Supplemental digital content is available in the text.
Key Words: COLLAGEN, INJURY, FORCE TRANSFER, LIGAMENT, TENDON, PROTEIN SUPPLEMENTATION
ABSTRACT
Purpose
This study aimed to assess the effect of dietary protein ingestion on intramuscular connective tissue protein synthesis rates during overnight recovery from a single bout of resistance exercise.
Methods
Thirty-six healthy, young males were randomly assigned to one of three treatments. One group ingested 30 g intrinsically L-[1-13C]-phenylalanine-labeled casein protein before sleep (PRO, n = 12). The other two groups performed a bout of resistance exercise in the evening and ingested either placebo (EX, n = 12) or 30 g intrinsically L-[1-13C]-phenylalanine-labeled casein protein before sleep (EX + PRO, n = 12). Continuous intravenous infusions of L-[ring-2H5]-phenylalanine and L-[1-13C]-leucine were applied, and blood and muscle tissue samples were collected to assess connective tissue protein synthesis rates and dietary protein-derived amino acid incorporation in the connective tissue protein fraction.
Results
Resistance exercise resulted in higher connective tissue protein synthesis rates when compared with rest (0.086 ± 0.017%·h−1 [EX] and 0.080 ± 0.019%·h−1 [EX + PRO] vs 0.059 ± 0.016%·h−1 [PRO]; P < 0.05). Postexercise casein protein ingestion did not result in higher connective tissue protein synthesis rates when compared with postexercise placebo ingestion (P = 1.00). Dietary protein-derived amino acids were incorporated into the connective tissue protein fraction at rest, and to a greater extent during recovery from exercise (P = 0.002).
Conclusion
Resistance exercise increases intramuscular connective tissue protein synthesis rates during overnight sleep, with no further effect of postexercise protein ingestion. However, dietary protein-derived amino acids are being used as precursors to support de novo connective tissue protein synthesis.
Skeletal muscle adaptation is regulated by the net balance between muscle protein synthesis and breakdown rates, with a protein turnover of 1%–2% per day (1). Physical activity and food ingestion are two major stimuli that increase muscle protein synthesis rates (2). Resistance exercise increases myofibrillar protein synthesis (3,4) and sensitizes skeletal muscle tissue to the anabolic properties of protein ingestion (5,6). Protein ingestion during recovery from exercise increases plasma essential amino acid availability, thereby further augmenting the acute postexercise increase in myofibrillar protein synthesis rate (7–10). Consequently, resistance exercise training is generally combined with postexercise protein supplementation to maximize muscle mass and strength gains (11,12).
Gains in muscle mass after exercise training are generally attributed to the postexercise accretion of myofibrillar protein (i.e., contractile elements), which contributes to the increased capacity to generate contractile force. However, the force generated by the muscle contractile apparatus must be transferred through the intra- and extracellular matrix of collagenous proteins to articulate the bone (13,14). In fact, up to 80% of the contractile force is transmitted laterally to the extracellular matrix (15). Therefore, it seems evident that connective tissue in skeletal muscle must undergo extensive remodeling after acute exercise (16,17) as well as during prolonged exercise training (18) to allow the concomitant gains in both muscle mass and muscle strength. In support, exercise has been reported to increase intramuscular connective tissue protein synthesis rates (16,17,19–22). However, whether protein ingestion further augments postexercise intramuscular connective tissue protein synthesis rates remains unclear. Dideriksen et al. (23) observed no effect of protein ingestion to further increase postexercise muscle connective tissue protein synthesis rates. However, more recently, Holm et al. (24) demonstrated that the ingestion of ~18 g whey protein further increased postexercise intramuscular connective tissue protein synthesis rates when compared with the ingestion of carbohydrate. However, the latter effect did not become evident until the late stages of the postprandial phase, i.e., between 3 and 5 h after protein ingestion (24). These data have led to the suggestion that protein ingestion may have a more delayed (>3 h) effect on stimulating intramuscular connective tissue protein synthesis rates. Consequently, we hypothesized that protein ingestion can increase postexercise intramuscular connective tissue protein synthesis rates when assessed over a more prolonged recovery period.
In the present study, we selected 36 healthy, male subjects (24 ± 3 yr) who either ingested 30 g casein protein before sleep (PRO), performed a bout of resistance exercise before sleep (EX), or carried out a combination of both (EX + PRO). We applied primed, continuous intravenous infusions of L-[ring-2H5]-phenylalanine and L-[1-13C]-leucine throughout the subsequent overnight recovery period. Subjects in PRO and EX + PRO ingested 30 g intrinsically L-[1-13C]-phenylalanine and L-[1-13C]-leucine-labeled casein protein before sleep. We collected muscle biopsies before and after 7.5 h of overnight sleep and assessed labeled amino acid enrichments in connective tissue proteins isolated from the collected muscle samples. This approach allowed us to compare the effect of protein ingestion, exercise, and exercise plus protein ingestion on connective tissue protein synthesis rates in vivo in humans. Furthermore, by ingested intrinsically L-[1-13C]-phenylalanine-labeled protein with a high enrichment level, we were also able to assess whether dietary protein-derived amino acids were used as precursors for de novo synthesis of intramuscular connective tissue protein at rest and/or after a bout of resistance exercise.
METHODS
Subjects
A total of 36 healthy, recreationally active, young men were selected to participate in this study (see Table, Supplemental Digital Content 1, Subject characteristics, http://links.lww.com/MSS/B952). Subjects were randomly assigned to perform a bout of resistance exercise in the evening (1945–2045 h) combined with the ingestion of a placebo before sleep (EX, n = 12), perform a bout of resistance exercise in the evening combined with the ingestion of 30 g intrinsically L-[1-13C]-phenylalanine and L-[1-13C]-leucine-labeled casein protein intrinsically before sleep (EX + PRO, n = 12), or ingested 30 g intrinsically labeled protein before sleep without prior exercise (PRO, n = 12). All subjects were informed of the nature and possible risks of the experimental procedures before their written informed consent was obtained. This study is part of a greater project investigating the effect of exercise and presleep protein ingestion on overnight muscle protein synthesis, parts of which have already been published (25,26). The project was registered at Netherlands Trial Registry as NTR3885; approved by the Medical Ethical Committee of the Maastricht University Medical Centre, The Netherlands; and conformed to standards for the use of human subjects in research as outlined in the most recent version of the Helsinki Declaration.
Pretesting
Bodyweight and body composition were determined by dual-energy x-ray absorptiometry (Discovery A; Hologic, Bedford, MA). Leg volume was determined by anthropometry measurements as described by Jones and Pearson (27). The subjects were then familiarized with the resistance exercise protocol and the exercise equipment. All exercises during pretesting and experimental trials were supervised by trained personnel. Subjects started by performing a 10-min cycling warm-up at 150 W before completing an estimation of their one-repetition maximum (1RM) on the leg press and leg extension exercises using the multiple repetitions testing procedure (28). For each exercise, subjects performed 10 submaximal repetitions to become familiarized with the equipment and to have lifting technique critiqued and properly adjusted. Sets were then performed at progressively increasing loads until failure to perform a valid estimation within 3–6 repetitions of the set. A repetition was valid if the subject was able to complete the entire lift in a controlled manner without assistance. A 2-min resting period between subsequent attempts was allowed. The pretesting and experimental trials were separated by at least 7 d.
Diet and physical activity
All subjects were instructed to refrain from exhaustive physical activity and exercise and to keep their diet as constant as possible for the 2 d preceding the experimental day. Food intake and physical activity questionnaires were collected for 2 d before the experiment. All subjects received a standardized diet throughout the experimental day (0.16 MJ·kg−1, providing 62 energy percentage (En%) carbohydrate, 13 En% protein, and 22 En% fat). The energy content of the standardized diet was based on individual energy requirements based on the Harris–Benedict equation and adjusted using a physical activity factor of 1.6 to ensure ample energy intake. During the experimental day, participants ingested 1.2 ± 0.1 g protein·kg BW−1·d−1 from the standardized diet and an additional 20 g (0.27 ± 0.03 g·kg−1) of protein provided at 2045 h. Subjects in the PRO and EX + PRO ingested an additional 30 g of protein (0.40 ± 0.03 g·kg−1) before sleep (2330 h).
Experimental Protocol
At 1730 h, participants reported to the laboratory and had Teflon catheters inserted into an antecubital vein of each arm. At 1830 h (t = −300 min), all the subjects consumed a standardized dinner (0.04 MJ·kg−1, providing 55 En% carbohydrate, 21 En% protein, and 20 En% fat; Sligro, Maastricht, The Netherlands). Subjects in the EX and EX + PRO groups then performed a resistance exercise bout between 1945 and 2045 h. Subjects in the PRO group rested in a sitting position during this period. Immediately after the exercise or rest session, all groups received drinks providing 20 g protein and 45 g carbohydrate (Gatorade G-series 03 Recover protein recovery shake; Gatorade, Chicago, IL) to optimize muscle protein synthesis rates in the hours before sleep (29). After protein ingestion, a background blood sample was taken before the initiation of the tracer infusion protocol, which was started at 2100 h (t = −150 min). Plasma and intracellular phenylalanine and leucine pools were primed with a single intravenous dose (priming dose) of L-[ring-2H5]-phenylalanine (2.0 μmol·kg−1), L-[ring-2H2]-tyrosine (0.615 μmol·kg−1), and L-[1-13C]-leucine (4.0 μmol·kg−1). Once primed, the continuous stable isotope infusion was initiated (infusion rate: 0.05 μmol·kg−1·min−1 L-[ring-2H5]-phenylalanine, 0.015 μmol·kg−1·min−1 L-[ring-2H2]- tyrosine, and 0.1 μmol·kg−1·min−1 L-[1-13C]-leucine; Cambridge Isotopes Laboratories, Andover, MA). Participants rested in a supine position for 2.5 h until 2330 h (t = 0 min), after which the first muscle biopsy was taken. Subsequently, subjects in the PRO and EX + PRO groups ingested 30 g intrinsically L-[1-13C]-phenylalanine and L-[1-13C]-leucine-labeled casein protein, and the EX group ingested a water placebo. Both beverages contained 450 mL water and 1.5 mL of vanilla extract (Dr. Oetker, Amersfoort, The Netherlands). Subjects went to sleep at 0000 h. During the night, blood samples (10 mL) were taken without waking up the subjects at t = 30, 60, 90, 150, 210, 270, 330, 390, and 450 min relative to the intake of the protein drink. A second muscle biopsy was obtained from the contralateral leg 7.5 h later at 0700 h (t = 450 min) (see Fig. 1).
FIGURE 1.
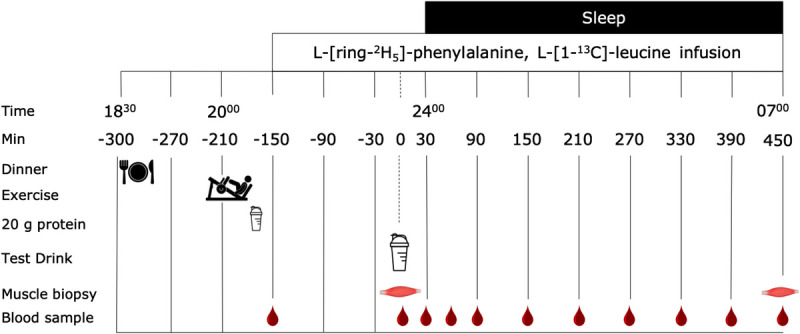
Experimental protocol. Thirty-six young male subjects performed a single bout of resistance exercise (EX and EX + PRO) or remained rested between 1945 and 2045 h. Subjects underwent an intravenous infusion of stable isotope amino acid tracers and ingested either 30 g intrinsically labeled casein protein (EX + PRO and PRO) or placebo (EX) at 2330 h. Skeletal muscle biopsies were collected before and after sleep to assess intramuscular connective tissue protein synthesis rates.
Blood samples were collected in EDTA tubes and centrifuged at 1000g for 10 min at 4°C. Aliquots of plasma were frozen in liquid nitrogen and stored at −80°C. Muscle biopsies were obtained from the middle region of the musculus vastus lateralis, 15 cm above the patella and approximately 4 cm below entry through the fascia, using the percutaneous needle biopsy technique (30). Muscle samples were dissected carefully and freed from any visible nonmuscle material. The muscle samples were immediately frozen in liquid nitrogen and stored at −80°C until further analysis.
Exercise protocol
The exercise protocol consisted of 60 min of lower-body resistance exercise. After 15 min of self-paced cycling at 150 W with a cadence of 60–80 rpm, subjects performed six sets of 10 repetitions on the horizontal leg press machine (Technogym BV, Rotterdam, Netherlands) and six sets of 10 repetitions on the leg extension machine (Technogym BV). The first two sets of both exercises were performed at 55% and 65% of 1RM, respectively. Sets 3–6 were performed at 75% of 1RM, and there were 2 min rest intervals between all sets.
Production of intrinsically labeled protein and tracer preparation
Details on the production of intrinsically L-[1-13C]-phenylalanine and L-[1-13C]-leucine-labeled casein protein and preparation of L-[ring-2H5]-phenylalanine, L-[1-13C]-leucine, and L-[ring-2H2]-tyrosine tracers are presented online (see Document, Supplemental Digital Content 2, Plasma and muscle connective tissue protein analyses, http://links.lww.com/MSS/B953).
Plasma and muscle connective tissue protein analyses
Details on the measurement of plasma glucose, insulin, and free L-[ring-2H5]-phenylalanine, L-[1-13C]-phenylalanine, and L-[1-13C]-leucine enrichments are presented online (see Document, Supplemental Digital Content 2, Plasma and muscle connective tissue protein analyses, http://links.lww.com/MSS/B953). Muscle connective tissue protein analyses could only be conducted in n = 11 in both the PRO and the PRO+EX treatments due to insufficient amount of muscle tissue sampled. Muscle connective protein-enriched fractions were isolated from ~60 mg of wet muscle tissue by hand homogenizing on ice using a pestle in a standard extraction buffer (10 μL·mg−1). The samples were spun for 15 min at 800g and 4°C. The pellet was washed with 400 μL of extraction buffer before vortexing and centrifugation at 800g and 4°C for 10 min. The supernatant was removed, and the pellet was washed with 500 μL ddH2O before vortexing and centrifugation at 800g and 4°C for 10 min. The supernatant was removed, and 1 mL of homogenization buffer was added and the material was suspended by vortexing before transferring into microtubes containing 1.4 mm ceramic beads and Lysing Matrix D (MP Biomedicals, Irvine, CA). The microtubes were vigorously shaken four times for 45 s at 5.5 m·s−1 (FastPrep-24 5G, MP Biomedicals) to mechanically lyse the protein network. Samples were then left to rest at 4°C for 3 h before centrifugation at 800g and 4°C for 20 min, discarding the supernatant and adding 1 mL of homogenization buffer. The microtubes were shaken for 40 s at 5.5 m·s−1 before centrifugation at 800g and 4°C for 20 min. The supernatant was discarded, and 1.5 mL of KCl buffer was added to dissolve connective tissue proteins overnight at 4°C. The next morning, samples were vortexed and centrifuged at 1600g for 20 min at 4°C. The supernatant was removed, and the pellet, containing both immature and mature connective tissue proteins, was mixed with 1 mL KCl buffer and left for 2 h at 4°C. The samples were vortexed and centrifuged at 1600g for 20 min at 4°C, and the supernatant was discarded. The remaining pellet was suspended in 1 mL of 6 M HCl in glass screw-cap tubes and left to hydrolyze overnight at 110°C. The free amino acids from the hydrolyzed connective protein pellet were dried under a nitrogen stream while being heated to 120°C. The free amino acids were then purified and converted into their N-ethoxycarbonyl ethyl ester derivatives before the measurement of enrichment on the GC-C-IRMS (see text, Supplemental Digital Content 2, http://links.lww.com/MSS/B953).
Calculations
The fractional synthetic rate (FSR) of intramuscular connective tissue protein was calculated by dividing the increment in connective tissue protein enrichment by the respective precursor amino acid tracer enrichments. Consequently, connective tissue protein FSR was calculated as follows (17):
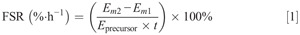
Em1 and Em2 represents protein-bound L-[ring-2H5]-phenylalanine or L-[1-13C]-leucine, Eprecursor represents the average plasma free L-[ring-2H5]-phenylalanine or L-[1-13C]-leucine enrichment during the tracer incorporation period, and t indicates the time interval (h) between biopsies.
Statistics
All data in text are expressed as mean ± SD. Baseline characteristics between groups were compared using a one-way ANOVA. Time-dependent variables (i.e., plasma glucose, insulin, amino acid concentrations, and tracer enrichments) were analyzed by a two-factor repeated-measures ANOVA with time as a within-subjects factor and treatment group as a between-subjects factor. The analysis was carried out for the period starting at the time of protein or placebo ingestion (t = 0 min) until the end of the experimental trial (t = 450 min). Non-time-dependent variables (i.e., intramuscular connective tissue protein FSR and L-[1-13C]-phenylalanine enrichment) were compared between treatment groups using a one-way ANOVA. Statistical significance was set at P < 0.05. Bonferroni-corrected post hoc comparisons were performed where appropriate. All calculations were performed using SPSS 24.0 (SPSS Inc., Chicago, IL).
RESULTS
Plasma glucose and insulin concentrations
Plasma glucose concentrations at t = 0 min averaged 5.5 ± 0.3, 5.2 ± 0.4, and 5.5 ± 0.4 mmol·L−1 in EX, EX + PRO, and PRO, respectively (P = 0.061). Plasma glucose concentrations declined slightly during the overnight period after the exercise treatments when compared with the rested treatment (time–treatment interaction: P = 0.010). Plasma insulin concentrations at t = 0 min averaged 7.8 ± 6.1, 5.4 ± 2.4, and 9.3 ± 5.5 mU·L−1 in EX, EX + PRO, and PRO, respectively (treatment: P = 0.164). Changes in plasma insulin concentrations did not differ between treatments (time–treatment interaction: P = 0.114), despite a small transient increase in plasma insulin concentrations at t = 30 min when casein protein was ingested before sleep.
Plasma amino acid concentrations and enrichments
Total (Fig. 2A) and essential (Fig. 2B) amino acid concentrations increased after protein intake when compared with placebo ingestion (time–treatment interactions: P < 0.001). Plasma glycine (Fig. 3A) concentrations showed a small decline after exercise when compared with rested conditions (time–treatment interaction: P = 0.006). In line, plasma glycine concentration incremental area under the curve (Fig. 3B) was significantly lower in EX when compared with the PRO treatment (post hoc comparison: P = 0.006), with intermediate values in the EX + PRO treatment (nonsignificant post hoc comparisons). Plasma proline concentrations (Fig. 3C) increased after protein ingestion when compared with placebo ingestion (time–treatment interaction: P < 0.001), resulting in greater overnight plasma proline concentration incremental areas under the curve (Fig. 3D) in the PRO and EX + PRO treatments when compared with EX (post hoc comparisons: P < 0.001).
FIGURE 2.
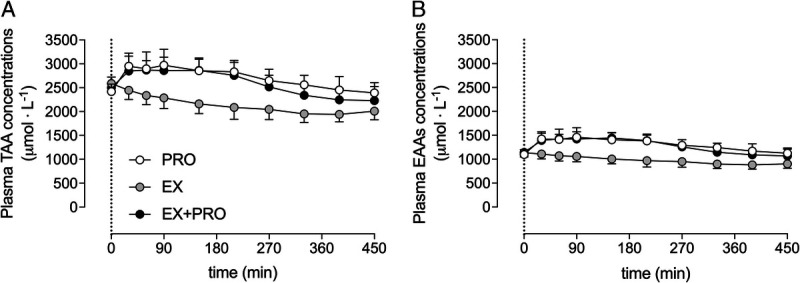
Overnight plasma total amino acid (A) and essential amino acid (B) concentrations (μmol·L−1). The dotted line represents the ingestion of the presleep protein. Values represent mean ± SD. Data were analyzed with a two-way repeated-measures (within-subject factor: time; between-subject factor: treatment) ANOVA. Total amino acids: time effect, P < 0.001; treatment effect, P < 0.001; time–treatment interaction, P < 0.001. Essential amino acids: time effect, P < 0.001; treatment effect, P < 0.001; time–treatment interaction, P < 0.001. TAA, total amino acids; EAA, essential amino acids; PRO, presleep protein ingestion without prior exercise; EX, placebo ingestion with prior exercise; EX + PRO, protein ingestion with prior exercise.
FIGURE 3.
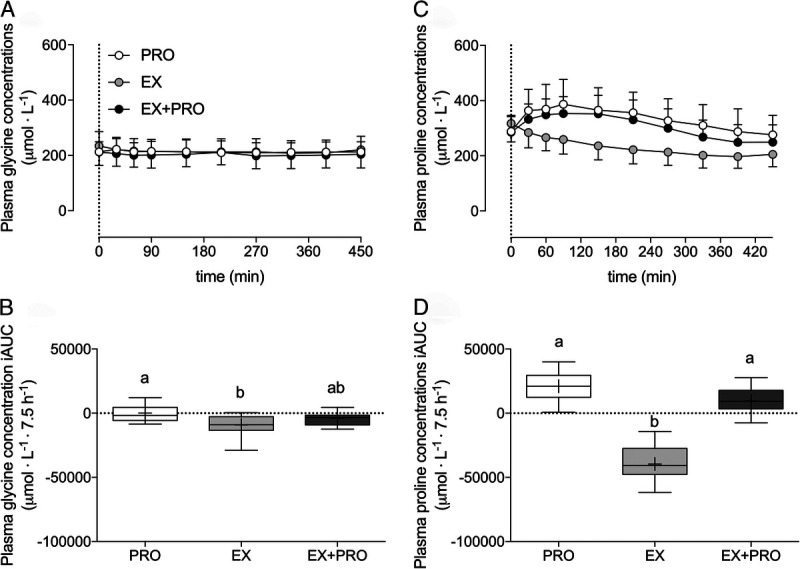
Overnight plasma glycine concentrations (A), glycine iAUC (B), proline concentrations (C), and proline iAUC (D) (μmol·L−1). The dotted line represents the ingestion of the presleep protein. Plasma concentrations over time are expressed as mean ± SD. Plasma iAUC is expressed as box and whisker plots with the median (line), mean (cross), interquartile range (box), and minimum and maximum values (tails), and treatments without a common letter differ, P < 0.05. Plasma concentrations over time were analyzed with a two-way repeated-measures (within-subject factor: time; between-subject factor: treatment) ANOVA. Plasma concentration iAUC was analyzed with a one-way (between-subject factor: treatment) ANOVA. Glycine concentrations: time effect, P < 0.001; treatment effect, P = 0.761; time–treatment interaction, P = 0.006. Glycine iAUC: main treatment effect, P = 0.008; EX vs PRO post hoc comparison, P = 0.006. Proline concentrations: time effect, P < 0.001; treatment effect, P < 0.001; time–treatment effect, P = 0.002. Proline iAUC: main treatment effect, P < 0.001; EX vs PRO and EX vs EX + PRO post hoc comparisons, P < 0.001. iAUC, incremental area under the curve; PRO, presleep protein ingestion without prior exercise; EX, placebo ingestion with prior exercise; EX + PRO, protein ingestion with prior exercise.
Plasma L-[ring-2H5]-phenylalanine, L-[1-13C]-leucine, and L-[1-13C]-phenylalanine (Fig. 4) enrichments did not differ between treatments before drink ingestion (t = 0 min). Plasma L-[ring-2H5]-phenylalanine enrichments increased slightly during the overnight period, but this increase was lower after protein ingestion (time–treatment interaction: P < 0.001). Plasma L-[1-13C]-leucine enrichments also increased slightly throughout the overnight period, but this increase was more profound after protein ingestion (time–treatment interaction: P < 0.001). Plasma L-[1-13C]-phenylalanine enrichments increased after protein ingestion, but not after placebo ingestion (time–treatment interaction: P < 0.001).
FIGURE 4.
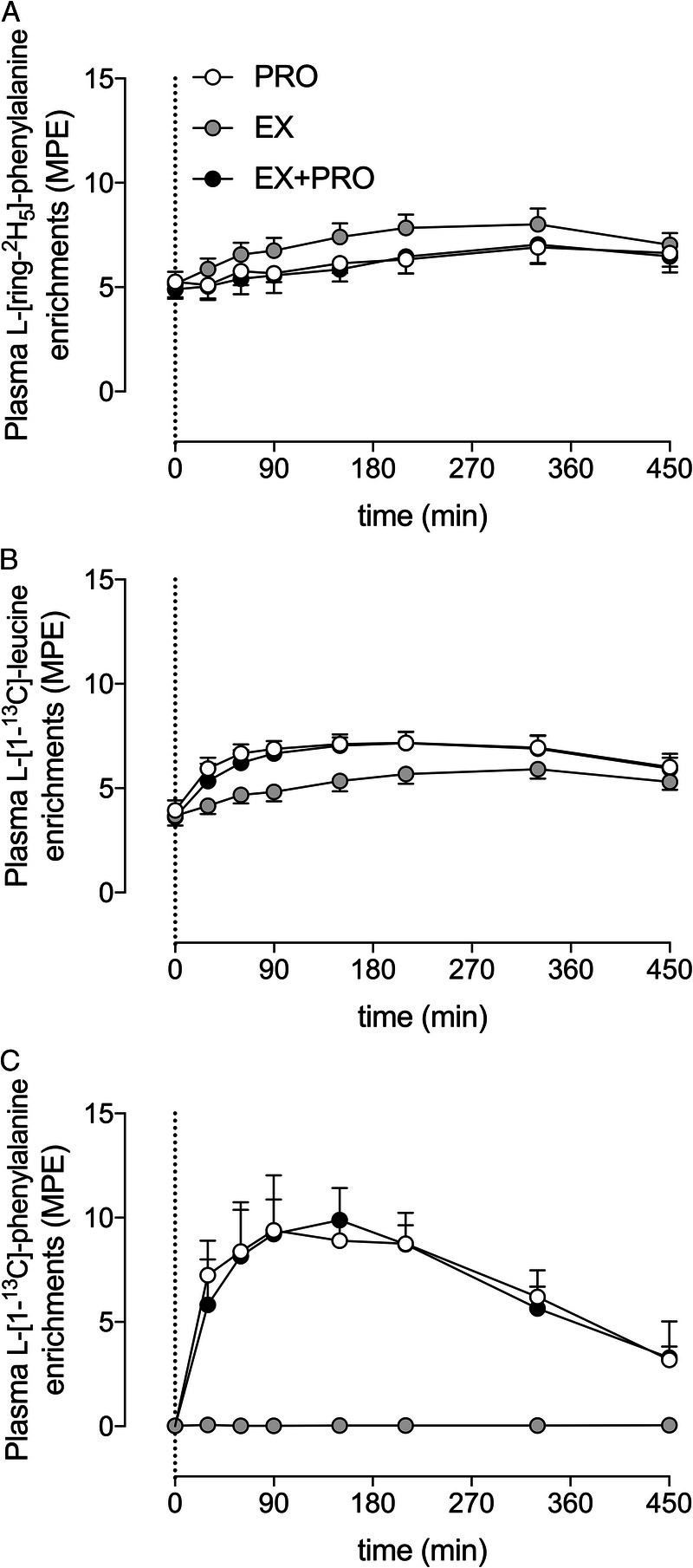
Overnight plasma L-[ring-2H5]-phenylalanine (A), L-[1-13C]-leucine (B), and L-[1-13C]-phenylalanine (C) enrichments in MPE. The dotted line represents the ingestion of the presleep protein. Values represent mean ± SD. Data were analyzed with a two-way repeated-measures (within-subject factor: time; between-subject factor: treatment) ANOVA. L-[ring-2H5]-phenylalanine enrichments: time effect, P < 0.001; treatment effect, P < 0.001; time–treatment effect, P < 0.001. L-[1-13C]-leucine enrichments: time effect, P < 0.001; treatment effect, P < 0.001; time–treatment effect, P < 0.001. L-[1-13C]-phenylalanine enrichments: time effect, P < 0.001; treatment effect, P < 0.001; time–treatment effect, P < 0.001. PRO, presleep protein ingestion without prior exercise; EX, placebo ingestion with prior exercise; EX + PRO, protein ingestion with prior exercise.
Intramuscular connective tissue protein FSR and protein-bound enrichments
Overnight intramuscular connective tissue protein FSR based on the infused L-[ring-2H5]-phenylalanine tracer (Fig. 5A) were higher in the exercise treatments when compared with the rested treatment (EX: 0.086 ± 0.017%·h−1, n = 12; EX + PRO: 0.080 ± 0.019%·h−1, n = 11; PRO: 0.059 ± 0.016%·h−1; n = 11; post hoc comparisons vs PRO: P < 0.05). A similar pattern was observed for overnight intramuscular connective tissue protein FSR based on the infused and ingested L-[1-13C]-leucine tracer (Fig. 5B), although the differences did not reach statistical significance (EX: 0.078 ± 0.026%·h−1, n = 12; EX + PRO: 0.073 ± 0.024%·h−1, n = 11; PRO: 0.060 ± 0.018%·h−1, n = 11; treatment effect: P = 0.166). Intramuscular connective tissue protein-bound L-[1-13C]-phenylalanine enrichments (Fig. 6), derived from the ingested protein, were substantially higher when protein ingestion was combined with prior exercise (PRO: 0.026 ± 0.006 mole percent excess [MPE], n = 11; EX + PRO: 0.041 ± 0.115 MPE, n = 11; treatment effect: P = 0.002).
FIGURE 5.
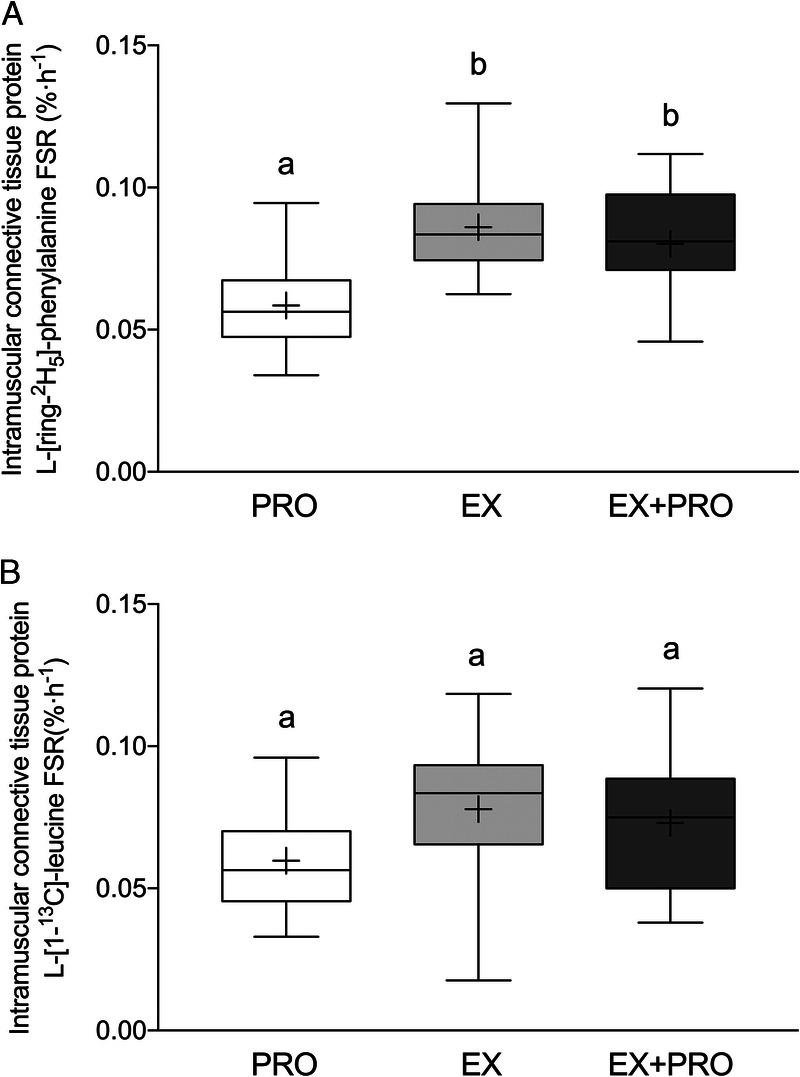
Overnight intramuscular connective tissue protein synthetic rates (FSR in %·h−1) as calculated using L-[ring-2H5]-phenylalanine or L-[1-13C]-leucine as tracer. The data are presented as box and whisker plots with the median (line), mean (cross), interquartile range (box), and minimum and maximum values (tails). Treatments without a common letter differ, P < 0.05. Data were analyzed with a one-way (between-subject factor: treatment) ANOVA. FSR based on L-[ring-2H5]-phenylalanine; main treatment effect, P = 0.002. EX vs PRO and EX + PRO vs PRO post hoc comparisons, P < 0.005. FSR based on L-[1-13C]-leucine: main treatment effect, P = 0.166. PRO, presleep protein ingestion without prior exercise; EX, placebo ingestion with prior exercise; EX + PRO, protein ingestion with prior exercise.
FIGURE 6.
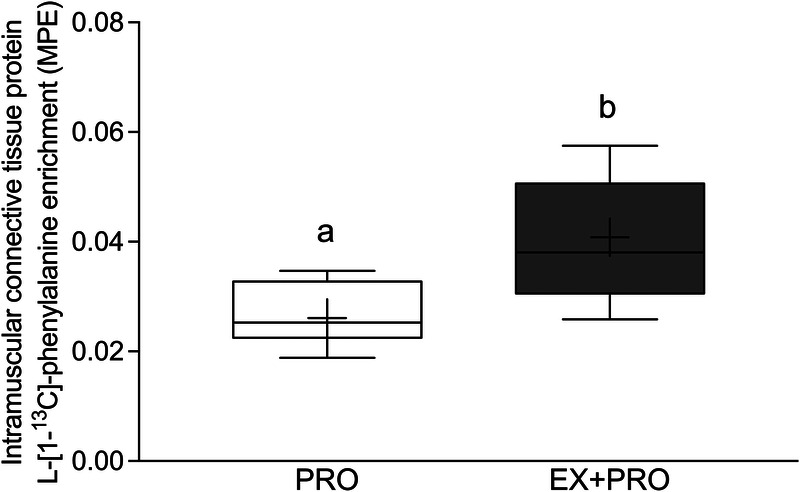
Overnight L-[1-13C]-phenylalanine incorporation into intramuscular connective tissue protein in MPE. The data are presented as box and whisker plots with the median (line), mean (cross), interquartile range (box), and minimum and maximum values (tails). Treatments without a common letter differ, P < 0.05. The data were analyzed with an independent sample t-test, P = 0.002. PRO, presleep protein ingestion without prior exercise; EX + PRO, protein ingestion with prior exercise.
DISCUSSION
In the present study, we observed that a single bout of resistance exercise increases intramuscular connective tissue protein synthesis rates during overnight recovery. Furthermore, presleep casein protein ingestion did not further increase intramuscular connective tissue protein synthesis rates during postexercise recovery. However, the dietary protein-derived amino acids are shown to act as precursors for de novo intramuscular connective tissue protein synthesis as they become incorporated in the protein fraction at rest and, more prominently, during recovery from exercise.
The postprandial rise in plasma (essential) amino acids is a key factor for driving the increase in postprandial muscle protein synthesis rates (31,32). Here, protein ingestion rapidly increased plasma insulin and essential amino acid (Fig. 2B) concentrations, indicating proper protein digestion and amino acid absorption during overnight sleep. Along with the rise in essential amino acid concentrations, we observed that presleep protein ingestion in both the rested and postexercise state increased plasma proline concentrations during sleep (Fig. 3C/D). By contrast, presleep casein protein ingestion did not substantially increase plasma glycine concentrations above baseline values after a day of standardized nutrition (Fig. 3A/B). Our findings align well with Alcock et al. (33), who recently demonstrated that the ingestion of calcium caseinate and hydrolyzed casein protein do not provide sufficient glycine to increase postprandial plasma glycine availability. This is attributed to the fact that casein, like many other protein sources, does not contain much glycine (2%) when compared with other amino acids, such as leucine (10%) or proline (11%) (34).
By intravenously infusing stable isotope amino acid tracers and collecting muscle biopsies before and after sleep, we were able to assess overnight intramuscular connective tissue protein synthesis rates. Our current approach revealed intramuscular connective tissue protein FSR slightly lower (0.080–0.086%·h−1) than rates previously reported by Holm et al. (0.086–0.119%·h−1) who used the same tissue protein extraction protocol to isolate both immature and mature connective tissue proteins (24). The slight discrepancy between findings can likely be explained by the study design (7.5 h of postprandial overnight sleep vs 5 h postprandial response in the morning, respectively).
As hypothesized, a bout of resistance exercise increased postprandial intramuscular connective tissue protein synthesis rates by ~40% (Fig. 5A). Our data in the overnight setting extend on previous work by demonstrating that resistance exercise performed in the evening increases intramuscular connective tissue synthesis rates throughout overnight sleep (16,17,19–22). Interestingly, plasma glycine levels showed a small decline after exercise when compared with rested conditions (Fig. 3A). In line, plasma glycine concentration incremental area under the curve was significantly lower after exercise when compared with the protein treatment, with intermediate values in the exercise and protein treatments (Fig. 3B). Lower plasma glycine availability after exercise may be attributed to lower endogenous glycine production, increased glycine degradation, and/or increased glycine uptake and/or utilization for the biosynthesis of compounds such as collagen, creatine, and uric acid (35). It has been suggested that de novo glycine synthesis may be insufficient for optimal growth and health in humans and, therefore, should be classified as a conditionally essential amino acid (35,36). Evidence is needed to assess whether exercise is a factor that further increases dietary glycine requirements.
Recently, Holm et al. (24) showed that the ingestion of ~18 g whey protein further increased intramuscular connective tissue protein synthesis rates during the later stages of postexercise recovery. Based on this delayed response, we hypothesized that protein ingestion may be effective at stimulating intramuscular connective tissue protein synthesis rates during more prolonged overnight recovery from exercise. In contrast to our hypothesis, we observed no further increase in postexercise intramuscular connective tissue protein synthesis rates after ingestion of 30 g casein protein when compared with the ingestion of a nonnitrogenous placebo. The absence of a further increase in intramuscular connective tissue protein synthesis rates may be related to the type and/or dose of dietary protein provided in the present study. For example, we have previously demonstrated that the ingestion of 40 g (37), as opposed to 30 g of casein (26), is required to increase myofibrillar protein synthesis rates during overnight recovery from exercise (38). It may be speculated that a protein dose ≥40 g is required to stimulate postexercise intramuscular connective tissue protein synthesis rates. Alternatively, casein protein may not provide sufficient amounts of key amino acids required to support the synthesis of new connective tissue proteins. For example, intramuscular connective tissue proteins, such as collagen, contain approximately 5% leucine and phenylalanine, but 45% glycine and proline (39). Although casein protein contains ample leucine and phenylalanine (~9%) (34), the absence of an increase in overnight plasma glycine availability after casein ingestion suggests that casein may not deliver sufficient glycine to support a greater increase in postexercise intramuscular connective tissue protein synthesis rates.
By applying specifically produced highly L-[1-13C]-phenylalanine-enriched (>35 MPE) casein protein, we were able to directly assess the metabolic fate of dietary protein-derived amino acids. Here, we demonstrate for the first time that dietary protein-derived amino acids are incorporated into de novo intramuscular connective tissue protein (Fig. 6). Furthermore, we show that resistance exercise performed in the evening allows more of the ingested protein-derived amino acids (~60% increase vs the PRO only condition) to be directed toward the de novo synthesis of intramuscular connective tissue protein during overnight recovery. These data reinforce our findings that intramuscular connective tissue is actively remodeling after exercise. Although we did not observe an added effect of casein protein ingestion on the rate of intramuscular connective tissue protein synthesis per se, our observation of dietary protein-derived amino acid incorporation into connective tissue protein implies that it should be possible to modulate postexercise remodeling with nutritional intervention(s).
It could be speculated that the ingestion of a dietary protein source other than casein may be (more) effective at stimulating postexercise intramuscular connective tissue protein synthesis rates. For instance, proteins that contain greater amounts of glycine and proline may deliver more amino acid precursors required to support higher postexercise intramuscular connective tissue protein synthesis rates. Dietary collagen protein sources, such as collagen hydrolysate and gelatin, have been reported to contain approximately 26% and 12% of glycine and proline, respectively (40). Recent work by Shaw et al. (41) demonstrates that 15 g gelatin ingestion robustly increases plasma glycine and proline concentrations, leading to greater collagen synthesis rates in tissue constructs in an ex vivo setting. Whether or not the ingestion of dietary collagen sources, or perhaps other protein sources coingested with added glycine and/or proline, promotes in vivo intramuscular connective tissue protein synthesis rates remains a topic for future exploration. Such information would be critical to establish effective nutritional strategies to optimize skeletal muscle remodeling after prolonged exercise training.
In conclusion, resistance exercise increases intramuscular connective tissue protein synthesis rates during overnight sleep, with no further effect of postexercise protein ingestion. However, dietary protein-derived amino acids are being used as precursors to support de novo intramuscular connective tissue protein synthesis.
Supplementary Material
Acknowledgments
The authors acknowledge the enthusiastic support of the subjects who volunteered to participate in these experiments. The project was funded by the TI Food and Nutrition, a public–private partnership on precompetitive research in food and nutrition. The researchers are responsible for the study design, data collection and analysis, decision to publish, and preparation of the manuscript. The industrial partners have contributed to the project through regular discussion. J. Trommelen, L. B. Verdijk, and L. J. C. van Loon have received speaker’s fee, research grants, consulting fees, or a combination of these, from Friesland Campina, Nutricia Research, and PepsiCo. None of the other authors has any conflicts of interest, financial or otherwise, to disclose. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
J. T. and A. M. H. contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
REFERENCES
- 1.Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. Journal Appl Physiol. 2009;106(6):2040–8. [DOI] [PubMed] [Google Scholar]
- 2.Burd NA, Gorissen SH, van Vliet S, Snijders T, van Loon LJ. Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: a randomized controlled trial. Am J Clin Nutr. 2015;102(4):828–36. [DOI] [PubMed] [Google Scholar]
- 3.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995;268(3 Pt 1):E514–20. [DOI] [PubMed] [Google Scholar]
- 4.Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol (1985). 1992;73(4):1383–8. [DOI] [PubMed] [Google Scholar]
- 5.Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol. 2009;587(Pt 4):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 2011;93(2):322–31. [DOI] [PubMed] [Google Scholar]
- 7.Trommelen J, Betz MW, van Loon LJC. The muscle protein synthetic response to meal ingestion following resistance-type exercise. Sports Med. 2019;49(2):185–97. [DOI] [PubMed] [Google Scholar]
- 8.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41(2):215–9. [DOI] [PubMed] [Google Scholar]
- 9.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc. 2009;109(9):1582–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond MJ Dreyer HC Pennings B, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104(5):1452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snijders T Res PT Smeets JS, et al. Protein ingestion before sleep increases muscle mass and strength gains during prolonged resistance-type exercise training in healthy young men. J Nutr. 2015;145(6):1178–84. [DOI] [PubMed] [Google Scholar]
- 12.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96(6):1454–64. [DOI] [PubMed] [Google Scholar]
- 13.Huijing PA. Muscle as a collagen fiber reinforced composite: a review of force transmission in muscle and whole limb. J Biomech. 1999;32(4):329–45. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Gao Y. Effects of aging on the lateral transmission of force in rat skeletal muscle. J Biomech. 2014;47(5):944–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramaswamy KS Palmer ML van der Meulen JH, et al. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol. 2011;589(Pt 5):1195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuthbertson DJ Babraj J Smith K, et al. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab. 2006;290(4):E731–8. [DOI] [PubMed] [Google Scholar]
- 17.Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;288(6):E1153–9. [DOI] [PubMed] [Google Scholar]
- 18.Oertzen-Hagemann V Kirmse M Eggers B, et al. Effects of 12 weeks of hypertrophy resistance exercise training combined with collagen peptide supplementation on the skeletal muscle proteome in recreationally active men. Nutrients. 2019;11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dideriksen K Reitelseder S Malmgaard-Clausen NM, et al. No effect of anti-inflammatory medication on postprandial and postexercise muscle protein synthesis in elderly men with slightly elevated systemic inflammation. Exp Gerontol. 2016;83:120–9. [DOI] [PubMed] [Google Scholar]
- 20.Mikkelsen UR Dideriksen K Andersen MB, et al. Preserved skeletal muscle protein anabolic response to acute exercise and protein intake in well-treated rheumatoid arthritis patients. Arthritis Res Ther. 2015;17:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holm L van Hall G Rose AJ, et al. Contraction intensity and feeding affect collagen and myofibrillar protein synthesis rates differently in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298(2):E257–69. [DOI] [PubMed] [Google Scholar]
- 22.Hansen M Miller BF Holm L, et al. Effect of administration of oral contraceptives in vivo on collagen synthesis in tendon and muscle connective tissue in young women. J Appl Physiol. 2009;106(4):1435–43. [DOI] [PubMed] [Google Scholar]
- 23.Dideriksen KJ Reitelseder S Petersen SG, et al. Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scand J Med Sci Sports. 2011;21(6):e372–83. [DOI] [PubMed] [Google Scholar]
- 24.Holm L, Rahbek SK, Farup J, Vendelbo MH, Vissing K. Contraction mode and whey protein intake affect the synthesis rate of intramuscular connective tissue. Muscle Nerve. 2017;55(1):128–30. [DOI] [PubMed] [Google Scholar]
- 25.Trommelen J Holwerda AM Kouw IW, et al. Resistance exercise augments postprandial overnight muscle protein synthesis rates. Med Sci Sports Exerc. 2016;48(12):2517–25. [DOI] [PubMed] [Google Scholar]
- 26.Trommelen J Kouw IWK Holwerda AM, et al. Presleep dietary protein-derived amino acids are incorporated in myofibrillar protein during postexercise overnight recovery. Am J Physiol Endocrinol Metab. 2018;314(5):E457–e67. [DOI] [PubMed] [Google Scholar]
- 27.Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol. 1969;204(2):63p–6p. [PubMed] [Google Scholar]
- 28.Mayhew JL, Prinster JL, Ware JS, Zimmer DL, Arabas JR, Bemben MG. Muscular endurance repetitions to predict bench press strength in men of different training levels. J Sports Med Phys Fitness. 1995;35(2):108–13. [PubMed] [Google Scholar]
- 29.Beelen M Tieland M Gijsen AP, et al. Coingestion of carbohydrate and protein hydrolysate stimulates muscle protein synthesis during exercise in young men, with no further increase during subsequent overnight recovery. J Nutr. 2008;138(11):2198–204. [DOI] [PubMed] [Google Scholar]
- 30.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35(7):609–16. [PubMed] [Google Scholar]
- 31.Dreyer HC Drummond MJ Pennings B, et al. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294(2):E392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alcock RD, Shaw GC, Tee N, Burke LM. Plasma amino acid concentrations after the ingestion of dairy and collagen proteins, in healthy active males. Front Nutr. 2019;6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorissen SHM Crombag JJR Senden JMG, et al. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018;50(12):1685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Wu Z, Dai Z, Yang Y, Wang J, Wu G. Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids. 2013;45(3):463–77. [DOI] [PubMed] [Google Scholar]
- 36.Jackson AA. The glycine story. Eur J Clin Nutr. 1991;45(2):59–65. [PubMed] [Google Scholar]
- 37.Res PT Groen B Pennings B, et al. Protein ingestion before sleep improves postexercise overnight recovery. Med Sci Sports Exerc. 2012;44(8):1560–9. [DOI] [PubMed] [Google Scholar]
- 38.Snijders T, Trommelen J, Kouw IWK, Holwerda AM, Verdijk LB, van Loon LJC. The impact of pre-sleep protein ingestion on the skeletal muscle adaptive response to exercise in humans: an update. Front Nutr. 2019;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eastoe JE. The amino acid composition of mammalian collagen and gelatin. Biochem J. 1955;61(4):589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skov K, Oxfeldt M, Thogersen R, Hansen M, Bertram HC. Enzymatic hydrolysis of a collagen hydrolysate enhances postprandial absorption rate-a randomized controlled trial. Nutrients. 2019;11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw G, Lee-Barthel A, Ross ML, Wang B, Baar K. Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am J Clin Nutr. 2017;105(1):136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


