Supplemental Digital Content is Available in the Text.
Glyceryl trinitrate can provoke premonitory symptoms, namely yawning, nausea, photophobia, and concentration difficulties, which are most specific for an impending migraine-like headache.
Keywords: Migraine, Glyceryl trinitrate, Premonitory symptoms, Prodromal symptoms
Abstract
Spontaneous and pharmacologically provoked migraine attacks are frequently preceded by nonheadache symptoms called premonitory symptoms. Here, we systematically evaluated premonitory symptoms in migraine patients and healthy controls after glyceryl trinitrate (GTN) infusion. In women with migraine without aura (n = 34) and age-matched female controls (n = 24), we conducted systematically a semistructured interview assessing 21 possible premonitory symptoms every 15 minutes in the 5 hours after GTN infusion (0.5 µg/kg/min over 20 minutes). Migraine-like headaches occurred in 28/34 (82.4%) migraineurs (GTN responders). After GTN, 26/28 (92.9%) responders, 6/6 (100%) nonresponders, and 13/24 (54.2%) controls reported at least one possible premonitory symptom. Concentration difficulties (P = 0.011), yawning (P = 0.009), nausea (P = 0.028), and photophobia (P = 0.001) were more frequently reported by those migraineurs who developed a migraine-like attack vs healthy controls. Importantly, concentration difficulties were exclusively reported by those who developed a migraine-like attack. Thus, our findings support the view that GTN is able to provoke the naturally occurring premonitory symptoms and show that yawning, nausea, photophobia, and concentration difficulties are most specific for an impending GTN-induced migraine-like headache. We suggest that these symptoms may also be helpful as early warning signals in clinical practice with concentration difficulties exclusively reported by those who develop a migraine-like attack.
1. Introduction
Migraine is a highly prevalent episodic neurovascular brain disorder, causing severe disability worldwide.15,28,42 In up to 90% of patients, the onset of a migraine attack is characterized by a premonitory phase with accompanying nonheadache symptoms called premonitory symptoms.24,40,44 Premonitory symptoms are able to precede the migraine headache up to 72 hours12 and may persist in the subsequent aura (if applicable) and headache phase, even up to the postdromal phase.11,12,20,24 Some of these nonheadache symptoms as photophobia, phonophobia, nausea, and vomiting are also included as associated symptoms in the criteria for migrainous headache.21
Reported premonitory symptoms are numerous and span various categories such as homeostatic alterations, sensory sensitivities, mood, cognitive, and fatigue symptoms, which are regularly mislabelled by patients as triggers.16,24,25 Frequently reported symptoms include concentration impairment, tiredness/fatigue, food cravings, irritability, yawning, photophobia, and neck stiffness.7,27,40 Given the nature and often circadian rhythmicity of premonitory symptoms, the hypothalamus with orexinergic (sleep regulation and feeding) and dopaminergic (yawning and nausea) systems has been suggested to be implicated.16 This is supported by neuroimaging findings showing hypothalamic involvement preceding and during spontaneous9,41 and pharmacologically triggered migraine(-like) attacks.30 The presence and endurance of premonitory symptoms into consecutive migraine phases have also been suggested to occur when triggered by pharmacological substances.17,18 The most well-known migraine provocation model relies on intravenous infusion with the nitric oxide donor glyceryl trinitrate (GTN),6 and premonitory symptoms have also been reported after GTN infusion,1,30 with activation of hypothalamic regions preceding the onset of migraine-like headaches.30
Premonitory symptoms may go unrecognized by patients unless specifically asked for, due to their limited specificity which is illustrated by the reported prevalence ranging from 7% to 88% of patients.24,39,44 Common and nonspecific nonheadache symptoms as yawning, food craving, and tiredness/fatigue are likely also experienced on a day-to-day basis by the general population not suffering from migraine.12 However, because healthy controls logically do not suffer from migraine attacks, no prevalence studies or pharmacological provocation studies systematically and prospectively investigated the onset and specificity of these symptoms in migraine patients compared with healthy volunteers. Pharmacological migraine models provide an opportunity for clinical investigation of symptoms occurring before and during an attack under precisely controlled and regulated conditions5,6 and allow for detailed prospective monitoring of the presence and timing of symptom onset. Detailed knowledge on premonitory symptoms could offer insights into pathophysiological mechanisms involved in the early phases of a migraine attack.
In this study, we aimed to compare the presence of premonitory symptoms after GTN under matched conditions between age-matched female migraine patients and healthy controls, and between attack responders and nonresponders. Second, we aimed to investigate the timing and persistence of these nonheadache symptoms after GTN administration.
2. Methods
2.1. Participants
We included 2 age-matched study groups consisting of female migraine without aura patients (n = 37) and healthy female controls (n = 25). Migraine without aura was diagnosed in accordance with the International Classification of Headache Disorders (ICHD-3).21 Participants with migraine experienced at least one migraine attack per month during the 6 months before the investigation, did not have chronic migraine or medication-overuse headache, and were otherwise healthy. Healthy female controls were free of any neurological or psychiatric disorders and primary or secondary headaches apart from occasional episodic tension-type headache. Furthermore, healthy controls did not report a first degree family member with migraine or trigeminal autonomic cephalalgia. None of the participants used any chronic medication other than possibly oral contraceptives. Participants in this study were part of an imaging study consisting of 3 scan sessions during a single day, aimed at the onset of migraine-like attacks triggered by GTN. Participants were recruited from the Leiden University Medical Center Migraine Neuro Analysis project in which migraineurs and controls from the Dutch population are listed who have agreed to participate in migraine-related scientific research and by public advertisement.37 The study was approved by the medical ethics committee of the Leiden University Medical Center. All participants provided written informed consent before participation.
2.2. Study design
In this longitudinal prospective study, participants arrived at 07:30 am at the hospital in a nonfasting state. Participants were instructed to abstain from alcoholic beverages, caffeinated beverages, and smoking for at least 8 hours before the first scan session and were allowed to eat during the course of the day. Migraineurs did not use any prophylactic medication for at least 4 weeks and were at least 3 days attack-free before the investigation. At the hospital, participants were interviewed and underwent a neurological examination before undergoing 3 magnetic resonance imaging scans. The interview included questions regarding migraine and headache frequency, presence of headache, and 21 premonitory symptoms. Between 9:45 and 10:45 am, GTN (0.5 µg/kg/min over 20 minutes) was administered by intravenous infusion with the participant in supine position. Participants were informed GTN could potentially induce headache but were not informed of the timing or characteristics of the headache. Five minutes before the start of the GTN infusion, the presence of possible premonitory symptoms, headache, and associated symptoms was registered. During the actual GTN infusion (20 minutes), headache and associated symptoms were documented every 5 minutes. After this infusion period, the occurrence of premonitory symptoms, headache, and associated symptoms were documented every 15 minutes until 5 hours after GTN infusion, except for the time around MR scanning (directly before MR scanning [only headache and associated symptoms] and during MR scanning [no questionnaire]). After the study day, participants were asked to fill in a headache diary at home for 7 days after the study day (participants with migraine also filled in a headache diary 7 days before the study day).
2.3. Premonitory symptoms and migraine-like attacks
The ICHD-3 defines premonitory symptoms as symptoms warning of a migraine attack and occurring 48 hours before onset.21 In migraine with aura, this means before the first aura signs and in migraine without aura before the onset of pain. We included only migraine without aura patients. Migraine provocation with GTN typically follows a biphasic pattern; it first induces immediate headache in migraine patients as well as healthy controls, after which migraineurs may develop a delayed headache fulfilling the criteria for migraine without aura within 12 hours (Fig. 1).5 This biphasic pattern limits the applicability of the ICHD-3 criteria for premonitory symptoms.21 Therefore, in accordance with other provocation studies, the following definition for pharmacological triggered premonitory symptoms was applied: premonitory symptoms are nonheadache symptoms before (preictal) the onset of a provoked migrainous headache.1,30 Migraine-like attack onset (ictal) was determined according to criteria as used in similar previously published provocation studies4,6,18,19 as attacks fulfilling either (1) moderate to severe headache (verbal rating scale [VRS] ≥4) fulfilling ICHD-3 criteria C and D for migraine without aura or (2) headache described as mimicking patients usual migraine attack and treated with acute migraine medication.
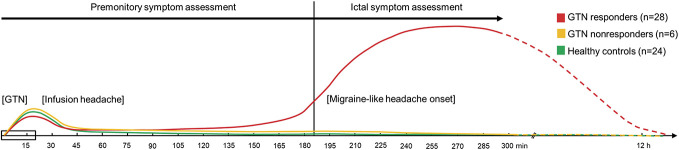
Figure 1.
Schematic headache pattern after the start of the GTN infusion in the 3 study groups. In red, a typical headache pattern for migraine patients who respond to GTN (GTN responders) is shown, combined with typical patterns for migraine patients who do not respond to GTN (GTN nonresponders) in orange and healthy controls in green. Data on the presence of nonheadache symptoms were collected for 5 hours after GTN infusion indicated by solid lines in the headache response and symptom assessments (premonitory and ictal). GTN, glyceryl trinitrate infusion (GTN; 0.5 µg/kg/min for 20 minutes).
2.4. Questionnaires
In this study, we recorded the presence of 21 possible premonitory symptoms, possible aura symptoms, and a detailed headache assessment including known migrainous headache associated symptoms, verbal pain score, type of pain, and location. Premonitory symptoms included fatigue, yawning, thirst, concentration difficulties, craving for sweets, fluid retention, neck stiffness, face or extremity stiffness, feeling depressed, feeling irritated, decreased appetite, increased appetite, mental restlessness, physical restlessness, decreased urination, altered bowel habits, sleep disturbances, photophobia, phonophobia, nausea, and vomiting. Associated nonheadache symptoms as photophobia, phonophobia, nausea, and vomiting were also listed as part of the headache assessment. After conclusion of the study day in the hospital (5 hours after GTN infusion), participants registered the onset of headache in a headache dairy. Participants were also asked for headache fitting migraine-like attack onset in the telephone follow-up ±3 days after participation, to determine GTN responder status.
2.5. Statistical analysis
Descriptive statistics were reported as mean ± SDs or percentages. Differences in mean values were compared between groups using an independent-samples t test or Mann–Whitney U test for continuous data and a Fisher's exact test for categorical data. In this study, clinical data collected as part of an extensive imaging study was used; therefore, no a priori power calculation was performed. As primary outcome, we investigated the incidence of nonheadache symptoms between GTN responders (developed a migraine-like attack) vs GTN nonresponders and GTN responders vs healthy controls using a Fisher's exact test. Explored intervals included before (preictal) and during the onset of the migraine-like attack (ictal) and over the entire study period (0-5 hours). As GTN nonresponders and healthy controls do not develop a migraine-like, we determined the median interval for the responder group and applied this to the other groups to ensure the number of observations matched between groups for the preictal and ictal intervals. P-values < 0.05 were considered to indicate statistical significance and were not adjusted for multiple testing. All statistical analyses were performed using SPSS 23.0 (SPSS, Inc, IBM).
3. Results
3.1. Clinical characteristics
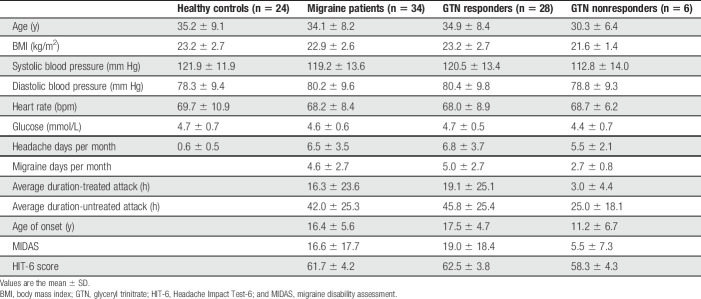
In total, we started with n = 37 participants with migraine and n = 25 healthy controls in this study. Four participants were excluded; 3 participants with migraine and 1 healthy control (Fig. 2). The healthy control was excluded because of a provoked migraine-like headache. One migraine patient was excluded because we could not classify the provoked headache attack (did not fully fulfill migraine-like headache but could also not be classified as a nonresponder); 2 migraine patients were excluded because GTN infusion was not performed due to claustrophobia as both patients refused further participation after the first scan session. Thus, data from n = 34 participants with migraine and n = 24 healthy controls were suitable for analysis. Participants with migraine and healthy controls were of similar age (34.1 ± 8.2 vs 35.2 ± 9.1 years) and body mass index (22.9 ± 2.6 vs 23.2 ± 2.7). Among the clinical variables, there were no significant differences between groups except for the average headache days per month, which was higher for participants with migraine (Table 1).
Figure 2.
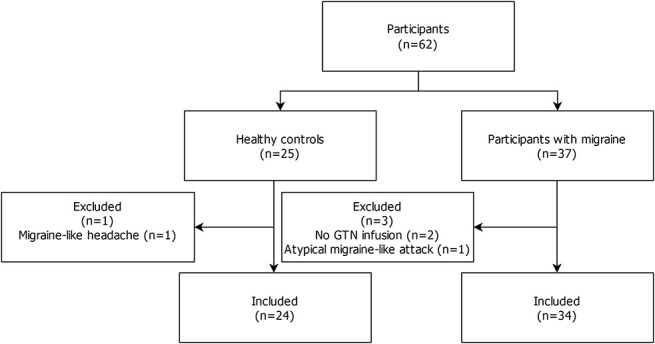
Study flowchart. GTN, glyceryl trinitrate.
Table 1.
Clinical characteristics of the healthy controls and migraine patients, subdivided GTN responders and GTN nonresponders.
3.2. Migraine-like attack provocation
Twenty-eight participants with migraine (82.4%) experienced a migraine-like attack after GTN infusion and were defined as responders. Migraine-like attack characteristics of GTN responders are shown in e-table 1, supplementary materials (available at http://links.lww.com/PAIN/B2), reported onset ranged between 45 and 345 minutes. Headache experienced by the study groups during and after GTN infusion is shown in Figure 3. Glyceryl trinitrate responders were significantly older at disease onset (17.5 ± 4.7 vs 11.2 ± 6.7), had a higher disability expressed in migraine disability assessment (MIDAS) and HIT-6 scores, and experienced more migraine days per month (5.0 ± 2.7) compared with nonresponders, see Table 1.
Figure 3.

Verbal rating scale over time. (A) Individual and median verbal rating scores are depicted for participants with migraine developing a migraine-like attack after glyceryl trinitrate (GTN responders; individual cases = various colors with diamonds, median = black line with diamonds), (B) participants with migraine who did not develop an attack (GTN nonresponders; individual cases = various colors with squares, median = black line with squares), and (C) healthy controls (individual cases = various colors with circles, median = black line with circles). (D) Median verbal rating scores are depicted for GTN responders (dots), GTN nonresponders (left diagonal lines), and healthy controls (right diagonal lines) after glyceryl trinitrate. Note, 2 controls reported a high VRS of 7 at the end of the study (see C). Clinically, there was a discrepancy between the reported subjective VRS and how this objectively was interpreted by the investigator, and the headache did not fulfill criteria for migraine-like headache. GTN, glyceryl trinitrate; VRS, verbal rating scale.
3.3. Premonitory and ictal nonheadache symptoms
To inspect onset after GTN, symptoms were summed per assessment (0-5 hours) for responders and healthy controls (Fig. 4) and nonresponders and responders (e-figure 1, supplementary materials, available at http://links.lww.com/PAIN/B2). Yawning, fatigue, thirst, neck stiffness, concentration difficulties, nausea, and photophobia were among the earliest frequently reported symptoms. Yawning, nausea, and photophobia appeared to be more specific for responders, and concentration difficulties were even exclusively reported by responders. Fatigue, thirst, and neck stiffness were regularly reported by both responders and healthy controls in the first hours after GTN. Symptoms most frequently reported by responders included fatigue (82.1%), photophobia (82.1%), nausea (75.0%), yawning (64.3%), phonophobia (60.7%), thirst (57.1%), and neck stiffness (53.6%), see e-table 2, supplementary materials (available at http://links.lww.com/PAIN/B2). For symptoms reported by each individual participant, see e-figures 2 (responders), 3 (nonresponders), and 4 (controls), supplementary materials (available at http://links.lww.com/PAIN/B2).
Figure 4.

Reported nonheadache symptoms over time (0-5 hours) by GTN responders (red) and healthy controls (green). The reported median onset for migraine-like attacks (181 minutes; making this after the questionnaire taken at 195 minutes after GTN infusion) is depicted by a dashed line, illustrating the preictal and ictal period. GTN, glyceryl trinitrate.
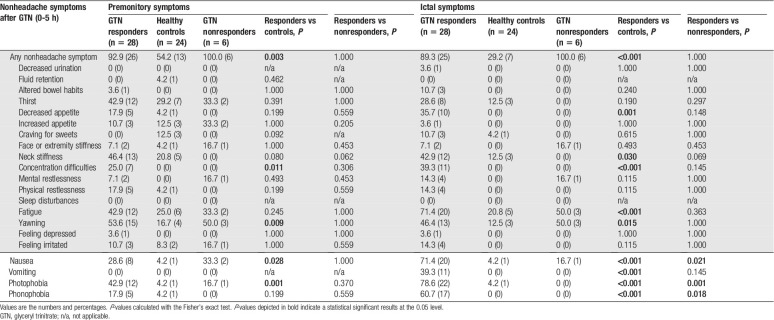
Almost all GTN responders (92.9%) reported at least one of the 21 premonitory symptoms after GTN infusion before the onset of migraine-like headache compared with 100% of GTN nonresponders and 54.2% of healthy controls. Responders reported concentration difficulties (P = 0.011), yawning (P = 0.009), nausea (P = 0.028), and photophobia (P = 0.001) more frequently compared with controls (Table 2). To validate our findings, we performed a sensitivity analysis excluding 2 controls with a high VRS at the end of the study day (Fig. 3); this had only marginal effects and did not affect our main findings except for nausea (which did not reach significance [P = 0.060]) due to reduced power as none of excluded controls suffered from nausea (data not shown). No significant differences in reported symptoms were found between responders and nonresponders apart from reported associated symptoms during the provoked migrainous headache phase with nausea, photophobia, and phonophobia in the responders, which is logical as these symptoms form part of the criteria for defining a provoked migraine-like attack. The significant premonitory symptoms persisted ictally, and fatigue, neck stiffness, decreased appetite, vomiting, and phonophobia were also reported more frequently by responders compared with controls during this phase (Table 2).
Table 2.
Nonheadache symptoms reported after the start of GTN infusion.
3.4. Symptoms in relation to migraine-like attack onset
Of the 4 identified specific symptoms, we accessed which symptom was reported first by each GTN responder (Fig. 5A). Yawning and nausea were reported as initial specific premonitory symptom by 8 responders, followed closely by photophobia (n = 7), while concentration difficulties were reported the fewest times (n = 4). To inspect the onset of yawning, concentration difficulties, nausea, and photophobia with respect to onset of the migraine-like attack, we plotted the interquartile range (middle 50%) of the average reported time across responders. Yawning is reported first followed by nausea, photophobia, and concentration difficulties (Fig. 5B). As the reaction to GTN often follows a biphasic headache pattern (an immediate headache that resides, and later, a migraine-like headache develops, Fig. 1), we checked whether these symptoms were (in)dependent from headache. Fourteen responders with pain-free intervals after GTN infusion ended were inspected and checked for the occurrence of these symptoms in the postinfusion phases without headache. Yawning, nausea, and photophobia were also reported in the headache-free intervals, while concentration difficulties were not mentioned in a pain-free state.
Figure 5.
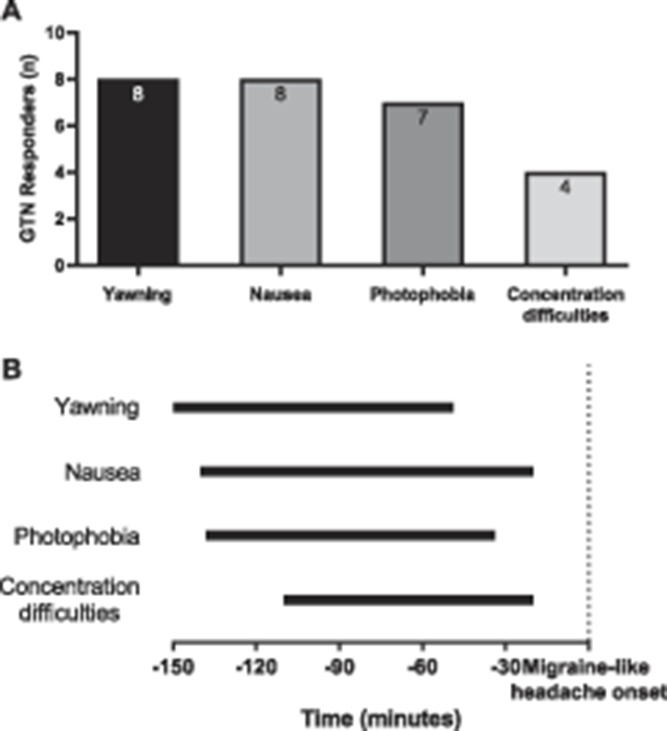
Timing of premonitory symptoms. (A) Premonitory symptoms reported first. Depicted is the number of GTN responders who reported a particular specific premonitory symptom first. In case 2 symptoms were reported simultaneously (P23 and P37, see e-figure 2, http://links.lww.com/PAIN/B2), both symptoms were plotted. Two GTN responders (P19 and P24) not reporting premonitory symptoms, and P3 that reported none of the identified specific premonitory symptoms, were not included (see e-figure 2, http://links.lww.com/PAIN/B2). (B) Timing of premonitory symptoms relative to onset of migraine-like headache. Depicted are the interquartile ranges plotted for yawning, nausea, photophobia, and concentration difficulties composed of all responders that reported these symptoms relative to the onset of the migraine-like headache attacks. GTN, glyceryl trinitrate.
4. Discussion
We performed a systematic assessment into the specificity and development of premonitory symptoms in GTN-provoked migraine-like attacks. Because of the inclusion of a control group and an extensive prospective assessment under matched conditions, we were able to show for the first time that concentration difficulties were exclusively reported by those who developed a migraine-like attack. Yawning and nausea were the earliest symptoms reported, followed by photophobia and concentration difficulties that occurred nearer to attack onset. Neck stiffness, fatigue, decreased appetite, vomiting, and phonophobia though associated with migraine-like headache lacked specificity preictally.
Having a closer look at the 4 most specific premonitory symptoms identified in the current study, excessive yawning is the earliest reported symptom in responders relative to attack onset, although there is considerable overlap between 4 identified symptoms, and on participant level, nausea and photophobia were also reported frequently as initial specific symptom. The anatomical basis of yawning in migraine is typically linked to the hypothalamus,24 which has been shown to be involved in spontaneous9,41 and provoked attack onset in imaging studies.30 Nausea, also reported early, might also be linked to this same brain region24,33 but may also be linked to the rostral dorsal medulla and the periaqueductal gray.32 Apart from imaging evidence, the circadian rhythmicity of attacks2,3,36 and the association with the menstrual cycle10,29,35 also point to hypothalamic involvement in migraine.33 Furthermore, on a neurotransmitter level, the dopaminergic system might be a driving force because dopaminergic agonists have been reported to increase among others yawning and nausea in migraine patients, while antagonists are used to treat nausea.34 Photophobia, also identified in the current study, is also among the early reported symptoms and has been suggested to involve the thalamus or cortical areas of the brain.24,31 Projections between the posterior hypothalamus and thalamus have been previously reported.22,33 This is consistent with brain activation of i.a. the thalamus and occipital cortex as shown in the late premonitory phase in GTN-triggered attacks.30 Concentration difficulties with a late onset, and likely involving cortical areas, are the most specific because it is only reported by responders.
Cognitive dysfunction in general, difficulty with focusing, concentration, speech, and reading, is among the most frequently reported complaint during the premonitory phase across studies.13 This impairment may remain during the migraine headache and postdromal phase and has been objectively measured to affect migraineurs during attacks.43 Interictal studies yield inconsistent results due to differences in clinical characteristics, recruitment methodology, migraine preventative usage, and comorbidities.14,43 In our study, concentration difficulties was an unique premonitory symptom only reported in migraine patients with a provoked attack. Notably, also our GTN nonresponders and controls who experienced infusion headache never reported concentration problems. Difficulty in concentrating has been reported for in other headache types such as cluster headache and tension-type headache, but in these conditions, it was purely related to severe pain during the headache,43 while in migraine concentration difficulty is frequently reported in the premonitory phase in the absence of headache.11,12 However, we also noticed that concentration difficulties were temporally the closest to the migraine headache onset in our study when a mild headache was already developing. Importantly, concentration difficulties were exclusively reported by those who developed a migraine-like attack and may be a good early warning signal that may be helpful in clinical practice for patients.
Our results indicate that the hypothalamus (yawning and nausea), the brainstem (nausea), thalamus (photophobia), and cortical areas (photophobia and concentration difficulties) are involved in the premonitory phase with concentration difficulties being unique to GTN responders. Whether the hypothalamus is indeed activated first, which is suggested on a group level, cannot be reliably deduced from the current study because nausea (also attributed to the brainstem), photophobia, and concentration difficulties are also reported as initial symptoms by a considerable number of GTN responders. Although all responders reported at least one of the 4 identified symptoms, excluding the 2 responders without premonitory symptoms and 1 responder that reported none of the specific symptoms, not all 4 symptoms were present in each participant. This may suggest different brain structures, in different migraineurs, in different temporal sequences may be involved in the premonitory phase of migraine. To investigate affected brain structures and brain networks, future provocation studies might use detailed imaging methods (such as [resting state] functional magnetic resonance imaging) to follow-up the sequence of reported symptoms. Interestingly, premonitory symptoms persist during ictal and postictal phases, as shown in this study and in literature,11,12,17 substantially lengthening the actual attack duration from 4 to 72 hours (headache) and making the headache only one of the symptoms in the intricate cascade. This chain of symptomology has led to the hypothesis of an oscillating system of complex brain networks influencing the susceptibility threshold of sensory signals and bodily functions toward the onset of migrainous headache.8,34,38
Previous studies with GTN as a pharmacological trigger reported many premonitory symptoms before the onset of migraine-like headache.1,23,30 However, several symptoms such as neck stiffness, tiredness/fatigue, thirst, feeling moody/irritated, and phonophobia were also frequently reported by our healthy volunteers and thus lack specificity as early warning signal for onset of a migrainous headache. Whether the reported symptoms present in half of the controls after GTN infusion represent other brain areas or networks, incompletely activated network(s), GTN substance-related symptomatology, or symptoms reported due to the repeated and intensive interviews during the study procedure, cannot be deduced with certainty as no placebo group was included. However, we consider the inclusion of a control group as one of the strengths of our study allowing to identify symptoms specifically associated with migraine. For case-control studies, there is a potential risk that healthy controls might experience migraine attack(s) in the future; this is especially true for young participants. However, although peak prevalence in females is around 40 years, most female patients manifest before the age of 35.26,28 Glyceryl trinitrate has been shown to be such a potent trigger for migraine that it is also able to trigger migraine-like attacks in controls with a familial occurrence of migraine.1 Controls in our study, apart from one participant who was excluded from the analysis, did not develop migraine-like headache. We feel, therefore, that is it less likely, compared with other case-control studies, that our controls will be at risk to develop migraine in the future. Our control group consisted of age-matched healthy female volunteers, minimizing possible age and sex influences. Because all participants followed the same study day protocol and stayed in the same hospital rooms, environmental influences were also minimalized. Furthermore, to the best of our knowledge, this is the first study to systematically investigate (using an extensive questionnaire) the occurrence of premonitory symptoms in a population of migraine patients not selected for the occurrence of premonitory symptoms. We also elaborately assessed timing of symptom onset in this study. It should be noted that because this study was part of larger imaging study, we could not gather information on the presence of nonheadache symptoms while participants were being scanned. However, because our assessment was extensive outside the 2 scan moments (every 15 minutes) and most symptoms were present on multiple assessments, we expect no symptoms were missed. A limitation in our study, as in other GTN model studies, is that nonsignificant findings for the comparison between responders and nonresponders might be caused by the low number of nonresponders because of the large response rate (>80%).1,6,23 Second, we included only females and only participants with migraine without aura, which may limit the generalizability of our results, and lastly, we did not ask the patients who experienced premonitory symptoms whether the induced symptoms mimicked their usual symptoms because this was not the interest of our study.
In conclusion, our findings support the view that GTN is able to provoke the naturally occurring premonitory symptoms of which yawning, nausea, photophobia, and concentration difficulties are most distinctly predicting an impending GTN-induced migraine-like headache, indicative of specific pathophysiological mechanism underlying the beginning of attacks. Importantly, concentration difficulties were exclusively reported by those who developed a migraine-like attack. Specific early warning signals may be helpful in clinical practice for patients and their treating physicians to prevent occurrence of full blown attacks by early treatment strategies.
Conflict of interest statement
M.D. Ferrari reports grants and consultancy or industry support from Medtronic, Novartis, Amgen, Lilly, Teva, electroCore, and independent support from NWO, ZonMW, NIH, European Community, and the Dutch Heart Foundation. G.M. Terwindt reports consultancy support from Novartis, Amgen, Lilly, Teva, and independent support from NWO, ZonMW, NIH, Dutch Heart Foundation, and Dutch Brain Foundation. The remaining authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B2.
Acknowledgements
The authors greatly acknowledge subjects who participated in the study, W.H. van Galen who assisted in logistics, and medical students J. van Son, and M.N. Minderhout who assisted with recruitment of subjects, data acquisition, and data analysis.
This study was supported by the Netherlands Organization for Scientific Research (VICI grant 918.56.601 and Spinoza 2009 to M.D.F.), the Netherlands Organization for Health Research and Development (Clinical Fellowship 90700217 and VIDI grant 917.11.31 to G.M.T.), and European Community (EC) FP7-EUROHEADPAIN—no. 602633.
Author contributions: G.L.J. Onderwater, M.D. Ferrari, and G.M. Terwindt contributed to design and conceptualization of the study. G.L.J. Onderwater, J. Dool, M.D. Ferrari, and G.M. Terwindt contributed to data collection and interpretation, G.L.J. Onderwater performed the analyses. G.L.J. Onderwater contributed to drafting a first version of the manuscript and figures. G.L.J. Onderwater, J.Dool, M.D. Ferrari, and G.M. Terwindt interpreted the data and revised the manuscript.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
M.D. Ferrari and G.M. Terwindt contributed equally to this work as last authors.
REFERENCES
- [1].Afridi SK, Kaube H, Goadsby PJ. Glyceryl trinitrate triggers premonitory symptoms in migraineurs. PAIN 2004;110:675–80. [DOI] [PubMed] [Google Scholar]
- [2].Alstadhaug K, Salvesen R, Bekkelund S. Insomnia and circadian variation of attacks in episodic migraine. Headache 2007;47:1184–8. [DOI] [PubMed] [Google Scholar]
- [3].Alstadhaug KB. Migraine and the hypothalamus. Cephalalgia 2009;29:809–17. [DOI] [PubMed] [Google Scholar]
- [4].Arngrim N, Schytz HW, Britze J, Amin FM, Vestergaard MB, Hougaard A, Wolfram F, de Koning PJH, Olsen KS, Secher NH, Larsson HBW, Olesen J, Ashina M. Migraine induced by hypoxia: a MRI spectroscopy and angiography study. Brain 2016;136:723–37. [DOI] [PubMed] [Google Scholar]
- [5].Ashina M, Hansen JM, á Dunga BO, Olesen J. Human models of migraine—short-term pain for long-term gain. Nat Rev Neurol 2017;13:713–24. [DOI] [PubMed] [Google Scholar]
- [6].Ashina M, Hansen JM, Olesen J. Pearls and pitfalls in human pharmacological models of migraine: 30 years' experience. Cephalalgia 2013;33:540–53. [DOI] [PubMed] [Google Scholar]
- [7].Cuvellier J-C, Mars A, Vallée L. The prevalence of premonitory symptoms in paediatric migraine: a questionnaire study in 103 children and adolescents. Cephalalgia 2009;29:1197–201. [DOI] [PubMed] [Google Scholar]
- [8].Dahlem MA, Kurths J, Ferrari MD, Aihara K, Scheffer M, May A. Understanding migraine using dynamic network biomarkers. Cephalalgia 2014;35:627–30. [DOI] [PubMed] [Google Scholar]
- [9].Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G. Hypothalamic activation in spontaneous migraine attacks. Headache 2007;47:1418–26. [DOI] [PubMed] [Google Scholar]
- [10].Ellis J, Aspinall L, Hackshaw A, MacGregor EA, Frith A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology 2006;67:2154–8. [DOI] [PubMed] [Google Scholar]
- [11].Giffin NJ, Lipton RB, Silberstein SD, Olesen J, Goadsby PJ. The migraine postdrome: an electronic diary study. Neurology 2016;87:309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Giffin NJ, Ruggiero L, Lipton RB, Silberstein SD, Tvedskov JF, Olesen J, Altman J, Goadsby PJ, Macrae A. Premonitory symptoms in migraine: an electronic diary study. Neurology 2003;60:935–40. [DOI] [PubMed] [Google Scholar]
- [13].Gil-Gouveia R, Martins IP. Clinical description of attack-related cognitive symptoms in migraine: a systematic review. Cephalalgia 2018;38:1335–50. [DOI] [PubMed] [Google Scholar]
- [14].Gil-Gouveia R, Martins IP. Cognition and cognitive impairment in migraine. Curr Pain Headache Rep 2019;23:84. [DOI] [PubMed] [Google Scholar]
- [15].Global Burden of Disease Study 2016 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Goadsby PJ, Holland PR, Martins-oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine—a disorder of sensory processing. Physiol Rev 2017;97:553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guo S, Haulund Vollesen AL, Olesen J, Ashina M. Premonitory and non-headache symptoms induced by CGRP and PACAP38 in migraine patients. PAIN 2016;157:2773–81. [DOI] [PubMed] [Google Scholar]
- [18].Guo S, Olesen J, Ashina M. Phosphodiesterase 3 inhibitor cilostazol induces migraine-like attacks via cyclic AMP increase. Brain 2014;137:2951–9. [DOI] [PubMed] [Google Scholar]
- [19].Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 2010;30:1179–86. [DOI] [PubMed] [Google Scholar]
- [20].Hansen JM, Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Goadsby PJ, Charles A. Migraine headache is present in the aura phase: a prospective study. Neurology 2012;79:2044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- [22].Kagan R, Kainz V, Burstein R, Noseda R. Hypothalamic and basal ganglia projections to the posterior thalamus: possible role in modulation of migraine headache and photopobia. Neuroscience 2013;248:359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Karsan N, Bose P, Goadsby PJ. The phenotype of premonitory symptoms and migraine headache triggered with nitroglycerin. Cephalalgia 2016;36:S53–54. [Google Scholar]
- [24].Karsan N, Goadsby PJ. Biological insights from the premonitory symptoms of migraine. Nat Rev Neurol 2018;14:699–710. [DOI] [PubMed] [Google Scholar]
- [25].Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007;27:394–402. [DOI] [PubMed] [Google Scholar]
- [26].Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology 1999;53:537–42. [DOI] [PubMed] [Google Scholar]
- [27].Laurell K, Artto V, Bendtsen L, Hagen K, Häggström J, Linde M, Söderström L, Tronvik E, Wessman M, Zwart JA, Kallela M. Premonitory symptoms in migraine: a cross-sectional study in 2714 persons. Cephalalgia 2016;36:951–9. [DOI] [PubMed] [Google Scholar]
- [28].Lipton RB, Diamond S, Reed M, Diamond ML, Stewart WF. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41:638–45. [DOI] [PubMed] [Google Scholar]
- [29].Macgregor EA. Oestrogen and attacks of migraine with and without aura. Lancet Neurol 2004;3:354–61. [DOI] [PubMed] [Google Scholar]
- [30].Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain 2014;137:232–41. [DOI] [PubMed] [Google Scholar]
- [31].Maniyar FH, Sprenger T, Schankin C, Goadsby PJ. Photic hypersensitivity in the premonitory phase of migraine—a positron emission tomography study. Eur J Neurol 2014;21:1178–83. [DOI] [PubMed] [Google Scholar]
- [32].Maniyar FH, Sprenger T, Schankin C, Goadsby PJ. The origin of nausea in migraine—A PET study. J Headache Pain 2014;15:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].May A. Understanding migraine as a cycling brain syndrome: reviewing the evidence from functional imaging. Neurol Sci 2017;38:125–30. [DOI] [PubMed] [Google Scholar]
- [34].May A, Burstein R. Hypothalamic regulation of headache and migraine. Cephalalgia 2019;39:1710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].van Oosterhout WPJ, Dekkers OM, MaassenVanDenBrink A, Ferrari MD, Schoonman GG, van Zwet EW, Terwindt GM. Female sex hormones in men with migraine. Neurology 2018;91:e374–81. [DOI] [PubMed] [Google Scholar]
- [36].van Oosterhout WPJ, van Someren EJW, Schoonman GG, Louter MA, Lammers GJ, Ferrari MD, Terwindt GM. Chronotypes and circadian timing in migraine. Cephalalgia 2018;38:617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].van Oosterhout WPJ, Weller CM, Stam AH, Bakels F, Stijnen T, Ferrari MD, Terwindt GM. Validation of the web-based LUMINA questionnaire for recruiting large cohorts of migraineurs. Cephalalgia 2011;31:1359–67. [DOI] [PubMed] [Google Scholar]
- [38].Peng KP, May A. Migraine understood as a sensory threshold disease. PAIN 2019;160:1494–501. [DOI] [PubMed] [Google Scholar]
- [39].Russell M, Rasmussen B, Fenger K, Olesen J. Migraine without aura and migraine with aura are distinct clinical entities: a study of four hundred and eighty-four male and female migraineurs from the general population. Cephalalgia 1996;16:239–45. [DOI] [PubMed] [Google Scholar]
- [40].Schoonman GG, Evers DJ, Terwindt GM, van Dijk JG, Ferrari MD. The prevalence of premonitory symptoms in migraine: a questionnaire study in 461 patients. Cephalalgia 2006;26:1209–13. [DOI] [PubMed] [Google Scholar]
- [41].Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016;139:1987–93. [DOI] [PubMed] [Google Scholar]
- [42].Stovner LJ, Zwart JA, Hagen K, Terwindt GM, Pascual J. Epidemiology of headache in Europe. Eur J Neurol 2006;13:333–45. [DOI] [PubMed] [Google Scholar]
- [43].Vuralli D, Ayata C, Bolay H. Cognitive dysfunction and migraine. J Headache Pain 2018;19:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Waelkens J. Warning symptoms in migraine : characteristics and therapeutic implications. Cephalalgia 1985;5:223–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B2.