN4),12 pain catastrophizing scale (PCS),80 Pittsburgh sleep quality index (PSQI),14 insomnia severity index (ISI),6 hospital anxiety and depression scale (HADS),92 European quality of life-5 dimensions (EQ-5D),38 Western Ontario and McMaster Universities osteoarthritis index (WOMAC OA),7 and the international physical activity questionnaire short form (IPAQ-sf).19 In addition, the quality of recovery 15 (QoR-15)79 questionnaire was administered to assess postoperative recovery throughout the follow-up period.
2.3.4.1. Pain perception
The BPI-sf yields 2 domain scores: a mean pain severity score, and a pain interference score. The scores range from 0 to 10 (0 = no pain, 10 = worst imaginable pain; 0 = no pain interference, 10 = complete pain interference).
The DN4 consists of 10 items: 7 related to pain quality and nonpainful symptoms, and 3 based on clinical examination, which yields a score 0 to 10. Scores ≥4 indicate that the pain is likely to be neuropathic in origin.
2.3.4.2. Pain catastrophizing
The PCS contains 13 items, each scored 0 to 4, yielding a total score 0 to 52, with higher scores indicating more pain catastrophizing thoughts. A total PCS score ≥30 suggests clinically relevant levels of catastrophizing.
2.3.4.3. Sleep
The PSQI is composed of 19 items, each scored 0 (no difficulty) to 3 (severe difficulty). Component scores are summed to a global score, ranging from 0 to 21. Higher scores indicate worse sleep quality; scores >5 indicate poor sleep quality.15
The ISI consists of 7 questions, each scored 0 to 4, yielding a total score of 0 to 28 (0-7: no clinically significant insomnia; 8-14: subthreshold insomnia; 15-21: clinical insomnia [moderate severity]; 22 to 28: clinical insomnia [severe]).
2.3.4.4. Anxiety and depression
The HADS consists of 14 items, 7 related to anxiety and 7 related to depression. Each item is scored 0 to 3, which yields a score 0 to 21 for either anxiety or depression. The scores can be interpreted as: 0 to 7 = normal, 8 to 10 = borderline abnormal, and 11 to 21 = abnormal.
2.3.4.5. Health-related quality of life
The EQ-5D has 5 dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), each with 5 response levels. The EQ-5D also comprises a visual analogue scale, which represents the subject's self-rated health on a scale of 0 to 100 (0 = the worst health you can imagine, 100 = the best health you can imagine). Single EQ-5D index values were obtained through the EQ-5D-5L index value calculator85 using value sets from Denmark.
2.3.4.6. Osteoarthritis-related symptoms
The WOMAC OA consists of 24 items regarding OA-related symptoms, each scored 0 to 4 (0 = none, 1 = slight, 2 = moderate, 3 = severe, and 4 = extreme). Three subscores are generated: pain (0-20), stiffness (0-8), and physical function (0-68); higher scores indicate worse symptomatology.
2.3.4.7. Physical activity
The IPAQ-sf was used to assess activity level. Separate scores are generated for walking, moderate-intensity, and vigorous-intensity activities. Both categorical (inactive, minimally active, health-enhancing physical activity active) and continuous (median MET-minutes per week; MET = multiple of the resting metabolic rate) measures of physical activity can be calculated. Kilocalories can be computed from MET-minutes: MET-minutes × (weight in kilograms/60 kg).
2.3.4.8. Postoperative recovery
The QoR-15 consists of 15 questions covering important domains related to recovery after surgery (eg, pain, sleep, physical comfort, physical independence, psychological support, and emotional state). Each question is scored 0 to 10, which generates a total score of 0 to 150 (0 = very poor recovery, 150 = excellent recovery).
2.3.5. Multiplex assay analysis of cerebrospinal fluid
All CSF samples were centrifuged at 2000 G for 10 minutes at 4°C and stored in 1-mL aliquots at −80°C until batch analysis could be performed. Cerebrospinal fluid concentrations of the neuroinflammatory and cerebrovascular biomarkers (Fms-related tyrosine kinase 1 [Flt-1], granulocyte-macrophage colony-stimulating factor, intercellular adhesion molecule 1 [ICAM-1], IL-6, IL-8, IL-15, interferon gamma-induced protein 10 [IP-10], MCP-1, phosphatidylinositol-glycan biosynthesis class F protein, vascular cell adhesion molecule 1 [VCAM-1], and vascular endothelial growth factor [VEGF]) were analyzed using ultrasensitive Mesoscale Discovery immunoassay and a customized V-PLEX kit according to the manufacturer's recommendations. Biomarkers were selected based on their known or potential relevance to pain processes and our previous findings regarding detectability. All inflammatory markers were evaluated in the same assay batch. All samples were analyzed in duplicates and randomized according to diagnosis across plates/runs to minimize the effects of run-to-run variation. The lower limits of detection and interassay coefficients of variance (CV) are shown in Appendix 1 (available online as supplemental digital content at http://links.lww.com/PAIN/B18). Most granulocyte-macrophage colony-stimulating factor values (65%) were below the lower limits of detection and consequently excluded from further analyses. With the exception of VEGF, all interassay CV were low (6.6% for VEGF, and 2.0%-3.6% for the other biomarkers). Interassay CV for the VEGF assay were between 20% and 30% for 4 samples.
2.3.6. Postoperative follow-up assessments
Four postoperative follow-up assessments were conducted: POD1, POD7, 3 months postoperative, and 6 months postoperative. On POD1, subjects were assessed in the orthopedic ward before discharge from the hospital; on this occasion, the QoR-15 was completed. Information regarding opioid consumption during the first 24 hours after surgery was extracted from the subject's electronic medical chart. On POD7, all subjects were contacted through telephone to acquire information regarding postoperative analgesic consumption (mg oxycodone). In addition, the QoR-15, BPI-sf, and a modified seven-day version of the PSQI were administered. Three and 6 months postoperatively, subjects were contacted through telephone to complete the following questionnaires: QoR-15, BPI-sf, PSQI, and WOMAC OA. All postoperative assessments were conducted by one physician (M.F.B.), before any data analyses.
2.4. Statistical analysis
The Mann–Whitney U test was used for nonparametric comparisons between groups. Bivariate correlations were evaluated using Spearman rho. Longitudinal clinical data were examined through repeated-measures analysis of variance (Bonferroni adjustment was applied for multiple pairwise comparisons). Logistic regression models were used to examine the association between CSF biomarkers and dichotomized outcomes (tested with and without adjustment for covariates, including age and sex). The following subgroup analyses were predetermined: CSF proinflammatory mediator subgroup analyses of subjects with (1) signs of CS as suspected by arm PPDT <250 kPa vs ≥250 kPa (<250 kPa cutoff as previously used,58 based on a level 2 SDs below the mean PPDT in a healthy, non-OA population89), (2) sensitization in the area of maximum pain according to WUR ≥2 vs WUR <2 (above/below median in the current data set because no relevant a priori cutoff was available), and (3) maximum pain NRS score during peripheral venous cannulation (PVC) <3 vs ≥3 (cutoff level based on PVC pain NRS score ≥3/10 being associated with increased postoperative pain intensity and opioid consumption71). There was almost no missing longitudinal follow-up data (0.2%). All data were explored and examined through numerical and graphical methods to ensure that test and model assumptions were fulfilled for the analyses. Data are presented as mean ± SD unless otherwise stated. Variables were log-transformed (natural log) as needed. Significance was accepted for P < 0.05. Analyses were performed in SPSS version 25 (IBM Corp, Armonk, NY).
2.4.1. Sample size calculation
Sample size power calculations were based on the hypothesis of biomarker differences between persistent pain patients and controls, and previously reported CSF IL-8 group differences (hip/knee OA subjects vs controls).59 Detection of a significant CSF IL-8 between-group difference, with a two-sided significance level of 5% and power of 90%, yielded an estimation of 30 pain subjects and 30 control participants. Given the a priori determined subgroup analyses, we aimed to recruit 50 persistent pain patients.
3. Results
3.1. Demographic data
The THA and control groups did not differ on mean age (70.4 ± 8.3 vs 67.8 ± 6.6 years, P = 0.21) or sex distribution (female:male 31:21 vs 16:14, P = 0.65), but the THA subjects had slightly higher body mass index (BMI) (28.0 ± 3.7 vs 25.3 ± 2.9 kg/m2, P = 0.002). All participants were Caucasian. Current smoking was rare (OA subjects 5.8%, controls 6.7%). Education level was lower among OA subjects compared to controls (7-9 years: 42.3 vs 25.0%; 10-12 years: 34.6 vs 21.4%; >12 years: 23.1 vs 53.6%, P = 0.02).
3.2. Clinical and questionnaire data
3.2.1. Comorbidities and medications
Most OA subjects were classified as ASA physical status 1 or 2; only 3 subjects were ASA 3. The most common medical comorbidities in the OA group were hypertension (n = 25) and obesity grade 1 (BMI 30-35, n = 12). The most commonly used medications were angiotensin-converting enzyme-inhibitors (n = 17) and beta-blockers (n = 12). A complete description of comorbidities and medications is shown in Appendix 2 (available online as supplemental digital content at http://links.lww.com/PAIN/B18). Control participants had received few medical diagnoses—only 3/30 exhibited any relevant clinical condition (localized lichen ruber, synovial chondromatosis of the temporomandibular joint, and unspecified joint problems). None of the 3 subjects reported current pain symptoms or used analgesic/anti-inflammatory medications.
3.2.2. Preoperative pain
Previous, but no longer ongoing, long-term pain (duration ≥3 months), besides the present OA-related pain, was reported by 55.8% (n = 29). The most common diagnoses were low back pain, knee OA, and contralateral hip OA. The mean duration of current hip OA-related pain was 2.4 ± 2.2 years. Although use of paracetamol and nonsteroidal anti-inflammatory drugs was common, preoperative opioid treatment was relatively rare (codeine [n = 6], morphine [n = 4], and oxycodone [n = 2]), and only 2 subjects were taking gabapentinoids (Appendix 2, available online as supplemental digital content at http://links.lww.com/PAIN/B18). Preoperative pain scores can be found in Table 1. The OA cohort had high pain levels with major impact on activities of daily life. According to the DN4, at least 2 neuropathic pain features were exhibited by 61.5%; a DN4 score ≥4, indicative of neuropathic pain, was detected in 15.4%. Clinically relevant pain catastrophizing was found among 9.6%. No participant in the control group had ongoing pain problems by subjective report; 23/30 never used analgesics and 7/30 reported infrequent use of nonopioid analgesics on a nondaily basis during the past week.
Table 1.
Preoperative baseline questionnaire variables for the hip OA pain subjects (n = 52).

3.2.3. Preoperative sleep status
Significant past sleep problems lasting more than 3 months were reported by 44.2% of the pain subjects. The duration of past sleep problems was 7.3 ± 8.8 years. Most OA subjects (73.1%) had poor preoperative sleep quality, as demonstrated by PSQI scores >5. Baseline PSQI and ISI scores are found in Table 1. Subjective sleep duration was 6.2 ± 1.4 hours (range, 3.0-9.0 hours). More than half of the subjects (61.5%) reported sleep problems ≥3 times/week due to night-time pain. Sleep medications such as zolpidem and zopiclone were intermittently used by 23.5%. The control participants reported very good (22.2%) or fairly good (63.0%) sleep quality during the past month; only 14.8% had fairly bad sleep quality and no controls reported very bad sleep quality.
3.2.4. Psychopathology, health-related quality of life, and physical activity
The prevalence of psychopathologies was low (Table 1). One OA subject showed evidence of depression, and 3 subjects had abnormal anxiety levels according to the HADS. As expected, HADS scores were low also for the control group (anxiety 3.7 ± 4.5; depression 2.3 ± 3.2). Only one control subject was taking an antidepressant medication (citalopram). For OA subjects, EQ-5D index scores indicated relatively poor quality of life, and as anticipated for a cohort awaiting THA, physical activity level was generally low (Table 1).
3.3. Quantitative sensory testing
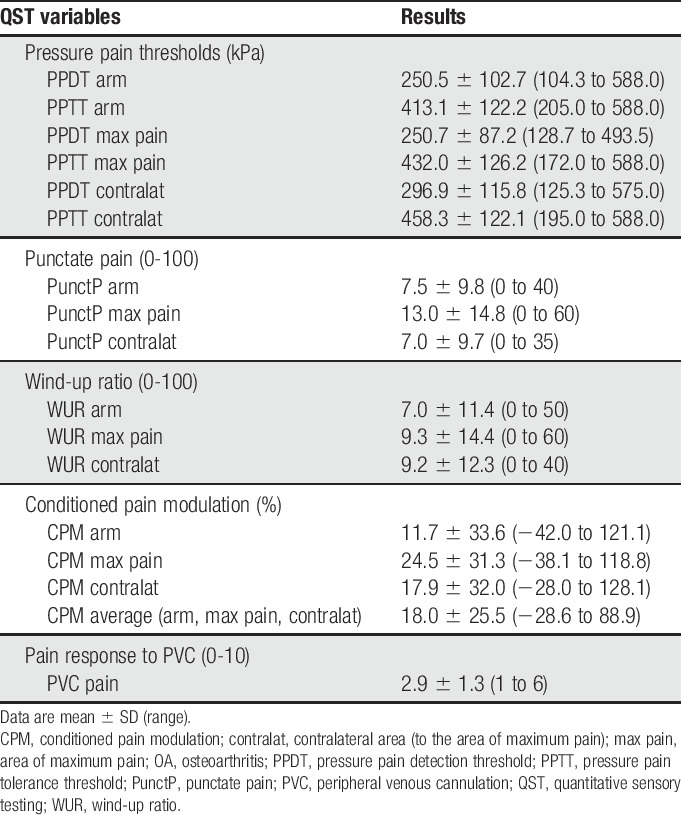
PPDTs and PPTTs are shown in Figure 1. More than half (61.5%, n = 32) of the OA subjects showed evidence of CS according to arm PPDT <250 kPa.58,89 All QST results, including pressure pain, punctate pain, WUR, conditioned pain modulation, and pain response to PVC, can be found in Table 2. Two subjects exhibited mechanical allodynia; no subject exhibited wind-up due to cotton brush stimulation. Approximately one-third of subjects (n = 15, 28.8%) showed evidence of impaired pain inhibitory capacity, as demonstrated by negative CPM% when the test stimulus was applied to the arm.
Figure 1.

Pressure pain detection and pressure pain tolerance thresholds for the hip OA pain cohort (n = 52). Area max pain, area of maximum pain; OA, osteoarthritis; PPDT, pressure pain detection threshold; PPTT, pressure pain tolerance threshold.
Table 2.
Preoperative quantitative sensory testing results for the OA pain cohort (n = 52).
3.4. Cross-sectional comparison of cerebrospinal fluid inflammatory mediators: osteoarthritis subjects vs pain-free controls
Between-group comparisons of CSF proinflammatory mediators are shown in Table 3 and Figure 2. Mean concentrations of IL-8, ICAM-1, and VCAM-1 were significantly higher in the OA pain group compared to controls. Notably, there were also relatively large numerical differences between groups regarding Flt-1 and IP-10, with higher levels of the markers in the pain group. Excluding the VEGF samples with high interassay CV did not affect the results. Several biomarkers were significantly associated with each other in both the OA and control groups (results for the OA group are shown in Table 4).
Table 3.
Cerebrospinal fluid multiplex analyses of proinflammatory mediators: OA pain group vs pain-free controls.

Figure 2.
Cerebrospinal fluid IL-8, ICAM-1, and VCAM-1: OA pain cohort (n = 52) vs healthy controls (n = 30). Between-group comparison P-value (Mann–Whitney): (A) P = 0.002, (B) P = 0.007, (C) P = 0.006. Black dots represent the mean value. CSF, cerebrospinal fluid; ICAM1, intercellular adhesion molecule 1; IL-8, interleukin 8; OA, osteoarthritis; VCAM1, vascular cell adhesion molecule 1.
Table 4.
Bivariate correlations between CSF proinflammatory mediators in the hip OA pain cohort (n = 52).
3.5. Cerebrospinal fluid inflammation related to objective measures of pain in the osteoarthritis subjects
3.5.1. Central sensitization as suspected by pressure pain threshold
Comparison of CSF biomarkers between subjects with PPDT possibly indicating CS (PPDT arm <250 kPa; n = 32) vs subjects with PPDT above the defined threshold (PPDT ≥250 kPa indicating moderate-low sensitization; n = 20) showed significantly higher mean levels of Flt-1 (40.9 ± 12.0 vs 34.4 ± 11.9 pg/mL, P = 0.044) and IP-10 (366.3 ± 219.1 vs 239.6 ± 86.1 pg/mL, P = 0.024) in the CS group (Fig. 3). Age and BMI did not differ between groups with low vs high PPDT, but there were significantly more females in the CS group compared to the moderate-low sensitization group (25 vs 6 females, χ2 P = 0.001). There were no significant between-sex differences for the whole group regarding mean Flt-1 or IP-10.
Figure 3.

Subgroup analyses within the OA pain cohort: Flt-1 and IP-10 in patients with arm PPDT <250 kPa (indicative of central sensitization; n = 32) vs arm PPDT ≥250 kPa (moderate-low sensitization; n = 20). Between-group comparison P-value (Mann–Whitney): (A) P = 0.044, (B) P = 0.024. Black dots represent the mean value. Flt-1, Fms-related tyrosine kinase 1; IP-10, interferon gamma-induced protein 10; OA; osteoarthritis; PPDT, pressure pain detection threshold; sens, sensitization.
Using logistic regression models, IP-10 predicted the binary sensitization outcome variable (ln IP-10 unadjusted odds ratio [OR] 3.9 [95% confidence interval 1.04-14.5], P = 0.044), but the effect was attenuated when sex was added to the model (sex OR 8.3 [2.1-32.7], P = 0.002; ln IP-10 OR 4.6 [0.98-21.5], P = 0.052). By contrast, sex and Flt-1 independently predicted the CS outcome (sex OR 10.6 [2.6-43.0], P = 0.001; ln Flt-1 OR 12.8 [1.2-130.6], P = 0.032).
3.5.2. High sensitization defined by wind-up ratio
Subjects with WUR ≥2 (n = 29) in the area of maximum pain had significantly higher levels of CSF MCP-1 compared to those with WUR <2 (n = 22) (341.9 ± 94.7 vs 277.2 ± 88.2 pg/mL, P = 0.011) (Fig. 4). Comparison of these 2 subgroups showed no significant differences regarding age, BMI, or sex. Multiple logistic regression showed that MCP-1 predicted WUR ≥2 (ln MCP-1 adjusted OR 10.6 [1.3-88.7], P = 0.029; model adjusted for sex, age, and BMI).
Figure 4.

Subgroup analysis within the OA pain cohort: MCP-1 in subjects with WUR ≥2 (n = 29) vs WUR <2 (n = 22) in the area of maximum pain. Between-group comparison P-value (Mann–Whitney): P = 0.011. Black dots represent the mean value. MCP-1, monocyte chemoattractant protein 1; OA, osteoarthritis; WUR, wind-up ratio.
3.5.3. High pain response to peripheral venous cannulation
Subjects who reported pain NRS score ≥3 (n = 28) vs pain NRS score <3 (n = 24) during PVC had significantly higher CSF levels of Flt-1 (40.9 ± 10.4 vs 35.4 ± 13.7 pg/mL, P = 0.025) (Fig. 5). There were no demographic differences between the 2 subgroups. There were several significant associations between pain NRS during PVC and QST outcomes: punctate pain arm (ρ = 0.476, P < 0.0001), punctate pain contralateral area (ρ = 0.314, P = 0.023), PPTT arm (ρ = −0.296, P = 0.033), and PPTT contralateral area (ρ = −0.301, P = 0.030). In a multiple logistic regression model adjusting for sex and age, Flt-1 predicted the categorical PVC pain outcome (ln Flt-1 adjusted OR 8.4 [1.1-65.4], P = 0.043).
Figure 5.

Subgroup analysis within the OA pain cohort: Flt-1 in subjects stratified according to high (NRS score ≥3) or low (NRS score <3) pain response to peripheral venous cannulation. Between-group comparison P-value (Mann–Whitney): P = 0.025. Black dots represent the mean value. Flt-1, Fms-related tyrosine kinase 1; NRS, numerical rating scale (0 = no pain, 10 = worst imaginable pain); OA, osteoarthritis.
3.6. Cerebrospinal fluid inflammation related to self-report measures of pain in the osteoarthritis subjects: explorative analyses
Analyses of baseline and follow-up subjective pain measures related to the 3 predefined objective pain phenotype categories revealed multiple significant findings.
The subjects with possible indications of CS according to arm PPDT, with elevated mean levels of Flt-1 and IP-10, had more pain-related symptoms, as shown by BPI-sf pain interference scores (POD7 4.7 ± 1.8 vs 3.7 ± 1.8, P = 0.016; 3 months postoperative 1.4 ± 2.0 vs 0.6 ± 1.5, P = 0.028; 6 months postoperative 2.1 ± 2.6 vs 0.7 ± 1.8, P = 0.052), BPI-sf pain severity scores (3 months postoperative 1.2 ± 1.2 vs 0.6 ± 1.2, P = 0.012; 6 months postoperative 2.0 ± 1.8 vs 0.9 ± 1.2, P = 0.042), and QoR-15 pain scores (3 months postoperative 17.5 ± 2.6 vs 18.9 ± 2.8, P = 0.005; 6 months postoperative 16.8 ± 4.2 vs 18.6 ± 2.5, P = 0.059). Notably, although only 4 subjects received intravenous oxycodone during the first 24 hours after surgery, all these subjects belonged to the CS group.
Compared to the group with WUR <2 in the area of maximum pain, subjects with WUR ≥2, with higher mean CSF MCP-1 levels, had more baseline pain (BPI-sf pain severity 5.7 ± 1.2 vs 4.9 ± 1.4, P = 0.042; WOMAC pain score 12.0 ± 2.7 vs 10.1 ± 3.8, P = 0.044; EQ5D pain score 3.7 ± 0.5 vs 3.3 ± 0.6, P = 0.018).
High pain response during PVC, associated with elevated CSF Flt-1 concentrations, correlated with poorer postoperative pain control on POD7 (BPI-sf pain severity ρ = 0.388, P = 0.004; pain interference ρ = 0.286, P = 0.040), more pain-related sleep problems on POD7 (PSQI subscore ρ = 0.326, P = 0.018), and higher pain levels 6 months after surgery (BPI-sf pain severity ρ = 0.308, P = 0.026).
Overall, there were few other clear patterns of significant bivariate correlations between IL-8, ICAM-1, VCAM-1, Flt-1, IP-10, and demographic, clinical, or neurophysiological baseline or follow-up variables in the whole pain cohort, or among males and females analyzed separately.
4. Discussion
4.1. Main findings
This study provides an assessment of neuroinflammatory mediators and neurophysiological pain profiles in OA subjects undergoing THA due to disabling pain. To the best of our knowledge, differential expression of multiple CSF proinflammatory mediators depending on objective characteristics of pain in humans has previously not been reported. Osteoarthritis subjects with possible evidence of CS according to the predefined pressure pain threshold cutoff level had significantly elevated CSF concentrations of Flt-1 and IP-10. Flt-1 was also increased among subjects reporting high PVC pain, a clinically relevant evoked measure of CS. Moreover, subjects who exhibited increased mechanical TS, that is, enhancement of pain during repeated noxious stimulation, had elevated CSF levels of MCP-1. Cross-sectional comparison of CSF inflammatory mediators between OA subjects and pain-free controls showed that IL-8, ICAM-1, and VCAM-1 were higher among pain subjects, which strengthens and expands the evidence implicating neuroinflammation, as characterized by multiple markers, is associated with long-term pain.
4.2. Pain mechanisms underlying persistent osteoarthritis pain
Pain is the most disabling symptom of OA.42,69 In the periphery, pain is generated due to tissue injury, inflammation, angiogenesis, and ectopic growth of sensory and sympathetic nerve fibers in the affected joint.62 Immune cells and nonneuronal cells increase the excitability of primary sensory neurons (peripheral sensitization).18 In the CNS, CS may contribute to the pain experience. Indeed, the correlation between structural joint affection and presence of pain is only weak-moderate,42 7% to 23% of hip OA patients continue to experience long-term pain after joint replacement,8 and meta-analyses show decreased PPDTs at remote sites in OA subjects compared to healthy controls.56,81 In OA rodent models, mechanisms underlying CS include macrophage infiltration of the DRG, activation of spinal cord glial cells, and increased production of proinflammatory mediators.43,45,48,61,72,81 Congruent with our results, impairment of endogenous descending pain inhibition is common in OA populations.56 Features of neuropathic pain are prevalent among patients with hip OA,30 and increasing evidence demonstrates an association between neuroinflammation and persistent neuropathic pain in humans.5,34 Comorbidities to OA, particularly sleep disturbance and affective disorders, are also related to proinflammatory changes in the CNS.44
4.3. The role of IL-8, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in pain processes
We and others have previously reported elevated CSF levels of IL-8 in persistent pain populations such as fibromyalgia,47 failed back surgery syndrome,33 lumbar disk herniation/radiculopathy,54,68,70 hip/knee OA,52,59 and postherpetic neuralgia.49,53 IL-8 (also known as CXCL8) exerts its actions through binding to the receptors CXCR1 and CXCR2.20 In the human CNS, CXCR2 is expressed on neurons, microglia, and astrocytes; glial release of IL-8 is stimulated by immune activation and afferent nociceptive input.39,65 Pain-promoting effects of IL-8 include activation of CXCR2 on nociceptors, leading to upregulation of sodium channels,87 and enhanced transient receptor potential vanilloid 1 (TRPV1) channel function,22 increasing neuronal excitability. Importantly, IL-8 drives inflammation through chemotactic effects on T lymphocytes and neutrophils.75 Although activated glia cells at multiple levels of the CNS may be the main source of IL-8,3,4,57 disruption of the blood–spinal cord barrier, through nerve injury or circulating cytokines, can facilitate transmission of inflammatory mediators and leukocytes into the CNS.25,40,55,82 Recently, reparixin, a CXCR1/2 inhibitor, was found to attenuate nocifensive behavior in a mouse model of chronic low back pain.54
In contrast to IL-8, less is known about the role of ICAM-1 and VCAM-1 in pain populations, with few studies in humans. Intercellular adhesion molecule-1 and VCAM-1 mediate interactions between vascular endothelium and leukocytes, promoting recruitment of leukocytes to inflammatory foci. Inflammatory cytokines trigger increases of ICAM-1 on the luminal surface of endothelial cells and multiple other cell types.23 In a peripheral inflammatory pain model, upregulation of ICAM-1 in the endothelium was associated with enhanced migration of β-endorphin-containing immune cells, and decreased pain behavior.60 In another study, inflammatory pain increased ICAM-1 expression at the blood–brain barrier, and induced region-specific ICAM-1 expression in cerebral microvessels correlated with microglia activation in brain areas involved with pain modulation.41 Interestingly, in a population-based study, baseline blood levels of soluble VCAM-1 independently predicted the 15-year probability of knee and hip joint replacement in OA patients.76
4.4. Links between cerebrospinal fluid neuroinflammatory mediators and pain phenotype
To the best of our knowledge, no previous study has incorporated both comprehensive QST and CSF proinflammatory mediator assessment in a pain population. Two studies have, however, included pressure pain testing in remote body sites in conjunction with examination of CSF markers.52,70 In a recent study, subgroup analysis showed a negative correlation between CSF IL-8 and spinal pressure pain threshold in male lumbar disk herniation subjects.70 By contrast, another study reported that increased CSF IL-8 levels were associated with reduced pressure pain sensitivity, albeit for only 1 out of 3 pressure pain measures.52 Importantly, in both of these studies, pressure pain testing was conducted “within a week from surgery”, whereas CSF was sampled immediately preoperative. Our QST protocol was completed approximately one hour before CSF acquisition, which optimizes interpretation of associations between pain phenotype and neuroimmune profile.
Cerebrospinal fluid Flt-1 was elevated in OA subjects demonstrating signs of CS as determined by PPDT and PVC pain response in 2 different body sites remote from the affected joint. Notably, although the overlap between the groups with CS according to low PPDT or high PVC-pain was low (18/52 = 34.6%), CSF Flt-1 was still coherently increased in both groups. Flt-1 (or VEGF receptor 1) is 1 of 3 types of receptor kinases that binds members of the VEGF family of glycoproteins. VEGF conveys multiple functions, including angiogenesis and chemotaxis. Aberrant VEGF signaling in OA tissues correlates with disease severity and pain.37 Pronociceptive mechanisms of Flt-1 signaling include indirect effects through stimulation of sensory nerve ingrowth, and direct pain sensitizing actions on neurons. Selvaraj and colleagues recently showed that Flt-1 is upregulated in sensory neurons in human and mouse cancer models, and that activation of Flt-1 leads to sensitization of TRPV1.77 Notably, systemic anti-Flt-1 antibody treatment reversed mechanical and thermal hyperalgesia.77 In a mouse knee OA model, IT inhibition of Flt-1 reduced mechanical hyperalgesia,21 indicating a CNS role of Flt-1 in pain transmission.
In addition to Flt-1, IP-10 was increased among subjects with PPDT possibly indicating CS. IP-10 is a chemokine that mediates leukocyte entry into the CNS. Although the role of IP-10 is established in several neuroinflammatory diseases,64 little is known about CNS IP-10 in human pain populations. In rodent models of neuropathic pain, mechanical allodynia and thermal hyperalgesia are associated with increased expression of IP-10, and its receptor CXCR3, in the DRG and spinal cord.9,16 The induced pain hypersensitivity is attenuated by IT injection of a CXCR3 inhibitor.16 In a metastatic cancer bone pain model, blockade of IP-10/CXCR3 decreased pain behavior and microglial activation.13
We also found that subjects with increased TS had elevated CSF MCP-1. Interestingly, the high-TS subgroup showed low overlap with both high-CS subgroups (low arm PPDT 17/52 = 32.7%; high PVC pain 15/52 = 28.8%). TS arises in part due to sensitization of second-order dorsal horn C fibers in the spinal cord.26 N-methyl-D-aspartate-receptor activation is central for TS, and MCP-1 potentiates N-methyl-D-aspartate-induced currents.31 Ample preclinical evidence implicates MCP-1 in processes underlying CS. For example, IT MCP-1 causes mechanical and thermal hypersensitivity, and conversely, IT inhibition of MCP-1 attenuates neuropathic pain sensitization.31,83 Monocyte chemoattractant protein-1 is a chemokine that promotes infiltration of leukocytes through the BSCB, and increases BSCB permeability after nerve injury.24 Monocyte chemoattractant protein-1 is released in the periphery by multiple cell types and primary afferent neurons,86 and in the CNS by activated BSCB endothelial cells,88 and spinal cord astrocytes.31 After peripheral nerve injury, MCP-1 is an important factor for microglial activation in the spinal cord, and stimulates proliferation and differentiation of macrophages/monocytes to microglia.83,91
4.5. Methodological considerations
First, the QST protocol and full set of questionnaires were only applied to the pain cohort. Although the study focused on within-group analyses among OA subjects, more detailed characterization of the control group, including neurophysiological examination, could have increased our understanding of the investigated pain processes. Second, the arm PPDT threshold <250 kPa, indicating CS, is not an established cutoff level, but was chosen based on statistics and previous research58,89; results should be interpreted accordingly. Third, no objective sleep measures were collected. For future studies, we encourage inclusion of actigraphy for acquisition of objective sleep continuity data, to enable detailed evaluation of links between perioperative sleep and pain.11 Finally, besides our predetermined group and subgroup analyses, our correlation analyses were not adjusted for multiple comparisons and should be regarded as explorative.
4.6. Conclusions
In conclusion, our results suggest that persistent pain phenotype may be associated with distinct CSF neuroinflammatory profiles in hip OA patients. Although there is no gold standard for determination of CS, our findings indicate a connection between Flt-1, IP-10, MCP-1, and measures of sensitization. Elevated CSF levels of IL-8, ICAM-1, and VCAM-1 in pain subjects compared to pain-free controls strengthens the evidence of neuroinflammation contributing to long-term pain. Given the salient links between CNS inflammation and pain perception, future mechanistic studies assessing neuroimmune mediators and QST measures of pain are encouraged. Targeting of mediators and pathways underlying neuroinflammation and glia activation may provide improved pain control for patients suffering from persistent pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B18.
Acknowledgments
Work at the authors' laboratory was supported by Stiftelsen Olle Engkvist Byggmästare, the Skåne University Hospital Foundation, the Wallenberg Center for Molecular Medicine, The Knut and Alice Wallenberg Foundation, The Medical Faculty at Lund University, Region Skåne, the European Research Council, the Swedish Research Council, the Marianne and Marcus Wallenberg foundation, the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson's disease) at Lund University, the Swedish Alzheimer Foundation, the Swedish Brain Foundation, the Torsten Söderberg Foundation, the Parkinson foundation of Sweden, The Parkinson Research Foundation, the Swedish Medical Association, the Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, and the Swedish federal government under the ALF agreement. O. Hansson has acquired research support (for the institution) from Roche, Pfizer, GE Healthcare, Biogen, AVID Radiopharmaceuticals, and Euroimmun. In the past 2 years, O. Hansson has received consultancy/speaker fees (paid to the institution) from Biogen and Roche. The authors thank all anesthesiologists, orthopedic surgeons, and nurse anesthetists at the Department of Orthopedics, Hässleholm Hospital, who contributed to the data collection of the study. The authors also thank Maria Jönsson for excellent logistical assistance and technical help.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A 2003;100:7947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ahn DK, Lee KR, Lee HJ, Kim SK, Choi HS, Lim EJ, Park JS. Intracisternal administration of chemokines facilitated formalin-induced behavioral responses in the orofacial area of freely moving rats. Brain Res Bull 2005;66:50–8. [DOI] [PubMed] [Google Scholar]
- [3].Albrecht DS, Ahmed SU, Kettner NW, Borra RJH, Cohen-Adad J, Deng H, Houle TT, Opalacz A, Roth SA, Melo MFV, Chen L, Mao J, Hooker JM, Loggia ML, Zhang Y. Neuroinflammation of the spinal cord and nerve roots in chronic radicular pain patients. PAIN 2018;159:968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Albrecht DS, Forsberg A, Sandstrom A, Bergan C, Kadetoff D, Protsenko E, Lampa J, Lee YC, Hoglund CO, Catana C, Cervenka S, Akeju O, Lekander M, Cohen G, Halldin C, Taylor N, Kim M, Hooker JM, Edwards RR, Napadow V, Kosek E, Loggia ML. Brain glial activation in fibromyalgia—a multi-site positron emission tomography investigation. Brain Behav Immun 2019;75:72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Backryd E, Lind AL, Thulin M, Larsson A, Gerdle B, Gordh T. High levels of cerebrospinal fluid chemokines point to the presence of neuroinflammation in peripheral neuropathic pain: a cross-sectional study of 2 cohorts of patients compared with healthy controls. PAIN 2017;158:2487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2:297–307. [DOI] [PubMed] [Google Scholar]
- [7].Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt L. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes following total hip or knee arthroplasty in osteoarthritis. J Orthopaedic Rheumatol 1988;1:95–108. [PubMed] [Google Scholar]
- [8].Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012;2:e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, Li B, Monahan PE, Chan DM, Ripsch MS, White FA. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain 2007;3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bjurstrom MF, Giron SE, Griffis CA. Cerebrospinal fluid cytokines and neurotrophic factors in human chronic pain populations: a comprehensive review. Pain Pract 2016;16:183–203. [DOI] [PubMed] [Google Scholar]
- [11].Bjurstrom MF, Irwin MR. Perioperative pharmacological sleep-promotion and pain control: a systematic review. Pain Pract 2019;19:552–69. [DOI] [PubMed] [Google Scholar]
- [12].Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lanteri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). PAIN 2005;114:29–36. [DOI] [PubMed] [Google Scholar]
- [13].Bu H, Shu B, Gao F, Liu C, Guan X, Ke C, Cao F, Hinton AO, Jr, Xiang H, Yang H, Tian X, Tian Y. Spinal IFN-gamma-induced protein-10 (CXCL10) mediates metastatic breast cancer-induced bone pain by activation of microglia in rat models. Breast Cancer Res Treat 2014;143:255–63. [DOI] [PubMed] [Google Scholar]
- [14].Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- [15].Buysse DJ, Reynolds CF, III, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep 1991;14:331–8. [PubMed] [Google Scholar]
- [16].Chen Y, Yin D, Fan B, Zhu X, Chen Q, Li Y, Zhu S, Lu R, Xu Z. Chemokine CXCL10/CXCR3 signaling contributes to neuropathic pain in spinal cord and dorsal root ganglia after chronic constriction injury in rats. Neurosci Lett 2019;694:20–8. [DOI] [PubMed] [Google Scholar]
- [17].Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129–38. [PubMed] [Google Scholar]
- [18].Conaghan PG, Cook AD, Hamilton JA, Tak PP. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat Rev Rheumatol 2019;15:355–63. [DOI] [PubMed] [Google Scholar]
- [19].Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- [20].Cui GB, An JZ, Zhang N, Zhao MG, Liu SB, Yi J. Elevated interleukin-8 enhances prefrontal synaptic transmission in mice with persistent inflammatory pain. Mol Pain 2012;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Das V, Kc R, Li X, O-Sullivan I, van Wijnen AJ, Kroin JS, Pytowski B, Applegate DT, Votta-Velis G, Ripper RL, Park TJ, Im HJ. Blockade of vascular endothelial growth factor receptor-1 (Flt-1), reveals a novel analgesic for osteoarthritis-induced joint pain. Gene Rep 2018;11:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dong F, Du YR, Xie W, Strong JA, He XJ, Zhang JM. Increased function of the TRPV1 channel in small sensory neurons after local inflammation or in vitro exposure to the pro-inflammatory cytokine GRO/KC. Neurosci Bull 2012;28:155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].DosSantos MF, Holanda-Afonso RC, Lima RL, DaSilva AF, Moura-Neto V. The role of the blood-brain barrier in the development and treatment of migraine and other pain disorders. Front Cell Neurosci 2014;8:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Echeverry S, Shi XQ, Rivest S, Zhang J. Peripheral nerve injury alters blood-spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J Neurosci 2011;31:10819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Echeverry S, Shi XQ, Yang M, Huang H, Wu Y, Lorenzo LE, Perez-Sanchez J, Bonin RP, De Koninck Y, Zhang J. Spinal microglia are required for long-term maintenance of neuropathic pain. PAIN 2017;158:1792–801. [DOI] [PubMed] [Google Scholar]
- [26].Eide PK. Wind-up and the NMDA receptor complex from a clinical perspective. Eur J Pain 2000;4:5–15. [DOI] [PubMed] [Google Scholar]
- [27].Eijkelkamp N, Steen-Louws C, Hartgring SA, Willemen HL, Prado J, Lafeber FP, Heijnen CJ, Hack CE, van Roon JA, Kavelaars A. IL4-10 fusion protein is a novel drug to treat persistent inflammatory pain. J Neurosci 2016;36:7353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fine PG. Long-term consequences of chronic pain: mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Med 2011;12:996–1004. [DOI] [PubMed] [Google Scholar]
- [29].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].French HP, Smart KM, Doyle F. Prevalence of neuropathic pain in knee or hip osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum 2017;47:1–8. [DOI] [PubMed] [Google Scholar]
- [31].Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci 2009;29:4096–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Giron SE, Bjurstrom MF, Griffis CA, Ferrante FM, Wu II, Nicol AL, Grogan TR, Burkard JF, Irwin MR, Breen EC. Increased central nervous system interleukin-8 in a majority postlaminectomy syndrome chronic pain population. Pain Med 2018;19:1033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol 2014;14:217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Haight ES, Forman TE, Cordonnier SA, James ML, Tawfik VL. Microglial modulation as a target for chronic pain: from the bench to the bedside and back. Anesth Analg 2019;128:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hall S, Janelidze S, Surova Y, Widner H, Zetterberg H, Hansson O. Cerebrospinal fluid concentrations of inflammatory markers in Parkinson's disease and atypical parkinsonian disorders. Sci Rep 2018;8:13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hamilton JL, Nagao M, Levine BR, Chen D, Olsen BR, Im HJ. Targeting VEGF and its receptors for the treatment of osteoarthritis and associated pain. J Bone Miner Res 2016;31:911–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Horuk R, Martin AW, Wang Z, Schweitzer L, Gerassimides A, Guo H, Lu Z, Hesselgesser J, Perez HD, Kim J, Parker J, Hadley TJ, Peiper SC. Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol 1997;158:2882–90. [PubMed] [Google Scholar]
- [40].Hu P, Bembrick AL, Keay KA, McLachlan EM. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun 2007;21:599–616. [DOI] [PubMed] [Google Scholar]
- [41].Huber JD, Campos CR, Mark KS, Davis TP. Alterations in blood-brain barrier ICAM-1 expression and brain microglial activation after lambda-carrageenan-induced inflammatory pain. Am J Physiol Heart Circ Physiol 2006;290:H732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019;393:1745–59. [DOI] [PubMed] [Google Scholar]
- [43].Im HJ, Kim JS, Li X, Kotwal N, Sumner DR, van Wijnen AJ, Davis FJ, Yan D, Levine B, Henry JL, Desevre J, Kroin JS. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum 2010;62:2995–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol 2019;19:702–15. [DOI] [PubMed] [Google Scholar]
- [45].Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science 2016;354:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018;129:343–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kadetoff D, Lampa J, Westman M, Andersson M, Kosek E. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J Neuroimmunol 2012;242:33–8. [DOI] [PubMed] [Google Scholar]
- [48].Kawarai Y, Orita S, Nakamura J, Miyamoto S, Suzuki M, Inage K, Hagiwara S, Suzuki T, Nakajima T, Akazawa T, Ohtori S. Changes in proinflammatory cytokines, neuropeptides, and microglia in an animal model of monosodium iodoacetate-induced hip osteoarthritis. J Orthop Res 2018;36:2978–86. [DOI] [PubMed] [Google Scholar]
- [49].Kikuchi A, Kotani N, Sato T, Takamura K, Sakai I, Matsuki A. Comparative therapeutic evaluation of intrathecal versus epidural methylprednisolone for long-term analgesia in patients with intractable postherpetic neuralgia. Reg Anesth Pain Med 1999;24:287–93. [DOI] [PubMed] [Google Scholar]
- [50].Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. PAIN 2000;88:69–78. [DOI] [PubMed] [Google Scholar]
- [51].Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J Pain 2000;4:229–38. [DOI] [PubMed] [Google Scholar]
- [52].Kosek E, Finn A, Ultenius C, Hugo A, Svensson C, Ahmed AS. Differences in neuroimmune signalling between male and female patients suffering from knee osteoarthritis. J Neuroimmunol 2018;321:49–60. [DOI] [PubMed] [Google Scholar]
- [53].Kotani N, Kushikata T, Hashimoto H, Kimura F, Muraoka M, Yodono M, Asai M, Matsuki A. Intrathecal methylprednisolone for intractable postherpetic neuralgia. N Engl J Med 2000;343:1514–19. [DOI] [PubMed] [Google Scholar]
- [54].Krock E, Millecamps M, Anderson KM, Srivastava A, Reihsen TE, Hari P, Sun YR, Jang SH, Wilcox GL, Belani KG, Beebe DS, Ouellet J, Pinto MR, Kehl LJ, Haglund L, Stone LS. Interleukin-8 as a therapeutic target for chronic low back pain: upregulation in human cerebrospinal fluid and pre-clinical validation with chronic reparixin in the SPARC-null mouse model. EBioMedicine 2019;43:487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lim TK, Shi XQ, Martin HC, Huang H, Luheshi G, Rivest S, Zhang J. Blood-nerve barrier dysfunction contributes to the generation of neuropathic pain and allows targeting of injured nerves for pain relief. PAIN 2014;155:954–67. [DOI] [PubMed] [Google Scholar]
- [56].Lluch E, Torres R, Nijs J, Van Oosterwijck J. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. Eur J Pain 2014;18:1367–75. [DOI] [PubMed] [Google Scholar]
- [57].Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, Riley M, Wasan AD, Zurcher NR, Albrecht DS, Vangel MG, Rosen BR, Napadow V, Hooker JM. Evidence for brain glial activation in chronic pain patients. Brain 2015;138:604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Luna IE, Kehlet H, Jensen CM, Christiansen TG, Lind T, Stephensen SL, Aasvang EK. The effect of preoperative intra-articular methylprednisolone on pain after TKA: a randomized double-blinded placebo controlled trial in patients with high-pain knee osteoarthritis and sensitization. J Pain 2017;18:1476–87. [DOI] [PubMed] [Google Scholar]
- [59].Lundborg C, Hahn-Zoric M, Biber B, Hansson E. Glial cell line-derived neurotrophic factor is increased in cerebrospinal fluid but decreased in blood during long-term pain. J Neuroimmunol 2010;220:108–13. [DOI] [PubMed] [Google Scholar]
- [60].Machelska H, Mousa SA, Brack A, Schopohl JK, Rittner HL, Schafer M, Stein C. Opioid control of inflammatory pain regulated by intercellular adhesion molecule-1. J Neurosci 2002;22:5588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Malfait AM, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol 2013;9:654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol 2012;8:390–8. [DOI] [PubMed] [Google Scholar]
- [63].McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol 2005;192:444–62. [DOI] [PubMed] [Google Scholar]
- [64].Michlmayr D, McKimmie C. Role of CXCL10 in central nervous system inflammation. Int J Interferon Cytokine Mediator Res 2014;6:1–18. [Google Scholar]
- [65].Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 2009;10:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Milligan ED, Penzkover KR, Soderquist RG, Mahoney MJ. Spinal interleukin-10 therapy to treat peripheral neuropathic pain. Neuromodulation 2012;15:520–6; discussion 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth 2019;123:e273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nagashima H, Morio Y, Yamane K, Nanjo Y, Teshima R. Tumor necrosis factor-alpha, interleukin-1beta, and interleukin-6 in the cerebrospinal fluid of patients with cervical myelopathy and lumbar radiculopathy. Eur Spine J 2009;18:1946–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013;21:1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Palada V, Ahmed AS, Finn A, Berg S, Svensson CI, Kosek E. Characterization of neuroinflammation and periphery-to-CNS inflammatory cross-talk in patients with disc herniation and degenerative disc disease. Brain Behav Immun 2019;75:60–71. [DOI] [PubMed] [Google Scholar]
- [71].Persson AK, Pettersson FD, Dyrehag LE, Akeson J. Prediction of postoperative pain from assessment of pain induced by venous cannulation and propofol infusion. Acta Anaesthesiol Scand 2016;60:166–76. [DOI] [PubMed] [Google Scholar]
- [72].Raoof R, Willemen H, Eijkelkamp N. Divergent roles of immune cells and their mediators in pain. Rheumatology (Oxford) 2018;57:429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain 2000;4:247–57. [DOI] [PubMed] [Google Scholar]
- [74].Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. PAIN 2006;123:231–43. [DOI] [PubMed] [Google Scholar]
- [75].Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol 2008;9:949–52. [DOI] [PubMed] [Google Scholar]
- [76].Schett G, Kiechl S, Bonora E, Zwerina J, Mayr A, Axmann R, Weger S, Oberhollenzer F, Lorenzini R, Willeit J. Vascular cell adhesion molecule 1 as a predictor of severe osteoarthritis of the hip and knee joints. Arthritis Rheum 2009;60:2381–9. [DOI] [PubMed] [Google Scholar]
- [77].Selvaraj D, Gangadharan V, Michalski CW, Kurejova M, Stosser S, Srivastava K, Schweizerhof M, Waltenberger J, Ferrara N, Heppenstall P, Shibuya M, Augustin HG, Kuner R. A functional role for VEGFR1 expressed in peripheral sensory neurons in cancer pain. Cancer Cell 2015;27:780–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Smith A, Pedler A. Conditioned pain modulation is affected by occlusion cuff conditioning stimulus intensity, but not duration. Eur J Pain 2018;22:94–102. [DOI] [PubMed] [Google Scholar]
- [79].Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology 2013;118:1332–40. [DOI] [PubMed] [Google Scholar]
- [80].Sullivan MJL, Bishop S, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- [81].Suokas AK, Walsh DA, McWilliams DF, Condon L, Moreton B, Wylde V, Arendt-Nielsen L, Zhang W. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage 2012;20:1075–85. [DOI] [PubMed] [Google Scholar]
- [82].Sweitzer SM, Hickey WF, Rutkowski MD, Pahl JL, DeLeo JA. Focal peripheral nerve injury induces leukocyte trafficking into the central nervous system: potential relationship to neuropathic pain. PAIN 2002;100:163–70. [DOI] [PubMed] [Google Scholar]
- [83].Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain 2009;13:263–72. [DOI] [PubMed] [Google Scholar]
- [84].Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet 2011;377:2226–35. [DOI] [PubMed] [Google Scholar]
- [85].van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, Lloyd A, Scalone L, Kind P, Pickard AS. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708–15. [DOI] [PubMed] [Google Scholar]
- [86].Van Steenwinckel J, Reaux-Le Goazigo A, Pommier B, Mauborgne A, Dansereau MA, Kitabgi P, Sarret P, Pohl M, Melik Parsadaniantz S. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J Neurosci 2011;31:5865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wang JG, Strong JA, Xie W, Yang RH, Coyle DE, Wick DM, Dorsey ED, Zhang JM. The chemokine CXCL1/growth related oncogene increases sodium currents and neuronal excitability in small diameter sensory neurons. Mol Pain 2008;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wu F, Liu L, Zhou H. Endothelial cell activation in central nervous system inflammation. J Leukoc Biol 2017;101:1119–32. [DOI] [PubMed] [Google Scholar]
- [89].Wylde V, Palmer S, Learmonth ID, Dieppe P. Test-retest reliability of Quantitative Sensory Testing in knee osteoarthritis and healthy participants. Osteoarthritis Cartilage 2011;19:655–8. [DOI] [PubMed] [Google Scholar]
- [90].Yarnitsky D, Bouhassira D, Drewes AM, Fillingim RB, Granot M, Hansson P, Landau R, Marchand S, Matre D, Nilsen KB, Stubhaug A, Treede RD, Wilder-Smith OH. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain 2015;19:805–6. [DOI] [PubMed] [Google Scholar]
- [91].Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci 2007;27:12396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B18.




