Acute kidney injury is a commonly described complication of COVID-19 that has been linked to increased morbidity and mortality. Although severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been found in the kidney,1 the clinical effect remains unclear.2 Here, we present data from a post-mortem series of 63 patients who had SARS-CoV-2 respiratory infection (appendix pp 2–3), linking SARS-CoV-2 renal tropism to clinical outcome and acute kidney injury.
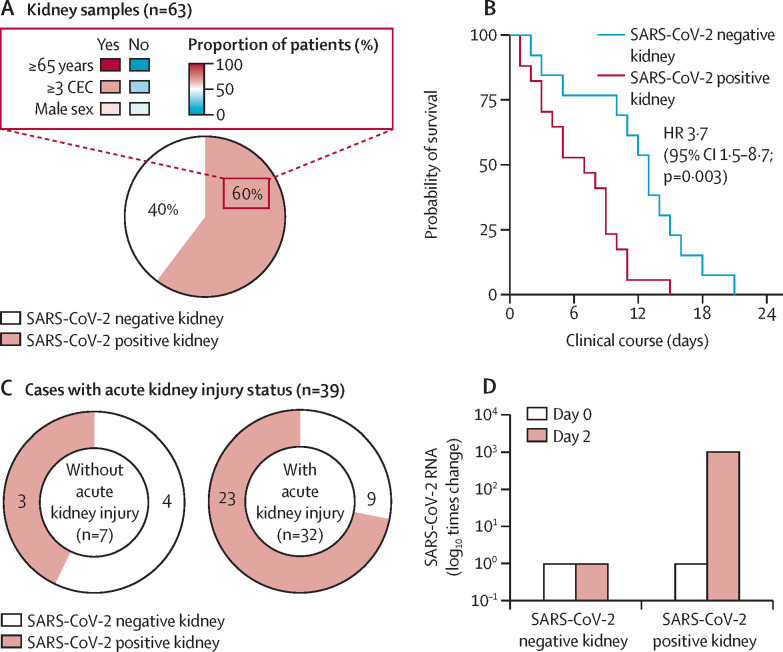
In this cohort, SARS-CoV-2 RNA was found in 38 (60%) of 63 patients. Presence of SARS-CoV-2 RNA in the kidney was associated with older age and an increased number of coexisting conditions (figure ). Furthermore, SARS-CoV-2 RNA was associated with a reduction in patients' survival time, obtained by calculating the time interval between COVID-19 diagnosis and date of death (figure). These findings support a potential correlation between extra-respiratory viral tropism, disease severity, and increased risk of premature death within the first 3 weeks of disease.
Figure.
Association between SARS-CoV-2 renal tropism, disease severity, and acute kidney injury
SARS-CoV-2 tropism was associated with older age and a number of coexisting conditions (A). Survival graph comparing patients with (n=19) and without (n=13) SARS-CoV-2 renal tropism (B). High frequency of SARS-CoV-2 renal tropism in patients with acute kidney injury (C). Successful isolation of infectious SARS-CoV-2 from a post-mortem kidney tissue sample (D). SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. CEC=coexisting conditions. HR=hazard ratio.
Previous studies have identified an increased risk of acute kidney injury in patients with COVID-19.3 Within our cohort, clinical kidney status was defined in 39 (62%) patients during the course of their disease progression (appendix pp 4–5). SARS-CoV-2 RNA was detected in the kidneys of 23 (72%) of 32 patients with acute kidney injury. By contrast, patients without acute kidney injury showed a lower frequency of SARS-CoV-2 renal tropism, with viral RNA only found in three (43%) of seven patients (figure).
SARS-CoV-2-mediated acute kidney injury might be explained by indirect factors (eg, cytokine-mediated injury) and by direct viral infection and replication in kidney epithelial cells.4 We isolated SARS-CoV-2 from an autopsied kidney, which produced a 1000-times increase in viral RNA after 48 h of cell infection in vitro (figure; appendix p 1), thus confirming the presence of infective virus in the kidney, even under post-mortem conditions. Furthermore, we found that patient-derived SARS-CoV-2 replicates in non-human primate kidney tubular epithelial cells (the main cellular target of acute kidney injury) using indirect immunofluorescence imaging of SARS-CoV-2 non-structural protein 3, one of the SARS-CoV replicase cleaving products (appendix p 5).5
Our findings indicate that SARS-CoV-2 renal tropism is associated with disease severity (ie, premature death) and development of acute kidney injury. This suggests that SARS-CoV-2 is able to target the kidney, pointing towards the importance of early urinary testing and eventual therapeutic prevention of kidney infection.
Acknowledgments
FB reports grants and personal fees from Amicus Therapeutics; personal fees from Takeda/Shire; and travel support from Sanofi Genzyme and Astellas, unrelated to this Correspondence. TBH reports grants from the German Research Foundation (CRC/1192, HU 1016/8-2, HU 1016/11-1, HU 1016/12-1), the Federal Ministry of Education and Research (STOP-FSGS-01GM1518C), and the European Research Council (grant 616891) during the study; grants and personal fees from Fresenius Medical Care; grants from Amicus Therapeutics and Sanofi Genzyme; and personal fees from Boehringer Ingelheim, Goldfinch Bio, Novartis Pharma, DaVita Germany, and Bayer Vital, unrelated to this Correspondence. SK reports grants and personal fees from Pfizer; personal fees from Biotest, Cytosorbents, Gilead, Merck Sharp & Dohme, Bayer, Astellas, Baxter, and Fresenius, unrelated to this Correspondence. VGP reports grants from the German Research Foundation (DFG: CRC/1192) and the Federal Ministry of Education and Research (BMBF: eMed Consortia Fibromap) during the study. All other authors declare no competing interests. FB, ML, SP, MNW, CE, MA, VGP, and TBH contributed equally as co-first or senior authors.
Supplementary Material
References
- 1.Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multi-organ and renal tropism of SARS-CoV-2. N Engl J Med. 2020 doi: 10.1056/NEJMc2011400. https://doi.org.10.1056/NEJMc2011400 published online May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross O, Moerer O, Weber M, Huber TB, Scheithauer S. COVID-19-associated nephritis: early warning for disease severity and complications? Lancet. 2020;395:e87–e88. doi: 10.1016/S0140-6736(20)31041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selby NM, Forni LG, Horne KL, et al. Covid-19 and acute kidney injury in hospital: summary of NICE guidelines. BMJ. 2020;369 doi: 10.1136/bmj.m1963. [DOI] [PubMed] [Google Scholar]
- 4.Batlle D, Soler MJ, Sparks MA, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. JASN. 2020;31:1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim D, Lee J-Y, Yang J-S, Kim JW, Kim VN, Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181:914–921. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.