Elagolix treatment for moderate to severe endometriosis-associated pain results in dose-dependent, clinically meaningful improvements in health-related quality of life.
Abstract
OBJECTIVE:
To evaluate the effects of elagolix on clinically meaningful improvements in health-related quality of life (HRQOL) measured by the EHP-30 (Endometriosis Health Profile-30).
METHODS:
Data from two phase III trials of elagolix for moderate to severe pain associated with endometriosis were pooled and analyzed as three groups: placebo, elagolix 150 mg once daily, or elagolix 200 mg twice daily. Patients were administered the EHP-30 questionnaire at baseline, and at months 1, 3, and 6 of treatment. Previously established responder definitions were applied to determine percentages of patients with clinically meaningful EHP-30 improvements. The probability of meeting EHP-30 responder definitions with elagolix compared with placebo at months 3 and 6 was determined by Poisson regression analysis, controlling for baseline scores.
RESULTS:
At month 6, the probabilities of meeting EHP-30 subscale responder definitions for pain, control and powerlessness, self-image, social support, emotional well-being, and sexual intercourse were 169% (adjusted relative risk [aRR]: 2.69, 95% CI 2.26–3.21), 129% (aRR 2.29, 95% CI 1.96–2.67), 80% (aRR 1.80, 95% CI 1.54–2.11), 70% (aRR 1.70, 95% CI 1.47–1.97), 67% (aRR 1.67, 95% CI 1.45–1.92), and 62% (aRR 1.62, 95% CI 1.36–1.92) greater, respectively (all P<.001), in the 200-mg group than in the placebo group. Although lower in magnitude than the 200-mg group, the 150-mg group also had greater probabilities of meeting responder definitions than the placebo group for all subscales except sexual intercourse. The probabilities of meeting responder definitions for pain, control and powerlessness, self-image, social support, and emotional well-being were 75% (aRR 1.75, 95% CI 1.44–2.14), 50% (aRR 1.50, 95% CI 1.25–1.80), 22% (aRR 1.22, 95% CI 1.01–1.47), 30% (aRR 1.30, 95% CI 1.09–1.53), and 35% (aRR 1.35, 95% CI 1.16–1.57) greater, respectively (all P<.05), in the 150-mg group than in the placebo group.
CONCLUSION:
Patients with moderate to severe pain associated with endometriosis and were treated with elagolix experienced clinically meaningful HRQOL improvements.
CLINICAL TRIAL REGISTRATION:
FUNDING SOURCE:
AbbVie Inc.
Endometriosis is a chronic gynecologic condition characterized by growth of endometrial tissue outside the uterus.1 Pain symptoms are a central feature of endometriosis, along with infertility and chronic fatigue,1,2 and current treatment guidelines prioritize pain management.3,4 Endometriosis reduces women’s health-related quality of life (HRQOL),5 resulting in impairments in physical functioning,6–9 diminished social life, difficulties in intimate relationships,6,7,10,11 and decreased productivity.6–8,12
Patient-reported outcome measures such as the EHP-30 (Endometriosis Health Profile-30)13–15 are increasingly employed to evaluate therapeutic efficacy and guide clinical decision making.16 In 2009, the U.S. Food and Drug Administration published guidance on using patient-reported outcome measures in clinical trials, emphasizing the establishment of meaningful changes at the patient level.17 Individual patient-reported outcome score changes (responder definitions) over a predetermined time period that would be interpreted as treatment benefits can be used to assess treatment effects perceived as meaningful by the patient.17,18
Few studies have defined clinically meaningful score changes for patient-reported outcomes used in endometriosis.19–22 Responder definitions were previously determined for EHP-30 subscales based on data from patients with moderate to severe pain associated with endometriosis in the ELARIS EM-I, and EM-II trials.23 In the current post hoc analysis, responder definitions for EHP-30 subscales were applied to pooled ELARIS trial data24 to determine whether treatment with elagolix, an oral nonpeptide gonadotropin-releasing hormone antagonist,25 was associated with clinically meaningful improvements in HRQOL compared with placebo.
ROLE OF THE FUNDING SOURCE
This study was funded by AbbVie Inc. AbbVie sponsored the study, contributed to the design, participated in collection, analysis, and interpretation of data, and participated in the writing, reviewing, and approval of the final version. The authors had access to relevant aggregated study data and other information (such as study protocol, analytic plan and report, validated data tables, and clinical study reports) required to understand and report research findings. The authors take responsibility for the presentation and publication of the research findings, have been fully involved at all stages of publication and presentation development, and are willing to take public responsibility for all aspects of the work. All individuals included as authors and contributors who made substantial intellectual contributions to the research, data analysis, and publication or presentation development are listed appropriately. The role of the sponsor in the design, execution, analysis, reporting, and funding is fully disclosed. The authors’ personal interests, financial or nonfinancial, relating to this research and its publication have been disclosed.
METHODS
Data were pooled from two similar phase III randomized controlled trials, ELARIS EM-I (NCT01620528) and ELARIS EM-II (NCT01931670), which evaluated the efficacy and safety of elagolix for the treatment of moderate to severe pain associated with endometriosis.24 The study designs and eligibility criteria have been previously described.24 Briefly, premenopausal women aged 18–49 years who received a surgical diagnosis of endometriosis in the previous 10 years and who had moderate to severe pain associated with endometriosis were eligible for the studies. Participants were randomized 2:2:3 to receive elagolix 150 mg once daily (150-mg cohort), 200 mg twice daily (200-mg cohort), or placebo for 6 months with a 12-month follow-up period. The primary endpoints in the studies were the proportion of patients with a clinical response for dysmenorrhea and nonmenstrual pelvic pain at month 3 of treatment, where response was defined as clinically meaningful reduction in pain score, and decreased or stable use of rescue analgesics.24 Secondary endpoints included efficacy evaluations per patient-reported outcomes, including assessment of HRQOL.
Patients in the two trials were administered the EHP-30 questionnaire15 at baseline, and at months 1, 3, and 6 of treatment. The EHP-30 is a validated disease specific questionnaire with 30 questions that encompass five core scales: pain, control and powerlessness, emotional well-being, social support, and self-image.15 The EHP-30 modular questionnaires address additional scales that may not apply to all women with endometriosis,14,15 including the sexual intercourse scale, which was administered to patients in these trials. The EHP-30 questions are asked regarding endometriosis in the previous 4 weeks and are answered on a 5-point Likert scale, where 0=never, 1=rarely, 2=sometimes, 3=often, and 4=always. Raw scores for the questions within a scale are summed and transformed to a 0–100 scale, with higher scores indicating worse HRQOL.14,15
Least squares mean change from baseline to months 1, 3, and 6 was calculated for the pooled data set of patients. Differences between elagolix dose groups and placebo were derived from an analysis of covariance model, with treatment as the main effect and baseline value as a covariate. A triangulation approach was used to assess responder thresholds for the sexual relationship module and each domain of EHP-30. The triangulation methodology involved three psychometric approaches, anchor- and distribution-based analyses, and use of clinically relevant indicators (improvement in dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia). The results for these three psychometric approaches as they apply to EHP-30 have been previously described.23 The following responder definitions for EHP-30 scales were used: pain, 30-point reduction; control and powerlessness, 35-point reduction; emotional well-being, social support, self-image, and sexual intercourse, 20-point reductions.23 The percentages of patients who met the responder definitions for each of the six EHP-30 scales at months 1, 3, and 6 were determined.
A binary Poisson regression analysis was performed to determine the probability of patients meeting EHP-30 responder definitions at months 3 and 6. In this model, baseline EHP-30 scores and treatment arm were independent variables, and patients given placebo were used as the reference group. Relative risk (RR) estimates, 95% CIs, and associated P values were generated; regression results were adjusted for differences in baseline EHP-30 domain scores. Continuous variables were summarized by means and SD and categorical variables were reported as percentages. The EHP-30 data were reported for only nonmissing responses. All data analyses were performed using SAS 9.4.
An institutional review board at each study center approved the clinical study protocol before the study was conducted. Shulman Associates IRB conducted the majority of the institutional review board approvals at each study center. The trial was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation guidelines.24 Informed consent was obtained from all individuals who participated in the ELARIS EM-I and EM-II trials.24
RESULTS
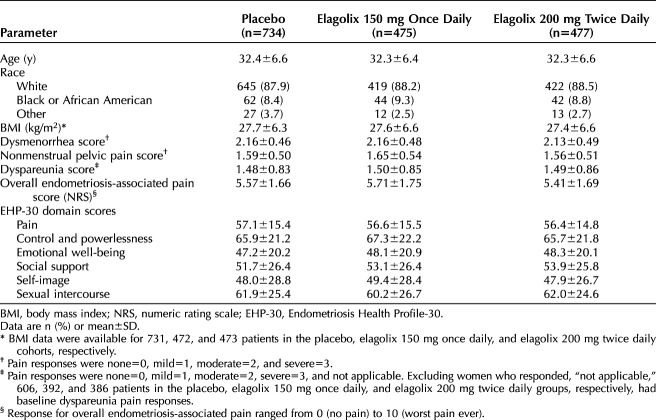
A total of 1,686 women were randomized and treated in the ELARIS EM-I and EM-II trials, with 734 receiving placebo, 475 receiving elagolix 150 mg once daily, and 477 receiving elagolix 200 mg twice daily (Table 1). The baseline demographics and clinical characteristics of the study populations were similar between trials and across treatment arms,24 allowing data from both trials to be pooled by treatment arm. Baseline pain scores for dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia were highly similar between treatment arms (Table 1)23,24
Table 1.
Baseline Demographics and Clinical Characteristics of the Study Population
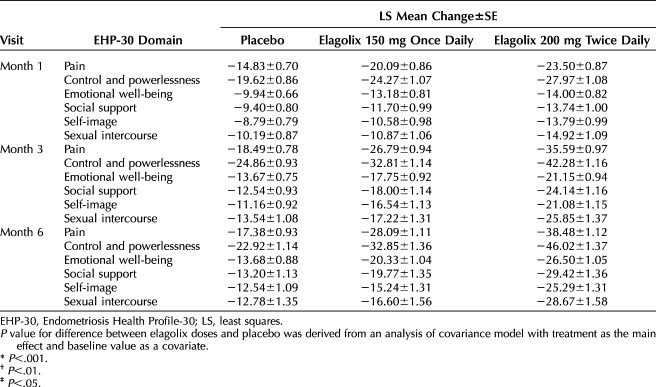
Women in the elagolix 200-mg cohort had significant improvements from baseline in all six EHP-30 subscale scores compared with women in the placebo group at months 1, 3 and 6 of treatment (Table 2). In the elagolix 200-mg cohort, the greatest improvements compared with placebo (least squares mean difference±SE) were reported for pain (−21.09±1.46; 95% CI −23.95 to −18.23; P<.001) and control and powerlessness (−23.10±1.78; 95% CI −26.59 to −19.61; P<.001) at month 6. Improvements at month 6 were also reported for the elagolix 150-mg cohort, but the magnitude was less than in the 200-mg cohort: pain (−10.70±1.45; 95% CI −13.54 to −7.87; P<.001) and control and powerlessness (−9.93±1.77; 95% CI −13.41 to −6.44; P<.001).
Table 2.
Change From Baseline in Endometriosis Health Profile-30 Domain Scores
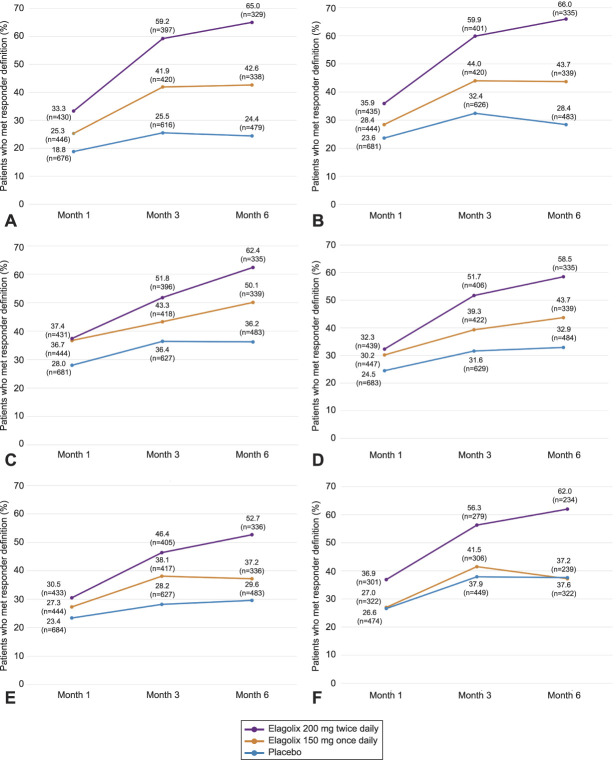
At month 1, a greater percentage of women treated with either dose of elagolix achieved responses for all EHP-30 subscales except sexual intercourse, compared with those given placebo (Fig. 1A–F). The percentage of patients meeting responder definitions increased from month 1 to month 3 for all EHP-30 subscales, with the greatest percentage of responders consistently in the elagolix 200-mg cohort (46.4–59.9%). By comparison, 38.1–44.0% of the elagolix 150-mg cohort and 25.5–37.9% of the placebo cohort met EHP-30 responder definitions at month 3. The placebo cohort had the lowest proportion of patients meeting responder definitions in the pain subscale at month 3 (25.5%; Fig. 1A). For both doses of elagolix, the percentage of patients meeting responder definitions at month 3 was greatest for the control and powerlessness subscale (150 mg, 44.0%; 200 mg, 59.9%; Fig. 1B). Among all EHP-30 subscales, the self-image subscale had the lowest proportion of patients meeting responder definitions for both elagolix doses at month 3 (150 mg, 38.1%; 200 mg, 46.4%; Fig. 1E).
Fig. 1. Proportion of patients meeting responder definitions for EHP-30 (Endometriosis Health Profile-30) subscales at months 1, 3, and 6 of treatment. The percentages of patients who met responder definitions for the following EHP-30 subscales: pain (A), control and powerlessness (B), emotional well-being (C), social support (D), self-image (E), and sexual intercourse (F). n values indicate the total number of patients with EHP-30 scores for the indicated subscale at each timepoint per treatment arm. Responder definitions were based on the following reductions in subscale scores from baseline: pain, 30-point reduction; control and powerlessness, 35-point reduction; emotional well-being, social support, self-image, and sexual intercourse, 20-point reductions.
Taylor. Elagolix and Meaningful EHP-30 Improvements. Obstet Gynecol 2020.
From months 3–6, the percentage of patients in the placebo cohort who met responder definitions generally plateaued across subscales (Fig. 1A–F). In contrast, the percentage of patients in the elagolix 200-mg cohort who met responder definitions increased from month 3–6 for all subscales (52.7–66.0%). The percentages of patients in the elagolix 150-mg cohort who met responder definitions (37.2–50.1%) were relatively sustained from month 3–6 for pain, control and powerlessness, self-image, and sexual intercourse subscales. Increased proportions of patients in the elagolix 150-mg cohort were responders at month 6 for the emotional well-being (50.1%; Fig. 1C) and social support (43.7%; Fig. 1D) subscales compared with month 3 (43.3% and 39.3%, respectively).
At month 6, comparing all EHP-30 subscales, the self-image and sexual intercourse subscales had the fewest responders among the elagolix 150-mg cohort (both 37.2%; Fig. 1E and F). Similarly, the fewest patients in the elagolix 200-mg cohort met responder definitions at month 6 for the self-image subscale (52.7%; Fig. 1E).
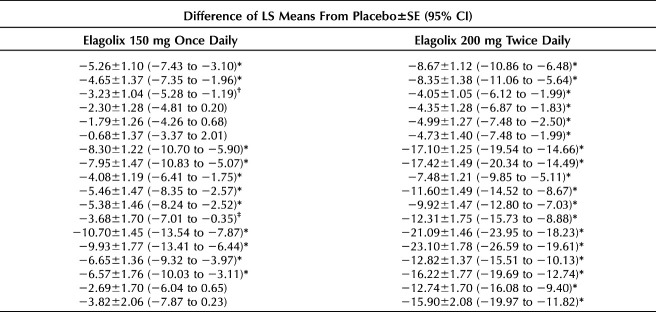
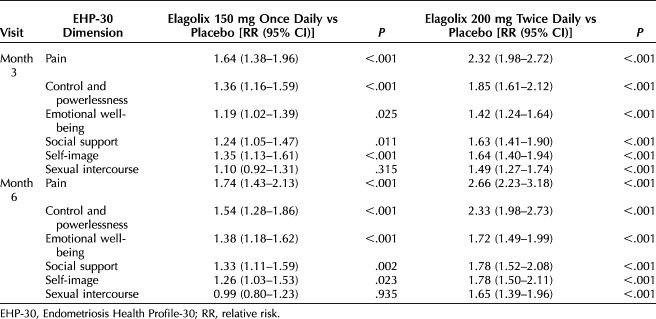
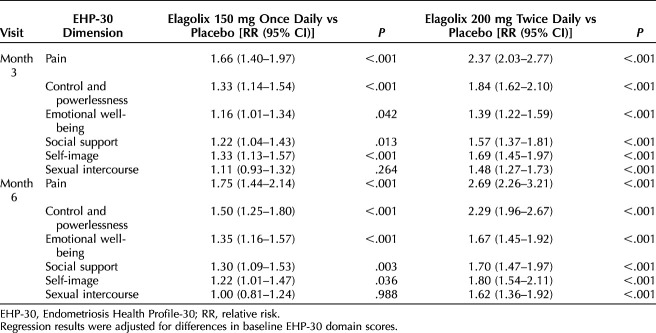
A Poisson regression analysis with robust standard errors was performed to determine the effect of both elagolix doses compared with placebo on the probability of meeting EHP-30 responder definitions at months 3 and 6 of treatment. Unadjusted RRs and adjusted relative risks (aRRs) are provided in Tables 3 and 4, respectively.
Table 3.
Unadjusted Poisson Relative Risk Estimates of Meeting Endometriosis Health Profile-30 Subscale Responder Definitions
Table 4.
Adjusted Poisson Relative Risk Estimates of Meeting Endometriosis Health Profile-30 Subscale Responder Definitions
After adjustment, the highest probability of meeting responder definitions was reported for the pain subscale at months 3 and 6 (Table 4). At month 3, patients in the elagolix 150-mg cohort had a 66% (aRR: 1.66; 95% CI 1.40–1.97; P<.001) greater probability of meeting the pain subscale responder definition than those given placebo, and patients in the elagolix 200-mg cohort had a 137% (aRR 2.37, 95% CI 2.03–2.77; P<.001) greater probability than those given placebo. The probability of meeting the pain subscale responder definition at month 6 increased for both doses of elagolix; the probability for the elagolix 150-mg cohort was 75% (aRR 1.75, 95% CI 1.44–2.14; P<.001) greater than placebo and 169% (aRR 2.69, 95% CI 2.26–3.21; P<.001) greater than placebo for the elagolix 200-mg cohort. The probabilities of women in both elagolix dose cohorts meeting responder definitions for the control and powerlessness subscale were also significantly higher than the placebo cohort. At month 3, the probability of patients in the elagolix 150-mg cohort meeting the control and powerlessness subscale responder definition was 33% (aRR 1.33, 95% CI 1.14–1.54; P<.001) greater than placebo and 84% (aRR 1.84, 95% CI 1.62–2.10; P<.001) greater than placebo for patients in the elagolix 200-mg cohort. At month 6, probabilities were 50% (aRR 1.50, 95% CI 1.25–1.80; P<.001) and 129% (aRR 2.29, 95% CI 1.96–2.67; P<.001) greater than placebo for the elagolix 150-mg and 200-mg cohorts, respectively.
DISCUSSION
In this post hoc analysis, patients with moderate to severe pain associated with endometriosis who were treated with elagolix 150 mg once daily or 200 mg twice daily achieved clinically meaningful improvements in HRQOL in a dose-dependent manner, as observed for primary efficacy endpoints.24 Higher percentages of patients in the elagolix 200-mg cohort met EHP-30 responder definitions than patients in the elagolix 150-mg cohort. At month 6, significant improvements from baseline were reported for all six subscale scores in the 200-mg cohort compared with placebo, and in four subscale scores (pain, control and powerlessness, emotional well-being, and social support) in the 150-mg cohort compared with placebo. Patients treated with elagolix perceived meaningful improvements in HRQOL as early as month 1 of treatment (200-mg cohort), with sustained or further improvements through months 3 and 6. Although both doses of elagolix increased the likelihood of meeting responder definitions across HRQOL domains compared with placebo, the RR estimates were greater in the 200-mg cohort. The changes in HRQOL scores mirror the improvements observed in pain scores dysmenorrhea and nonmenstrual pelvic pain.24
Patients treated with elagolix had the greatest probability of meeting responder definitions for pain and control and powerlessness subscales. Elagolix results in clinically meaningful reductions in dysmenorrhea and nonmenstrual pelvic pain24 and significantly reduces fatigue,26 both of which likely contribute to clinically meaningful improvements in the control and powerlessness scale. The probability of meeting responder definitions was lowest for the sexual intercourse subscale. This finding agrees with published data showing that treatment with elagolix 200-mg twice daily resulted in significant improvement in dyspareunia scores, whereas treatment with the 150-mg once-daily dose did not.11
Elagolix influences more than endometriosis-associated pain, as evidenced by the proportions of patients meeting responder definitions across EHP-30 subscales. Endometriosis is associated with feelings of powerlessness and the sense that symptoms dominate one's life.5,15,27 Effective symptom management with elagolix may restore a sense of control and improve emotional well-being by reducing depression, anxiety, and hopelessness.5,27 Treatment of endometriosis with elagolix may allow women to more fully engage in social activities and relationships, which often suffer owing to endometriosis symptoms.5,27 Alleviation of dyspareunia by elagolix improves women's sexual quality of life,11 positively influencing intimate relationships that may have deteriorated.5,27 It is common for women with endometriosis to defer or lose opportunities for career or educational advancement5,7,12,27,28; however, elagolix was shown to restore women's workplace productivity.26 The ability to participate in the workforce promotes mental well-being29 and reduces the financial burden of endometriosis.12 In these ways, elagolix mitigates the effects of endometriosis on women's HRQOL.
This study evaluated the effect of a standardized treatment regimen on clinically meaningful improvements in EHP-30 scores in women with moderate to severe endometriosis-associated pain. A PubMed search for “endometriosis EHP-30” found two other studies that evaluated clinically meaningful changes in EHP-30 scores. One study surveyed only 40 women with endometriosis who were recruited from one medical center before and after surgical intervention.22 The second study evaluated clinically meaningful score changes in the Dutch EHP-30 among Dutch women with endometriosis who were recruited from a medical center and patient support group who received various treatments.19 In contrast, our study highlights the utility of applying EHP-30 responder definitions to measure therapeutic efficacy in a large clinical trial setting.
A strength of this analysis is the large sample size derived from two phase III randomized, double-blind placebo-controlled trials. A high percentage of patients given placebo met responder definitions across EHP-30 subscales (28–38%). This placebo effect is not uncommon in a disease in which pain is the primary symptom, and the results of this analysis reflect placebo response rates observed for the trials' primary endpoints.24 Despite this, regression analyses found significantly higher probabilities of meeting responder definitions for patients treated with elagolix than for those who were given placebo. The responder definitions used here were based on a patient population with moderate to severe pain associated with endometriosis and are not applicable to patients with milder endometriosis. The EHP-30 responses may be subject to recall bias; however, recall was limited to the previous 4 weeks. Additional studies are needed to access whether the effects of elagolix are sustained long-term, because the present analysis was limited to 6 months.
In summary, this analysis demonstrated that the treatment of women with moderate to severe pain associated with endometriosis with elagolix 150 mg once daily or 200 mg twice daily resulted in greater HRQOL improvements than placebo that were clinically meaningful to patients. Treatment with elagolix 200 mg twice daily was associated with a higher chance of meeting responder definitions across EHP-30 scales than elagolix 150 mg once daily. These improvements are expected to positively affect the personal and professional lives of women with endometriosis.
Authors' Data Sharing Statement
Will individual participant data be available (including data dictionaries)? Yes.
What data in particular will be shared? Individual and trial-level data
What other documents will be available? Protocols, clinical study reports
When will data be available (start and end dates)? Data are made available at time of request and are accessible for 12 months, with possible extensions considered.
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Access to these clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. Access is provided to anonymized, patient and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports) from AbbVie-sponsored phase II-IV global interventional clinical trials conducted in patients (completed as of May 2004, for products and indications approved in either the United States or the European Union), as long as the trials are not part of an ongoing or planned regulatory submission). This includes requests for clinical trial data for unlicensed products and indications.
Access to this clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Footnotes
This study was funded by AbbVie Inc. AbbVie sponsored the study, contributed to the design, participated in collection, analysis, and interpretation of data, and participated in the writing, reviewing, and approval of the final version. Medical writing assistance was provided by Emily Mercadante, PhD, of JK Associates, Inc., a member of the Fishawack Group of Companies, Conshohocken, PA, and was funded by AbbVie Inc., North Chicago, IL.
Financial Disclosure Hugh S. Taylor has served as a consultant for AbbVie, Bayer, ObsEva, and DotLab. Ahmed M. Soliman, Beverly Johns, and Michael Snabes are AbbVie employees and have stock/stock options. Robin M. Pokrzywinski and Karin S. Coyne are employees of Evidera, a contract research organization that received funds from AbbVie to work on this research.
Data were presented in part at the American College of Obstetricians and Gynecologists’ Annual Clinical and Scientific Meeting, May 3–6, 2019, Nashville, Tennessee.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews are available at http://links.lww.com/AOG/B910.
REFERENCES
- 1.Johnson NP, Hummelshoj L; World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum Reprod 2013;28:1552–68. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod 2005;20:2698–704. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong C. ACOG updates guideline on diagnosis and treatment of endometriosis. Am Fam Physician 2011;83:84–5. [Google Scholar]
- 4.Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, De Bie B, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod 2014;29:400–12. [DOI] [PubMed] [Google Scholar]
- 5.Culley L, Law C, Hudson N, Denny E, Mitchell H, Baumgarten M, et al. The social and psychological impact of endometriosis on women's lives: a critical narrative review. Hum Reprod Update 2013;19:625–39. [DOI] [PubMed] [Google Scholar]
- 6.Fourquet J, Baez L, Figueroa M, Iriarte RI, Flores I. Quantification of the impact of endometriosis symptoms on health-related quality of life and work productivity. Fertil Steril 2011;96:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fourquet J, Gao X, Zavala D, Orengo JC, Abac S, Ruiz A, et al. Patients' report on how endometriosis affects health, work, and daily life. Fertil Steril 2010;93:2424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nnoaham KE, Hummelshoj L, Webster P, d'Hooghe T, de Cicco Nardone F, de Cicco Nardone C, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011;96:366–73.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012;27:1292–9. [DOI] [PubMed] [Google Scholar]
- 10.Bernuit D, Ebert AD, Halis G, Strothmann A, Gerlinger C, Geppert K, et al. Female perspectives on endometriosis: findings from the uterine bleeding and pain women's research study. J Endometr 2011;3:73–85. [Google Scholar]
- 11.Leyland N, Taylor HS, Archer DF, Peloso PM, Soliman AM, Palac HL, et al. Elagolix reduced dyspareunia and improved health-related quality of life in premenopausal women with endometriosis-associated pain. J Endometr Pelvic Pain Disord 2019;11:171–80. [Google Scholar]
- 12.Soliman AM, Coyne KS, Gries KS, Castelli-Haley J, Snabes MC, Surrey ES. The effect of endometriosis symptoms on absenteeism and presenteeism in the workplace and at home. J Manag Care Spec Pharm 2017;23:745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourdel N, Chauvet P, Billone V, Douridas G, Fauconnier A, Gerbaud L, et al. Systematic review of quality of life measures in patients with endometriosis. PLoS One 2019;14:e0208464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones G, Jenkinson C, Taylor N, Mills A, Kennedy S. Measuring quality of life in women with endometriosis: tests of data quality, score reliability, response rate and scaling assumptions of the Endometriosis Health Profile questionnaire. Hum Reprod 2006;21:2686–93. [DOI] [PubMed] [Google Scholar]
- 15.Jones G, Kennedy S, Barnard A, Wong J, Jenkinson C. Development of an endometriosis quality-of-life instrument: the Endometriosis Health Profile-30. Obstet Gynecol 2001;98:258–64. [DOI] [PubMed] [Google Scholar]
- 16.Johnston BC, Ebrahim S, Carrasco-Labra A, Furukawa TA, Patrick DL, Crawford MW, et al. Minimally important difference estimates and methods: a protocol. BMJ Open 2015;5:e007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services,Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH). Guidance for industry 2009. Patient-reported outcome measures: use in medical product development to support labeling claims. Available at: https://www.fda.gov/media/77832/download. Retrieved November 8, 2019.
- 18.McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res 2011;11:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van de Burgt TJ, Kluivers KB, Hendriks JC. Responsiveness of the Dutch Endometriosis Health Profile-30 (EHP-30) questionnaire. Eur J Obstet Gynecol Reprod Biol 2013;168:92–4. [DOI] [PubMed] [Google Scholar]
- 20.Gerlinger C, Schumacher U, Faustmann T, Colligs A, Schmitz H, Seitz C. Defining a minimal clinically important difference for endometriosis-associated pelvic pain measured on a visual analog scale: analyses of two placebo-controlled, randomized trials. Health Qual Life Outcomes 2010;8:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wickstrom K, Edelstam G. Minimal clinically important difference for pain on the VAS scale and the relation to quality of life in women with endometriosis. Sex Reprod Healthc 2017;13:35–40. [DOI] [PubMed] [Google Scholar]
- 22.Jones G, Jenkinson C, Kennedy S. Evaluating the responsiveness of the Endometriosis Health Profile questionnaire: the EHP-30. Qual Life Res 2004;13:705–13. [DOI] [PubMed] [Google Scholar]
- 23.Pokrzywinski R, Soliman AM, Chen J, Snabes MC, Taylor HS, Coyne KS. Responsiveness evaluation and recommendation for responder thresholds for Endometriosis Health Profile-30: analysis of two phase III clinical trials. J Womens Health (Larchmt) 2020;20:253–61. [DOI] [PubMed] [Google Scholar]
- 24.Taylor HS, Giudice LC, Lessey BA, Abrao MS, Kotarski J, Archer DF, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med 2017;377:28–40. [DOI] [PubMed] [Google Scholar]
- 25.Ng J, Chwalisz K, Carter DC, Klein CE. Dose-dependent suppression of gonadotropins and ovarian hormones by elagolix in healthy premenopausal women. J Clin Endocrinol Metab 2017;102:1683–91. [DOI] [PubMed] [Google Scholar]
- 26.Surrey ES, Soliman AM, Agarwal SK, Snabes MC, Diamond MP. Impact of elagolix treatment on fatigue experienced by women with moderate to severe pain associated with endometriosis. Fertil Steril 2019;112:298–304.e3. [DOI] [PubMed] [Google Scholar]
- 27.Moradi M, Parker M, Sneddon A, Lopez V, Ellwood D. Impact of endometriosis on women's lives: a qualitative study. BMC Womens Health 2014;14:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Graaff AA, D'Hooghe TM, Dunselman GA, Dirksen CD, Hummelshoj L, WERF EndoCost Consortium, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod 2013;28:2677–85. [DOI] [PubMed] [Google Scholar]
- 29.Schuring M, Robroek SJ, Burdorf A. The benefits of paid employment among persons with common mental health problems: evidence for the selection and causation mechanism. Scand J Work Environ Health 2017;43:540–9. [DOI] [PubMed] [Google Scholar]