Abstract
A replicated 5 × 5 Latin square design with a 2 × 2 + 1 factorial arrangement of treatments was used to determine the effects of bismuth subsalicylate (BSS) and encapsulated calcium ammonium nitrate (eCAN) on ruminal fermentation of beef cattle consuming bahiagrass hay (Paspalum notatum) and sugarcane molasses. Ten ruminally cannulated steers (n = 8; 461 ± 148 kg of body weight [BW]; average BW ± SD) and heifers (n = 2; 337 ± 74 kg of BW) were randomly assigned to one of five treatments as follows: 1) 2.7 g/kg of BW of molasses (NCTRL), 2) NCTRL + 182 mg/kg of BW of urea (U), 3) U + 58.4 mg/kg of BW of BSS (UB), 4) NCTRL + 538 mg/kg of BW of eCAN (NIT), and 5) NIT + 58.4 mg/kg of BW of BSS (NITB). With the exception of NCTRL, all treatments were isonitrogenous. Beginning on day 14 of each period, ruminal fluid was collected and rectal temperature was recorded 4× per day for 3 d to determine ruminal changes every 2 h from 0 to 22 h post-feeding. Ruminal gas cap samples were collected at 0, 3, 6, 9, and 12 h on day 0 of each period followed by 0 h on days 1, 2, 3, and 14. Microbial N flow was determined using Cr-Ethylenediaminetetraacetic acid, YbCl3, and indigestible neutral detergent fiber for liquid, small particle, and large particle phases, respectively. Data were analyzed using the MIXED procedure of SAS. Orthogonal contrasts were used to evaluate the effects of nonprotein nitrogen (NPN) inclusion, NPN source, BSS, and NPN source × BSS. There was no treatment effect (P > 0.05) on concentrations of H2S on day 0, 1, 2, or 14; however, on day 3, concentrations of H2S were reduced (P = 0.018) when NPN was provided. No effect of treatment (P = 0.864) occurred for ruminal pH. There was an effect of NPN source on total concentrations of VFA (P = 0.011), where a 6% reduction occurred when eCAN was provided. There were effects of NPN (P = 0.001) and NPN source (P = 0.009) on the concentration of NH3-N, where cattle consuming NPN had a greater concentration than those not consuming NPN, and eCAN reduced the concentration compared with urea. Total concentrations of VFA and NH3-N were not affected (P > 0.05) by BSS. There was an effect of BSS (P = 0.009) on rectal temperature, where cattle not consuming BSS had greater temperatures than those receiving BSS. No differences for NPN, NPN source, nor BSS (P > 0.05) were observed for microbial N flow. In conclusion, eCAN does not appear to deliver equivalent ruminal fermentation parameters compared with urea, and BSS has limited effects on fermentation.
Keywords: beef cattle, bismuth subsalicylate, calcium-ammonium nitrate, ruminal fermentation
Introduction
It is widely accepted that the addition of nitrate to ruminants diets can mitigate enteric CH4 production (van Zijderveld et al., 2010; Newbold et al., 2014; Olijhoek et al., 2016). The reduction in CH4 is due to nitrate acting as an H2 sink in the rumen and having negative effects on methanogenic archaea (Zhou et al., 2012; Duin et al., 2016). Researchers have evaluated the impacts of nitrate supplementation on ruminal fermentation, and ruminal volatile fatty acid (VFA) profiles have been reported to shift when nitrate was provided, often increasing molar proportions of acetate (El-Zaiat et al., 2014; Latham et al., 2016). This shift in fermentation is likely related to the availability of electrons to reduce nitrate to NH4 in the rumen (Ungerfeld and Kohn, 2006).
It has been speculated that nitrate could be substituted for urea as a nonprotein nitrogen (NPN) source while mitigating enteric CH4 production (Leng, 2008). Researchers have evaluated microbial crude protein (CP) synthesis in vivo and in vitro observing differing results (Li et al., 2012, 2013; Guyader et al., 2016); however, there has not been a focus on cattle consuming low-quality, forage-based diets, in which case NPN would be beneficial.
Nitrate has been evaluated extensively in the past in ruminants consuming diets that range from 50% to 90% concentrate (Olijhoek et al., 2016; Lee et al., 2017a, 2017b). The vast majority of this research has focused on mitigating enteric CH4 emissions and evaluating the performance of beef and dairy cattle, and sheep (van Zijderveld et al., 2010, 2011; Newbold et al., 2014). There is a lack of data evaluating the effects of nitrate on forage-fed animals, which produce nearly 3× as much CH4 as grain-fed cattle (Henry et al., 2015). The current experiment utilized bahiagrass hay and molasses, a common wintering diet for beef cattle in the southeast United States.
Cattle consuming forage-based diets rarely have issues with S-induced polioencephalomalacia (Gould, 1998) because the ruminal pH is not optimal for the undissociated sulfide (pKa 7.04); however, other negative effects of S have been reported, such as decreased trace mineral absorption (Arthington et al., 2002; Pogge et al., 2014). Bismuth subsalicylate (BSS) is not a novel compound in human health; yet, very little research has evaluated its effects in ruminants (Ruiz-Moreno et al., 2015). In humans, BSS has been reported to decrease H2S in the gut by binding the sulfide (Suarez et al., 1998) and to have antimicrobial effects (Manhart, 1990; Bland et al., 2004; Yakoob et al., 2013). It has been reported that, when BSS was included up to 1.0% of the substrate DM in in vitro systems, H2S was decreased by up to 61% without having negative effects on fermentation (Ruiz-Moreno et al., 2015).
It was hypothesized that the addition of encapsulated calcium ammonium nitrate (eCAN) to cattle consuming a bahiagrass hay-based diet would shift ruminal fermentation to acetate-producing pathways and have similar microbial CP synthesis compared with urea. Providing BSS was hypothesized to reduce H2S concentration in the ruminal gas cap while not shifting ruminal fermentation. The objective of this experiment was to evaluate the effects of eCAN and BSS, alone and in combination, on ruminal fermentation and microbial protein synthesis.
Materials and Methods
All procedures involving animals were approved by the University of Florida Institutional Animal Care and Use Committee (#201609298).
Experimental design, animals, and treatments
The experiment was conducted at the University of Florida-North Florida Research and Education Center Beef Unit (UF-NFREC BU) in Marianna, FL. Eight ruminally cannulated Angus-crossbred steers (461 ± 148 kg of body weight [BW]; average BW ± SD) and two heifers (337 ± 74 kg of BW) were used in a replicated 5 × 5 Latin square design with a 2 × 2 + 1 factorial arrangement of treatments. Factors included were: NPN source (eCAN vs. urea) and inclusion of BSS (0.0 or 58.4 mg/kg of BW), plus a negative control, where cattle did not receive any NPN source or BSS. In each of the five 28-d periods, days 0 to 13 were for adaptation to the diet and treatments; on days 0, 1, 2, 3, and 14, ruminal gas cap samples were collected; from day 13 to 17, cattle were brought into the UF-NFREC BU Pavilion to collect data for dry matter (DM) intake (DMI), blood and ruminal parameters, and omasal spot samples; on days 21 and 22, ruminal evacuations were performed and the cattle were dosed with Cr-Ethylenediaminetetraacetic acid (EDTA) and YbCL3 for digesta flow rate analysis; and days 23 to 28 were for washout when all the cattle only received bahiagrass hay and sugar cane molasses. At the beginning of the first period, all cattle were randomly assigned to one of five treatments: 1) NCTRL, no added NPN or BSS; 2) U, urea supplemented at 182 mg/kg of BW; 3) UB, urea supplemented at 182 mg/kg of BW and BSS supplemented at 58.4 mg/kg of BW; 4) NIT, nitrate, in the form of eCAN, supplemented at 350 mg/kg of BW; and 5) NITB, nitrate, in the form of eCAN, supplemented at 350 mg/kg of BW and BSS supplemented at 58.4 mg/kg of BW. Treatments U, NIT, UB, and NITB were isonitrogenous. The eCAN used in the current experiment contained 15.5% N and 75% NO3− on the DM basis (GRASP Ind. & Com. LTDA, Curitiba, Paraná, Brazil/EW|Nutrition GmbH, Visbek, Germany).
Prior to initiation of the experiment, dormant bahiagrass pastures to be used in the trial were mob grazed to remove residual dormant forage. Throughout the five periods, the cattle were kept in individual, previously mob grazed, dormant pastures (1 head/pasture; 0.69 ha; days 0 to 13, days 17 to 21, and days 23 to 28) and pens in the UF-NFREC BU Pavillion (1 head/pen; 13.4 m2; days 13 to 17 and days 21 to 22). The cattle had ad libitum access to Pensacola bahiagrass (Paspalum notatum) hay and were supplemented daily with molasses at 2.7 g/kg of BW (DM basis). When on pasture, cattle received full length bahiagrass hay as round bales; however, to be able to provide the same source of hay fed to cattle on dormant pastures, when the cattle were in the Pavilion, they were fed bahiagrass hay that was chopped using a Tub Grinder (Haybuster, Jamestown, ND) and square baled. Round bales of hay were sampled at the beginning of each period for nutrient analysis using a hand drill and core sampler, and square bales were sampled by hand as the hay was fed to the cattle. There was not sufficient residual forage for cattle to graze post-mob grazing; therefore, no nutrient composition of pasture or pasture forage disappearances was measured. The amount of molasses corresponding to each animal was weighed and offered daily using rubber containers inside pastures and pens. The sugar cane molasses was provided by Quality Liquid Feed (Dodgeville, WI). Chemical composition of bahiagrass hay and sugar cane molasses while on pasture and while confined in pens are presented in Table 1. Chemical composition of dietary intakes while confined in the UF-NFREC BU Pavillion are presented in Table 2.
Table 1.
Analyzed1 chemical composition of the basal diet fed to cattle while in the pasture and while confined in the UF-NFREC BU Pavilion
| Item, % DM | Bahiagrass hay on pasture (average ± SD2) | Bahiagrass hay while confined (average ± SD2) | Sugar cane molasses (average ± SD2) |
|---|---|---|---|
| DM, % as fed | 91.6 ± 0.15 | 94.0 ± 0.52 | 79.2 ± 0.71 |
| OM | 91.4 ± 0.11 | 93.9 ± 0.51 | 84.2 ± 0.35 |
| CP | 9.3 ± 0.25 | 9.6 ± 0.26 | 6.8 ± 0.57 |
| NDF | 71.1 ± 0.90 | 71.9 ± 1.87 | — |
| ADF | 38.5 ± 0.72 | 39.1 ± 0.27 | — |
| TDN | 54.0 ± 0.0 | 53.8 ± 0.44 | 76.5 ± 0.71 |
| Sulfur | 0.24 ± 0.01 | 0.31 ± 0.01 | 0.78 ± 0.98 |
| Nitrate | <0.033 | <0.033 | 0.08 ± 0.06 |
1Analyzed by a commercial laboratory using a wet chemistry package (Dairy One, Ithaca, NY).
2Average ± SD calculated from five samples; one composite sample per period.
30.03 represents the lower detection limit of nitrate.
Table 2.
Calculated1 chemical composition of treatment diets fed to cattle determined by intake while confined in the UF-NFREC BU Pavilion
| Treatment2 | |||||
|---|---|---|---|---|---|
| Item, % DM | NCTRL (average ± SD) | U (average ± SD) | NIT (average ± SD) | UB (average ± SD) | NITB (average ± SD) |
| DM, % as fed | 90.9 ± 0.85 | 91.2 ± 1.29 | 91.2 ± 1.63 | 91.0 ± 0.93 | 90.2 ± 1.13 |
| OM | 91.9 ± 0.56 | 90.8 ± 1.41 | 89.6 ± 2.05 | 90.6 ± 1.00 | 88.5 ± 1.53 |
| CP | 9.0 ± 0.16 | 12.8 ± 1.37 | 12.5 ± 1.15 | 12.5 ± 0.99 | 12.7 ± 0.82 |
| NDF | 57.0 ± 4.13 | 56.5 ± 6.54 | 54.7 ± 7.94 | 56.6 ± 5.74 | 52.5 ± 5.73 |
| ADF | 31.0 ± 2.25 | 30.7 ± 3.56 | 29.7 ± 4.32 | 30.8 ± 3.12 | 28.6 ± 3.12 |
| TDN | 58.5 ± 1.30 | 57.7 ± 1.66 | 57.4 ± 1.87 | 57.5 ± 1.86 | 57.3 ± 1.14 |
| Sulfur | 0.41 ± 0.03 | 0.40 ± 0.04 | 0.41 ± 0.05 | 0.40 ± 0.04 | 0.41 ± 0.03 |
| Nitrate | <0.033 | <0.033 | 1.75 ± 0.70 | <0.033 | 1.90 ± 0.51 |
1Calculated using the individual intakes of hay, molasses, and treatments from each animal. Hay and molasses were analyzed by a commercial laboratory using a wet chemistry package (Dairy One, Ithaca, NY). Urea, eCAN, and BSS were analyzed in house.
2NCTRL, treatment 2.7g/kg BW of molasses, NIT, NCTRL plus 538 mg/kg BW of eCAN, NITB, treatment NIT plus 58.4 mg/kg BW of BSS, U, treatment NCTRL plus 182 mg/kg BW of urea, UB, treatment U plus 58.4 mg/kg BW of urea. Average ± SD calculated from 10 animals per treatment.
30.03 represents the lower detection limit of nitrate.
To reduce any negative effects of nitrate on the cattle, cattle began an adaptation to eCAN and urea on day 0. On days 0 and 1, cattle received 20% of their total supplemental N; on days 2 and 3, cattle received 40% of their total supplemental N; on days 4 and 5, cattle received 60% of their total supplemental N; on days 6 and 7, cattle received 80% of their total supplemental N; and beginning on day 8, cattle were receiving 100% of their total supplemental N as urea or eCAN.
Sampling procedures
All protocols and procedures used for collecting samples and data from animals were used in an identical manner throughout all five periods.
Ruminal gas cap sampling
On day 0, ruminal gas cap samples were collected at 0, 3, 6, 9, and 12 h post-feeding of molasses. On days 1, 2, 3, and 14, samples were collected at 0 h, just prior to feeding molasses. To collect the sample, a 10-mL syringe fitted with a one-way valve and 16-gauge needle was inserted through the cannula plug to prevent as much contamination with environmental air as possible. Once the needle was inside the rumen, the plunger of the syringe was used five times to thoroughly mix the gas sample surrounding the needle. Thereafter, 5 mL of gas cap was drawn into the syringe, at which point, the one-way valve was closed to prevent contamination. The sample was then injected into a 10-mL evacuated tube (BD Vacutainer, Franklin Lakes, NJ) containing 5 mL of alkaline water (pH 8.5 to 9). The samples were transported to the laboratory and immediately analyzed for H2S concentration.
Ruminal fluid, pH, blood sampling, and rectal temperature
Ruminal fluid and blood were collected, and rectal temperature and ruminal pH were recorded over a 72-h period. Briefly, on day 14 of each period, samples were collected at 0800, 1400, and 2000 hours; on day 15 at 0200, 1000, 1600, and 2200 hours; on day 16 at 0400, 1200, and 1800 hours; and on day 17 at 0000 and 0600 hours. A representative sample of ruminal digesta was collected and strained through four layers of cheesecloth, and pH was immediately measured using a manual pH meter (Corning Pinnacle M530, Corning Inc., Corning, NY). A 10-mL sample of the ruminal fluid was transferred into a 15-mL conical tube and mixed with 0.1 mL of a 20% (vol/vol) H2SO4 to halt fermentation and preserve NH3-N and VFA. Samples of ruminal fluid were stored at −20°C for further analysis.
Blood samples were collected, at the same time as ruminal samples, via jugular venipuncture into 10-mL evacuated tubes, which contained Na heparin (BD Vacutainer; Franklin Lakes, NJ), placed on ice, and centrifuged at 1,500 × g for 15 min at 4 °C. Plasma was then transferred to polypropylene tubes (12 × 75 mm; Fisherbrand; Thermo Fisher Scientific Inc., Waltham, MA) and stored at −20 °C for further analysis.
Rectal temperature was measured and recorded at the same time points as ruminal and blood sampling.
Hay and total DMI
On day 13 of each period, the cattle were moved from their respective pastures into the UF-NFREC BU Pavilion. From days 13 to 17, hay DMI was measured by weighing bahiagrass hay (as-is) prior to feeding and then weighing orts after 24 h. Samples of hay and orts were collected each day for chemical analysis. The cattle consumed all of the molasses provided to them, within 15 min, while DMI was being measured; therefore, there were no molasses orts to be weighed.
Hay, molasses, and orts were also sampled on days 21 and 22 of each period to determine DM, organic matter (OM), and indigestible neutral detergent fiber (NDF) entering the rumen. These values were used for digesta flow calculations.
Omasal sampling
Omasal spot sampling occurred on days 14 to 17 at the same 12 time points as described in the Ruminal fluid, pH, blood sampling, and rectal temperature subsection. Omasal samples were collected with a device constructed as follows: first, a vacuum pump was connected using a rubber hose and rubber stopper to a 500-mL polypropylene side-arm flask; the first side-arm flask was connected to a second identical flask using a rubber hose. The collection hose, a 1.9-cm (inside diameter) clear rubber hose with fiber reinforcement, was fitted to the top of the second side-arm flask using a rubber stopper; a 50-mL scintillation vial was filled with ball bearings, sealed closed, and attached to the end of the collection hose to act as a weight aiding in the placement of the hose, without hindering flow.
To collect the sample, the omasal orifice was located, and the weight at the end of the collection hose was inserted into the orifice. The hose was then placed further into the omasum to collect strictly omasal samples; furthermore, the first 250-mL sample collected was discarded in case ruminal fluid had entered the collection tube. Samples were collected into the second side-arm flask until the volume reached 250 mL. The 250-mL sample was then transferred into a plastic freezer storage bag and placed on ice until transported to the laboratory. Omasal samples were stored at −20 °C for further analysis.
To separate the fractions of the omasal samples, equal volumes (150 mL) of each individual time point were combined to create one composite representative of 1 animal for 24 h post-feeding per period. The composites were filtered through 1 layer of cheesecloth and the residue left in the cheesecloth was considered the large particle fraction. The filtrate was centrifuged at 1,000 × g for 5 min at 4 °C and the supernatant was considered the liquid fraction, and the pellet was considered the small particle fraction (Ipharraguerre et al., 2007). Both solid fractions were weighed wet and subsamples were taken for DM, OM, indigestible NDF, N, and purine analysis. Volume and weight were recorded of the liquid fraction, and a subsample was collected for DM, OM, N, and purine analysis. Reconstruction of omasal composites, in DM, was calculated from the DM of the differing fractions.
Bacterial pellet for purine reference
On day 15 at 1000, 1600, and 2200 hours, a representative sample of whole rumen content was collected to isolate particle-associated bacteria and liquid-associated bacteria according to Martínez et al. (2009). The digesta was collected and placed into plastic freezer storage bags and placed on ice until transported to the laboratory. Once in the laboratory, samples were strained through four layers of cheesecloth to separate the liquid and solid fractions. The liquid fraction was then centrifuged at 1,000 × g for 5 min at 4 °C. The supernatant was collected and centrifuged at 25,000 × g for 15 min at 4 °C. The supernatant was discarded and the liquid-associated bacterial pellet was stored at −20 °C for further analysis. To isolate the particle-associated bacteria from the ruminal solids, a 50 g (as is) subsample was incubated at 39 °C under constant agitation (60 rpm) for 15 min with 150 mL of a saline solution (0.9% NaCl) which contained 0.1% methylcellulose. After incubation, 25 mL of cold saline-methylcellulose solution (4 °C) was added, and the solids were stored at 4 °C for 24 h. Once removed from the 4 °C environment, the solids were placed in a blender (Waring Products Division, New Hartford, CT) and homogenized for 10 s. The resulting solution was filtered through two layers of nylon cloth (50-μm pore size) and treated in an identical manner to the liquid fraction. The particle-associated bacterial pellet was stored at −20 °C for further analysis.
Ruminal evacuations and digesta flow marker dosing
On day 21, the cattle were brought into the UF-NFREC BU Pavilion at 0900 hours (1 h post-feeding of molasses). The cattle were immediately brought through the chute for ruminal evacuation. Ruminal contents were evacuated manually into large containers so that the weight and volume of the ruminal contents could be recorded. When the dimensions of the ruminal contents were recorded, the digesta was returned to the respective rumen. An aliquot representing 10% of the total digesta was separated and stored on ice until transported to the laboratory. Ruminal aliquots were stored at −20 °C for further analysis.
To determine the ruminal nutrient pool sizes, aliquots were thawed and fractionated into three phases: liquid fraction, large particles, and small particles. Briefly, the whole digesta sample (representing 10% of the total ruminal digesta) was strained through one layer of cheesecloth. The content remaining within the cheesecloth was considered the large particle phase. The filtrate was then centrifuged at 1,000 × g for 5 min at 4 °C. The supernatant was considered the liquid fraction phase and the pellet was considered the small particle phase. All three of the phases were weighed and subsamples were taken and lyophilized for further analysis. Ruminal pool sizes (kg) of DM and OM were determined by multiplying the concentration of each component by the DM mass of each of the three phases.
Chromium-EDTA and YbCl3, external and inert markers, were used to mark the liquid fraction and small particle phases of the digesta, respectively. Once the weight and volume of ruminal contents had been recorded and contents returned to their respective rumen, a 1-liter Cr-EDTA solution and 5 mL of a YbCl3 solution (2.77 g of Cr and 2.2 g Yb; modified from Binnerts et al., 1968) were put into the rumen. The Cr solution was poured into the rumen using a funnel and rubber hose (1 m), while the Yb solution was pipetted into the rumen through the cannula. Ruminal contents were mixed for 3 min to aid in the equilibration of the markers in the rumen. Whole ruminal contents were collected at 0 h and every 3 h post-dosing for 21 h and strained through one layer of cheesecloth to determine a ruminal liquid and small particle dilution rates (Romero et al., 2013). Indigestible NDF was determined in the large and small particle phases (not in the liquid fraction phase; Ahvenjärvi et al., 2000).
Laboratory analyses
All protocols and procedures used for analyzing samples and data from animals were used in an identical manner throughout all five periods.
Hydrogen sulfide concentration
The concentration of H2S in the ruminal gas cap was determined as described by Henry et al. (2015). Briefly, 0.5 mL of N,N dimethyl-p-phenylenediamine sulfate was injected into the tubes containing 5 mL of alkaline (pH = 8.5 to 9.0) water followed by 0.5 mL of a ferric chloride solution. Tubes were shaken vigorously and allowed to rest for 30 min for the reaction to occur (Smith et al., 2010). An aliquot (200 μL) was pipetted into a 96-well, flat bottom plate, and absorbance was read at 665 nm using a plate reader (DU 500; Beckman Coulter Inc., Palo Alto, CA).
VFA profile
A water-based solution using ethyl acetate extraction was used to determine VFA concentrations in the ruminal fluid samples. Samples were centrifuged at 10,000 × g for 15 min at 4 °C; 2 mL of the supernatant was mixed with 0.4 mL (5:1 ratio) of a metaphosphoric:crotonic acid (internal standard) solution and samples were frozen overnight. Samples were then thawed and centrifuged again at 10,000 × g for 15 min at 4 °C. Supernatant was transferred into 12 × 75 mm borosilicate disposable culture tubes (Fisherbrand; Thermo Fisher Scientific Inc., Waltham, MA) and mixed with ethyl acetate to form a 2:1 ethyl acetate:supernatant mixture. Culture tubes were vigorously shaken and then rested for 5 min to allow the separation of the ethyl acetate. A subsample of the ethyl acetate was transferred into small vials prior to analysis. Samples were analyzed, as described by Ciriaco et al. (2016), with a gas chromatograph (Agilent 7820A GC, Agilent Technologies) using a flame ionization detector and a capillary column (CP-WAX 58 FFAP 25 m × 0.53 mm, Varian CP7767; Varian Inc.). Column temperature was maintained at 110 °C, and the injector and detector temperatures were 200 and 220 °C, respectively.
Ammonia-N and blood urea N concentration
The phenol-hypochlorite reaction was used to determine NH3-N concentration as described by Broderick and Kang (1980). Ruminal fluid samples were centrifuged at 10,000 × g for 15 min at 4 °C (Avanti J-E, Beckman Coulter Inc.). Briefly, 1 mL of a phenol reagent was pipetted into 12 × 75 mm borosilicate disposable culture tubes (Fisherbrand; Thermo Fisher Scientific Inc., Waltham, MA). A 20-μL aliquot of the supernatant from the centrifuged ruminal fluid was then transferred to the phenol-containing culture tubes. After vortexing, 0.8 mL of a hypochlorite solution was added to the mixture and vortexed again. The culture tubes were then covered with glass marbles and placed in a water bath at 95 °C for 5 min. The only modification to the original protocol was that absorbance of 200 μL of sample-solution was read in 96-well, flat-bottom plates at 665 nm using a plate reader (DU 500; Beckman Coulter Inc.).
Plasma was analyzed for blood urea N (BUN) using a quantitative colorimetric kit (B7551-120; Pointe Scientific Inc., Canton, MI).
Chemical analyses of hay, orts, ruminal, and omasal digesta fractions
Hay and orts were analyzed for DM and OM on all days of collection (dayS 13 to 17 and days 21 and 22). On days 21 and 22, hay and orts were analyzed for indigestible NDF.
To determine DM of hay and orts, samples were weighed prior to being placed in a 55 °C forced air oven for 72 h. Dry, hot weight was used to calculate the DM of the sample. The sample was then ground to pass through a 2-mm screen in a Wiley mill (Thomas Scientific, Swedesboro, NJ). To determine OM, 0.5 g of ground sample (in duplicate) was weighed into ceramic crucibles and placed in a 105 °C forced air oven for 24 h to determine sample DM. Dried samples were then placed in a 650 °C muffle furnace for 6 h before returning to a 105 °C forced air oven. Hot, ashed samples were weighed and used to calculate OM.
To determine the DM of ruminal and omasal fractions, samples were weighed wet prior to lyophilization. After being lyophilized, samples were placed in a 55 °C oven for 24 h prior to being weighed. To determine OM, the same procedure as described for hay and orts was used.
The concentration of indigestible NDF in the hay, orts, ruminal, and omasal solid fractions was determined as described by Gregorini et al. (2008), Cole et al. (2011), and Krizsan and Huhtanen (2013). Briefly, 0.5 g of sample was weighed into Ankom F57 filter bags (Ankom Technology Corp. Macedon, NY) and then incubated into the rumen of a cannulated steer grazing a bahiagrass and bermudagrass (Cynodon dactylon) mixed pasture for 288 h to ensure complete digestion of potentially digestible NDF. After incubation, samples were rinsed twice with warm tap water followed by four rinses with water filtered through a reverse osmosis system. The rinsed samples were then analyzed for NDF, using heat-stable α-amylase and sodium sulfite as described by Van Soest et al. (1991) using an Ankom 200 Fiber Analyzer (Ankom Technology Corp.).
Ruminal and omasal fractions were analyzed for total N using a Carbon Hydrogen Nitrogen Sulfur analyzer by the Dumas dry combustion method (Vario Micro Cube; Elementar, Hanau, Germany). CP was calculated by multiplying the N concentration of the dry sample by 6.25.
Chromium concentrations
For concentrations of Cr, ruminal fluid samples were centrifuged at 1,000 × g at 4 °C for 5 min. The supernatant was transferred to 15-mL conical tubes and the small particle pellet was lyophilized for further analysis. Atomic absorption spectrophotometry (359.4 nm with an air-plus-acetylene flame; AAnalyst 200; PerkinElmer, Waltham, MA) was used to determine the concentration of Cr in the ruminal liquid fraction after dilution with deionized water.
Ytterbium concentrations
The small particle pellets were digested as described by Linden et al. (2014). Briefly, 0.2 g of the small particle phase was weighed into 20 × 150 mm borosilicate disposable culture tubes (Fisherbrand; Thermo Fisher Scientific Inc.) and dried at 105 °C for 24 h. The dried samples and tubes were then placed in a muffle furnace at 650 °C for 6 h. Ash was solubilized using 10 mL of an acid solution (2 M HNO3 + 3 M HCl) shaking the tubes every 3 h for 12 h. The tubes were allowed to settle for 12 h for the particles to settle. The Yb concentration of the liquid was determined by inductively coupled plasma spectrometry at UF/IFAS Analytical Services Laboratory (Gainsville, FL).
Purine analysis
The purine content of omasal digesta and ruminal bacteria (liquid and particle associated) was determined as described by Zinn and Owens (1986) using Torula Yeast RNA (Sigma-Aldrich, St. Louis, MO) as a standard. Briefly, 0.5 g of omasal sample and 0.2 g of bacterial sample were hydrolyzed by incubating the samples in 25-mL screw cap borosilicate culture tubes (Fisherbrand; Thermo Fisher Scientific Inc.) with 2.5 mL of a 70% perchloric acid in a 95 °C water bath. After 15 min, the samples were vortexed and returned to the water bath for an additional 45 min. A dilute buffer (28.5 mM NH4H2PO4; 17.5 mL) was added to the solution before vortexing. The tubes were then returned to the water bath for 15 min prior to filtration (Whatman #541; GE Healthcare UK Limited, Buckinghamshire, UK). To determine the purine content, 0.5 mL of the filtrate was transferred into 50-mL round-bottom centrifuge tubes followed by 0.5 mL of 0.4 M AgNO3 and 9 mL of a 0.2 M NH4H2PO4 buffer. The samples were then stored at 4 °C for 12 h before centrifugation at 10,000 × g for 10 min at 4 °C. The supernatant was removed and discarded. Without disturbing the pellet, 10 mL of acidic reverse-osmosis filtered water (made pH 2 using H2SO4) was used to wash the pellet. The tubes were centrifuged again at 10,000 × g for 10 min at 4 °C and the supernatant was removed and discarded. An HCl solution (0.5 N; 10 mL) was added and mixed using a Pasteur pipette. The tube was then incubated in a 95 °C water bath for 30 min. The samples were vortexed and centrifuged at 10,000 × g for 10 min at 4 °C. The absorbance of the supernatant was then read at 260 nm using a spectrophotometer (DU-530, Beckman Coulter, Palo Alto, CA).
Calculations
Flow of digesta
Large particle digesta flow was calculated as described by Linneen et al. (2015):
Large particle digesta flow (%/h) = indigestible NDF intake (g/h) ÷ [day 21 ruminal large particle indigestible NDF (kg) + day 22 ruminal large particle indigestible NDF (kg)] ÷ 2.
Small particle flow rate was calculated by regressing the natural logarithm of the Yb concentration against time. The absolute value of the slope was considered a flow rate in percent of volume per hour.
The flow rate of the liquid fraction was calculated by regressing the natural logarithm of the Cr concentration against time (Romero et al., 2013). The absolute value of the slope was considered flow rate in percent of volume per hour.
All fractional rates of passage were then multiplied by the mean ruminal DM mass of their respective fraction to calculate grams per hour and, further, kilograms per 24 h.
Flow and efficiency of microbial N
Microbial N flow to the omasum was calculated using liquid-associated bacteria and particle-associated bacteria as references and total purines as microbial markers as follows:
microbial N flow (g/d) = digesta flow (kg/d) × purines in digesta (μmol/g) × g of N/μmol of purines in bacterial reference.
The true digestibility of OM in the rumen was calculated as described by Gozho et al. (2009):
OM truly digested (% of total OM) = OM intake (kg/d) – {[OM flowing to the omasum (kg/d) –bacterial OM flowing to the omasum (kg/d)] ÷ OMI kg/d} × 100
Microbial N efficiency was calculated as described by Gozho et al. (2009):
Microbial N efficiency = microbial N flowing to the omasum (g/24 h) ÷ OM truly digested (kg/24 h).
Statistical analysis
Data were analyzed as a replicated 5 × 5 Latin square with repeated measures for blood and ruminal fermentation parameters (H2S on day 0, BUN, NH3-N, ruminal pH, and VFA) using the MIXED procedure of SAS (SAS Inst. Inc., Cary, NC). For hay and total DMI, H2S on days 1, 2, and 3, digesta flow, and microbial N efficiency, the model included the fixed effects of treatment, square, period, and animal within the square. For parameters analyzed using repeated measures, the model included the fixed effects of treatment, square, period, time, treatment × time, and animal within the square. The random effect of animal within treatment was used to designate the denominator degrees of freedom. Animal within period was considered the subject and the covariance structure used for all parameters was compound symmetry, which was selected based on the smallest Akaike information criterion value. To aid in the interpretation of data, the following contrasts were used: the effect of NPN = NCTRL vs. the mean of U, NIT, UB, and NITB; the effect of NPN source = the mean of U and UB vs. the mean of NIT and NITB; the effect of BSS = the mean of U and NIT vs. the mean of UB and NITB; and NPN source × BSS = the mean of U and NITB vs. the mean of NIT and UB. The contrasts were chosen to evaluate the treatments as a factorial arrangement along with the ability to discuss the effect that the addition of NPN had on parameters measured. Tukey’s adjustment was used for non-repeated models. Significance was declared at P ≤ 0.05.
Results and Discussion
Ruminal gas cap H2S concentration and rectal temperature data can be found in Table 3. There was no treatment × time interaction (P = 0.788) for ruminal H2S concentration nor was there an effect (P > 0.05) of NPN, NPN source, or BSS on day 0 when gas cap samples were collected at 0, 3, 6, 9, and 12 h post-supplementation. A decrease in H2S concentration was observed on day 3 for animals consuming NPN, regardless of source (P = 0.018). Neither NPN source nor BSS influenced H2S concentration of the ruminal gas cap on day 1, 2, 3, or 14 (P > 0.05). The authors speculate that the decrease of H2S concentration by NPN likely does not have a biological significance and occurred via random chance; however, being that the probability of this effect being random is only 1.8%, future experiments evaluating NPN supplementation should investigate impacts on H2S concentration in the ruminal gas cap. It was hypothesized that nitrate, which reduces CH4 via two methods—acting as an H2 sink (Ungerfeld and Kohn, 2006) and toxic effects on methanogens (Zhou et al., 2012; Duin et al., 2016), could mitigate H2S production in the rumen by acting as an H2 sink. BSS was also expected to reduce concentrations of H2S in the rumen. Data from a series of in vitro experiments (D. D. Henry, unpublished data) indicated that BSS was a strong inhibitor of H2S production. When provided at 0.33% of a bahiagrass hay and molasses substrate (DM basis), BSS reduced production of H2S by 61%. Furthermore, in vitro evaluation of BSS in a high-grain substrate observed a 34% reduction in the production of H2S (Ruiz-Moreno et al., 2015). In the current experiment, concentrations of H2S were analyzed prior to feeding molasses on days 1, 2, 3, and 14. It is possible that the effects of treatment were no longer observable. The fermentation of molasses, where the majority of the dietary S is found, is rapid, and ruminal pH reached the nadir 2 h post-feeding of molasses (Figure 1). Future studies evaluating the effects of nitrate and BSS on H2S production should consider evaluating ruminal gas cap concentrations 2 to 6 h post-feeding.
Table 3.
Effect of BSS and eCAN1 on ruminal gas cap H2S concentration and rectal temperature
| Treatment2 | P-value3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | NCTRL | U | NIT | UB | NITB | SEM | TRT × T | NPN | NS | B | B × N |
| H2S4 day 0, μg/mL | 0.44 | 0.52 | 0.31 | 0.39 | 0.37 | 0.121 | 0.788 | 0.518 | 0.093 | 0.599 | 0.158 |
| H2S5 day 1, μg/mL | 0.06 | 0.07 | 0.03 | 0.07 | 0.05 | 0.021 | — | 0.999 | 0.126 | 0.698 | 0.658 |
| H2S5 day 2, μg/mL | 0.13 | 0.03 | 0.02 | 0.05 | 0.10 | 0.036 | — | 0.056 | 0.678 | 0.211 | 0.392 |
| H2S5 day 3, μg/mL | 0.31 | 0.09 | 0.08 | 0.14 | 0.11 | 0.077 | — | 0.018 | 0.847 | 0.632 | 0.863 |
| H2S5 day 14, μg/mL | 0.06 | 0.07 | 0.07 | 0.07 | 0.08 | 0.012 | — | 0.532 | 0.997 | 0.576 | 0.757 |
| Temperature6, °C | 38.15 | 38.40 | 38.35 | 38.23 | 38.15 | 0.136 | 0.180 | 0.057 | 0.291 | 0.009 | 0.826 |
1eCAN = 5Ca(NO3)2-NH4NO3 – 65.1% nitrate DM basis.
2NCTRL, treatment 2.7g/kg BW of molasses; NIT, NCTRL plus 538 mg/kg BW of eCAN; NITB, treatment NIT plus 58.4 mg/kg BW of BSS; U, treatment NCTRL plus 182 mg/kg BW of urea; UB, treatment U plus 58.4 mg/kg BW of urea. Average ± SD calculated from 10 (NCTRL, U, NIT), 9 (UB) and 8 (NITB) animals per treatment. The largest SEM is provided.
3Observed significance levels for: TRT × T, treatment by time interaction; NPN, effect of NPN, NCTRL vs. the mean of U + NIT + UB + NITB; NS, effect of NPN source (excludes NCTRL); B, effect of BSS (excludes NCTRL); B × N, interaction of BSS and NPN source (excludes NCTRL).
4H2S on day 0 was analyzed in the ruminal gas cap at hours 0, 3, 6, 9, and 12 post-feeding molasses.
5H2S on days 1, 2, 3, and 14 was analyzed in the ruminal gas cap just prior to feeding molasses.
6Rectal temperature recorded at 12 time points over 72 h to represent every 2 h post-feeding for 24 h.
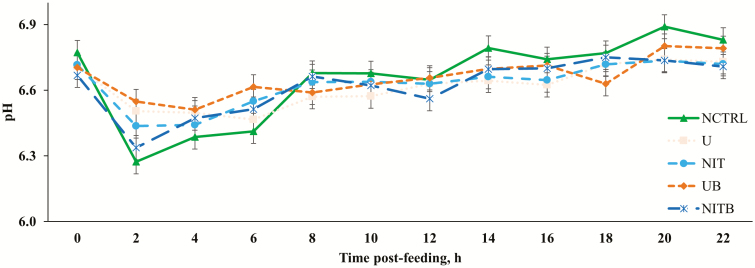
Figure 1.
Effect of BSS and eCAN (65.1% N DM basis) on ruminal pH of cannulated Angus-crossbred cattle fed bahiagrass hay ad libitum with 2.7 g/kg BW of molasses. A treatment × time post-feeding interaction was observed (P = 0.0002). Error bars represent the SEM for treatment × time post-feeding interaction. NCTRL, U, NIT, UB, and NITB had 10, 10, 10, 9, and 8 experimental units, respectively; the largest SEM was provided. * = pH of NCTRL was lesser compared with U, NIT, and UB (P < 0.03); U and UB had greater pH than NITB (P < 0.04). ¤ = NCRTL and U had lesser pH compared with UB (P < 0.05). § = NCTRL had greater pH than U (P < 0.05). ¥ = NCTRL had greater pH compared with NIT (P < 0.04).
There was no treatment × time interaction (P = 0.180) for rectal temperature. Rectal temperature was evaluated in this experiment because previous work in humans has indicated that nitrates lead to vasodilation (Butler and Feelisch, 2008). The authors speculated that if providing nitrate caused vasodilation in cattle, perhaps body temperature might be reduced. That hypothesis was not supported by the data in this experiment, as the addition of eCAN, in place of urea, in the diet of cannulated cattle did not affect (P = 0.291) rectal temperature; however, this experiment was conducted during the fall and winter when temperatures are quite mild in the Florida Panhandle. It is possible that the impacts of vasodilators on rectal temperature of cattle lessened with mild temperatures when the animal is not required to increase heat exchange capacity. Further research should investigate the use of nitrate in cattle under heat stress conditions. Interestingly, a decrease (P = 0.009) in rectal temperature was observed when cattle were provided BSS. A vast majority of research focusing on bismuth compounds (i.e., BSS) has been performed in human and rat models (Suarez et al., 1998; Levitt et al., 2002). To the best of the authors’ knowledge, this experiment represents the first data evaluating the effects of BSS in ruminants (in vivo). Salicylate can act as a nonsteroidal anti-inflammatory drug (NSAID) where, in humans, it can alter body temperature by inhibiting both Cyclooxygenase (COX)-1 and COX-2 enzymes, inhibiting the conversion of arachidonic acid into prostaglandin E2 (Cashman, 1996). Reduction of prostaglandin synthesis ultimately reduces systemic inflammation and, therefore, core body temperature. The efficacy of bismuth NSAID compounds have been reported in humans (Cashman, 1996; Hawksworth et al., 2014). Therefore, it may be possible that BSS had a similar anti-inflammatory effect in cattle.
Data related to DMI, ruminal pH, NH3-N, and BUN concentration are presented in Table 4. There were no effects observed on bahiagrass hay or total DMI (P > 0.05). There was a treatment × time interaction for ruminal pH (P < 0.001; Figure 1), ruminal concentration of NH3-N (P < 0.001; Figure 2), and BUN (P < 0.001; Figure 3). For ruminal pH, NCTRL exhibited a lesser pH compared with U, NIT, and UB at 2 h post-feeding of molasses (P < 0.03). Cattle consuming NCTRL also had lesser ruminal pH at 6 h post-feeding when compared with U and UB (P < 0.04). Likely, NCTRL had a more acidic ruminal environment because of the lack of NPN source to be converted to NH3, which acts as a buffer in the rumen. As time post-feeding progressed, NCTRL had greater ruminal pH when compared with U at 14 h (P < 0.05) and NIT at 20 h (P < 0.04).
Table 4.
Effect of BSS and eCAN1 on DMI and ruminal fermentation and blood parameters
| Treatment2 | P-value3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item4 | NCTRL | U | NIT | UB | NITB | SEM | TRT × T | NPN | NS | B | B × N |
| Hay DMI5, kg/d | 4.91 | 4.94 | 5.13 | 5.35 | 4.59 | 0.459 | — | 0.851 | 0.526 | 0.884 | 0.292 |
| Total DMI5, kg/d | 6.09 | 6.12 | 6.31 | 6.52 | 5.98 | 0.475 | — | 0.767 | 0.708 | 0.932 | 0.429 |
| Ruminal pH | 6.66 | 6.62 | 6.63 | 6.66 | 6.62 | 0.056 | <0.001 | 0.502 | 0.690 | 0.667 | 0.505 |
| NH3-N, mM | 0.84 | 3.34 | 2.08 | 3.49 | 2.48 | 0.565 | <0.001 | <0.001 | 0.001 | 0.375 | 0.673 |
| BUN, mg/dL | 6.41 | 10.49 | 9.73 | 10.33 | 10.16 | 0.984 | <0.001 | <0.001 | 0.463 | 0.836 | 0.640 |
1eCAN = 5Ca(NO3)2-NH4NO3 – 65.1% nitrate DM basis.
2NCTRL, treatment 2.7g/kg BW of molasses; NIT, NCTRL plus 538 mg/kg BW of eCAN; NITB, treatment NIT plus 58.4 mg/kg BW of BSS; U, treatment NCTRL plus 182 mg/kg BW of urea; UB, treatment U plus 58.4 mg/kg BW of urea. Average ± SD calculated from 10 (NCTRL, U, NIT), 9 (UB) and 8 (NITB) animals per treatment. The largest SEM is provided.
3Observed significance levels for: TRT × T, treatment by time interaction; NPN, effect of NPN, NCTRL vs. the mean of U + NIT + UB + NITB; NS, effect of NPN source (excludes NCTRL); B, effect of BSS (excludes NCTRL); B × N, interaction of BSS and NPN source (excludes NCTRL).
4Ruminal fluid and blood samples were collected at 12 time points over 72 h to represent every 2 h post-feeding for 24 h.
5DMI was recorded from days 13 to 17 of each period when cattle were housed in the UF-NFREC BU Pavilion.
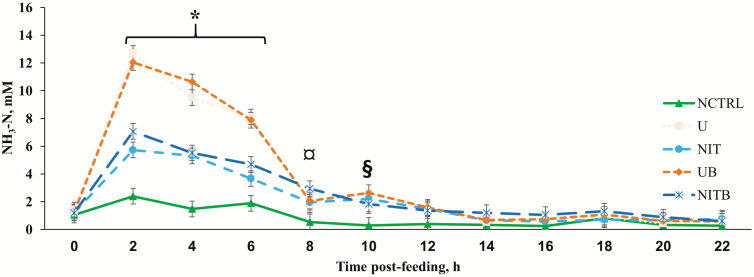
Figure 2.
Effect of BSS and eCAN (65.1% N DM basis) on ruminal NH3-N concentrations of cannulated Angus-crossbred cattle fed bahiagrass hay ad libitum with 2.7 g/kg BW of molasses. A treatment × time post-feeding interaction was observed (P < 0.0001). Error bars represent the SEM for treatment × time post-feeding interaction. NCTRL, U, NIT, UB, and NITB had 10, 10, 10, 9, and 8 experimental units, respectively; the largest SEM was provided. * = U and UB had greater concentrations of NH3-N compared with all other treatments (P < 0.0001); NIT and NITB had greater concentrations of NH3-N than NCTRL (P < 0.02). ¤ = NCTRL had lesser concentration of NH3-N compared with UB and NITB (P < 0.05). § = NCTRL had a lesser concentration of NH3-N compared with all other treatments (P ≤ 0.05).
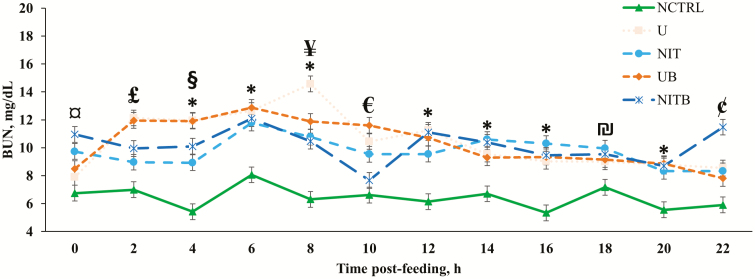
Figure 3.
Effect of BSS and eCAN (65.1% N DM basis) on BUN concentrations of cannulated Angus-crossbred cattle fed bahiagrass hay ad libitum with 2.7 g/kg BW of molasses. A treatment × time post-feeding interaction was observed (P < 0.0001). Error bars represent the SEM for treatment × time post-feeding interaction. NCTRL, U, NIT, UB, and NITB had 10, 10, 10, 9, and 8 experimental units, respectively; largest SEM was provided. ¤ = NCTRL had lesser concentrations of BUN compared with NIT and NITB (P < 0.05); U had lesser concentrations of BUN compared with NITB (P < 0.05). £ = NCTRL had lesser concentrations of BUN compared with U, UB, and NITB (P < 0.05); NIT had lesser concentrations compared with U and UB (P < 0.05). * = NCTRL had lesser concentrations of BUN compared with all other treatments (P < 0.05). § = U and UB had greater concentrations of BUN compared with NIT (P < 0.05). ¥ = U had greater concentrations of BUN compared with NIT, UB, and NITB (P < 0.05). € = NCTRL had lesser BUN concentration compared with U, NIT, and UB (P < 0.05); U and UB had greater concentrations of BUN compared with NITB (P < 0.05). ₪ = NIT had greater BUN concentration compared with NCTRL (P < 0.05). ¢ = NCTRL had lesser concentration of BUN compared with U and NITB (P ≤ 0.05); NITB had greater concentration of BUN compared with U, NIT, and UB (P < 0.05).
Obvious effects of the different NPN sources on ruminal NH3-N concentration can be observed in Figure 2. All treatments had a similar concentration of NH3-N at 0 h post-feeding of molasses, but from 2 to 6 h, U and UB had a greater concentration of NH3-N when compared with all other treatments (P < 0.001), and NIT and NITB were greater than NCTRL (P < 0.02). Eight hours post-feeding, NCTRL still had lesser concentrations of NH3-N when compared with U and UB (P < 0.05), and that difference remained until 10 h post-feeding when NCTRL had lesser concentration compared with all other treatments (P ≤ 0.05). The effect of NPN source on ruminal NH3-N concentration is one that has been reported by other research groups. Lee et al. (2015) provided ruminally cannulated beef heifers with a barley silage-based diet with either 0%, 1%, 2%, or 3% eCAN (DM). All diets were made isonitrogenous with urea. The authors observed a 16% reduction in NH3-N for 3% eCAN when compared with urea and went on to report that heifers provided eCAN had lesser ruminal NH3-N concentration at 3 and 6 h post-feeding. Furthermore, El-Zaiat et al. (2014) reported that ruminal concentration of NH3-N was decreased for Santa Inês lambs consuming eCAN rather than urea in a corn-based diet. In contrast, Veneman et al. (2015), providing dairy cattle urea or CAN, and Nolan et al. (2010), providing Merino wethers oaten chaff with urea or potassium nitrate, both reported no differences between urea and nitrate treatments for concentrations of ruminal NH3-N. Previous experiments (D. D. Henry, unpublished data) also observed reductions in NH3-N when unencapsulated CAN was provided compared with urea in vitro. The reduction in NH3-N concentrations in the current experiment may be due to the encapsulation of the nitrate, which causes a slow release in the rumen; however, Lee et al. (2017c) compared encapsulated urea and encapsulated nitrate and observed that when both NPN sources were encapsulated, NH3-N was still less for encapsulated nitrate compared with encapsulated urea. Another possibility is that nitrate may take longer to reduce to NH3 than urea, but if this was true, the sustained increase of concentrations of NH3-N would likely have been observed after the concentrations in the urea treatments decreased to pre-feeding levels; however, this was not detected.
The source of NPN and BSS did not impact the concentration of BUN (P > 0.05); however, the addition of NPN increased the concentration of BUN (P < 0.001; Figure 3). This was interesting due to the vast differences in ruminal NH3-N concentration between urea and eCAN. Contrary to data from the current experiment, Lee et al. (2017b) reported a reduction in BUN for steers provided a silage-based diet with either eCAN rather than urea as an NPN source. The authors followed these same steers to the finishing phase and observed the same patterns, where steers receiving urea had greater BUN compared with those consuming eCAN (Lee et al., 2017a).
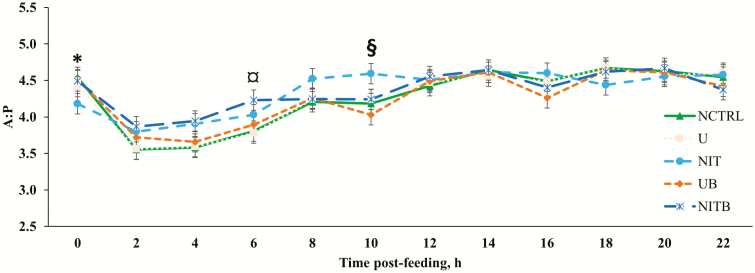
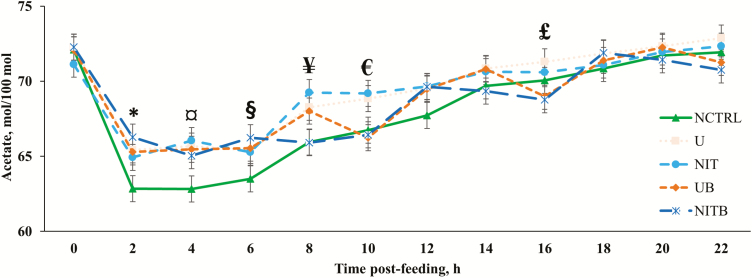
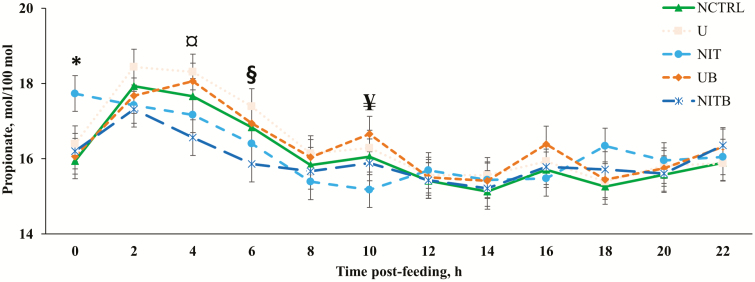
There were treatment × time interactions observed for the acetate to propionate ratio (A:P; P = 0.009; Figure 4) and acetate (P = 0.023; Figure 5) and propionate (P = 0.006; Figure 6) molar proportions. An important aspect of the treatment × time interactions observed is the decrease in acetate and increase in propionate molar proportions for approximately 8 h post-feeding. This is likely related to an increase in the ruminal concentration of H2 that is often found shortly after feeding (Janssen, 2010). Janssen (2010) described that when H2 concentration in the rumen is elevated, H2-utilizing pathways (i.e., propionate production) are increased, while H2-producing pathways (i.e., acetate production) are decreased. There was no effect of BSS on total VFA concentration (P = 0.171) or molar proportions of VFA analyzed (P ≥ 0.118). This is in agreement with data from a previous in vitro experiment (D. D. Henry, unpublished data) when BSS was included in the substrate DM at 0.33%. Ruiz-Moreno et al. (2015) also reported no difference in total VFA concentration when BSS was included at up to 2% of a high-grain substrate (DM basis) in an in vitro batch culture; however, in the same study, the authors observed a large decrease in VFA concentration when BSS was provided at 1% of the substrate DM in a continuous culture experiment. There were no observed effects of NPN (P = 0.359; Table 5) on the ruminal concentration of total VFA; however, the molar proportion of acetate was increased at the expense of butyrate when an NPN source was provided (P = 0.054). The addition of eCAN to the bahiagrass hay-based diet reduced (P = 0.011) the concentration of total VFA compared with urea. This is in agreement with Asanuma et al. (2015), which reported a decrease in total VFA concentration when a nitrate source was provided to goats at 9 g/d. An in vitro experiment utilizing alfalfa hay as the sole substrate had similar results with increasing nitrate concentration decreasing the total VFA concentration (Zhou et al., 2012). It should be noted that neither of these studies had isonitrogenous treatments (i.e., no urea control; Zhou et al., 2012; Asanuma et al., 2015). Several others have compared nitrate with urea and reported either no differences (de Raphélis-Soissan et al., 2014; Lee et al., 2015; Veneman et al., 2015) or increases (Nolan et al., 2010; El-Zaiat et al., 2014; Zhao et al., 2015) in concentrations of total VFA when a nitrate source was provided rather than urea. It is unclear why total VFA was reduced in the current experiment for cattle receiving eCAN compared with urea. In the literature, it has been recommended that the ruminal concentration of NH3-N be at or above 3.57 mM for optimal fermentation (Satter and Slyter, 1974). Although in the current experiment, ruminal NH3-N concentration was greater than this threshold for approximately 8 h post-feeding, the average concentration of NH3-N (Table 4), throughout the day, of all treatments was lesser than what has been recommended in the literature. More specifically, cattle consuming eCAN had lesser concentrations than those consuming urea; therefore, it is probable that total VFA concentration in the rumen was lesser for eCAN compared with urea in relation to a lack of nitrogen for optimal microbial fermentation. Furthermore, unpublished data from our laboratory indicate that eCAN decreases the apparent total tract digestibility of fiber compared with urea, which would likely reduce the total VFA concentration in the rumen, where the vast majority of fiber is to be digested (D. D. Henry, unpublished data). The addition of eCAN, in place of urea, also increased (P = 0.005) the molar proportion of valerate when compared with urea.
Figure 4.
Effect of BSS and eCAN (65.1% N DM basis) on the ruminal A:P of cannulated Angus-crossbred cattle fed bahiagrass hay ad libitum with 2.7 g/kg BW of molasses. A treatment × time post-feeding interaction was observed (P = 0.0087). Error bars represent the SEM for treatment × time post-feeding interaction. NCTRL, U, NIT, UB, and NITB had 10, 10, 10, 9, and 8 experimental units, respectively; the largest SEM was provided. * = NCTRL had a greater A:P compared with NIT (P < 0.05); NIT and NITB had greater concentrations of NH3-N than NCTRL (P < 0.02). ¤ = NCTRL and U had a lesser A:P compared with NITB (P < 0.03). § = NCTRL, U, and UB had a lesser A:P compared with NIT (P ≤ 0.05).
Figure 5.
Effect of BSS and eCAN (65.1% N DM basis) on ruminal acetate molar proportions of cannulated Angus-crossbred cattle fed bahiagrass hay ad libitum with 2.7 g/kg BW of molasses. A treatment × time post-feeding interaction was observed (P = 0.0227). Error bars represent the SEM for treatment × time post-feeding interaction. NCTRL, U, NIT, UB, and NITB had 10, 10, 10, 9, and 8 experimental units, respectively; largest SEM was provided. * = NCTRL had lesser concentration than U, UB, and NITB (P < 0.04). ¤ = NCTRL had lesser concentration compared with U, NIT, and NITB (P < 0.03). § = NCTRL had lesser concentration than NITB (P < 0.03). ¥ = NCTRL and NITB had lesser concentrations compared with U and NIT (P < 0.05). € = NCTRL, UB, and NITB had lesser concentrations than NIT (P < 0,03); UB and NITB had lesser concentrations than U and NIT (P < 0.05). £ = U had greater concentration compared with UB and NITB (P < 0.05).
Figure 6.
Effect of BSS and eCAN (65.1% N DM basis) on ruminal propionate molar proportions of cannulated Angus-crossbred cattle fed bahiagrass hay ad libitum with 2.7 g/kg BW of molasses. A treatment × time post-feeding interaction was observed (P = 0.0059). Error bars represent the SEM for treatment × time post-feeding interaction. NCTRL, U, NIT, UB, and NITB had 10, 10, 10, 9, and 8 experimental units, respectively; largest SEM was provided. * = NIT had greater concentration compared with all other treatments (P < 0.03). ¤ = NITB had less concentration than U and UB (P < 0.03). § = NITB had lesser concentration than U (P = 0.02). ¥ = NIT had lesser concentration compared with UB (P < 0.02).
Table 5.
Effect of BSS and eCAN1 on ruminal VFA molar proportions, total VFA concentration, and ruminal A:P
| Treatment2 | P-value3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item4 | NCTRL | U | NIT | UB | NITB | SEM | TRT × T | NPN | NS | B | B × N |
| VFA, mol/100 mol | |||||||||||
| Acetate | 67.99 | 69.55 | 69.34 | 68.90 | 68.66 | 0.868 | 0.023 | 0.054 | 0.670 | 0.220 | 0.979 |
| Propionate | 16.10 | 16.43 | 16.19 | 16.36 | 15.97 | 0.473 | 0.006 | 0.710 | 0.339 | 0.660 | 0.818 |
| Butyrate | 14.32 | 12.51 | 12.61 | 13.32 | 13.59 | 0.823 | 0.293 | 0.032 | 0.735 | 0.118 | 0.881 |
| BCVFA5 | 0.44 | 0.40 | 0.33 | 0.43 | 0.38 | 0.053 | 0.217 | 0.071 | 0.063 | 0.155 | 0.798 |
| Valerate | 1.12 | 1.10 | 1.50 | 0.97 | 1.35 | 0.181 | 0.086 | 0.446 | 0.005 | 0.297 | 0.981 |
| Total VFA, mM | 55.60 | 59.49 | 56.30 | 58.05 | 53.94 | 2.155 | 0.669 | 0.359 | 0.011 | 0.171 | 0.734 |
| A:P | 4.27 | 4.27 | 4.34 | 4.26 | 4.36 | 0.139 | 0.009 | 0.715 | 0.309 | 0.957 | 0.981 |
1eCAN = 5Ca(NO3)2-NH4NO3 – 65.1% nitrate DM basis.
2NCTRL, treatment 2.7g/kg BW of molasses; NIT, NCTRL plus 538 mg/kg BW of eCAN; NITB, treatment NIT plus 58.4 mg/kg BW of BSS; U, treatment NCTRL plus 182 mg/kg BW of urea; UB, treatment U plus 58.4 mg/kg BW of urea. Average ± SD calculated from 10 (NCTRL, U, NIT), 9 (UB) and 8 (NITB) animals per treatment. The largest SEM is provided.
3Observed significance levels for: TRT×T, treatment by time interaction; NPN, effect of NPN, NCTRL vs. the mean of U + NIT + UB + NITB; NS, effect of NPN source (excludes NCTRL); B, effect of BSS (excludes NCTRL); B×N, interaction of BSS and NPN source (excludes NCTRL).
4Ruminal fluid samples collected at 12 time points over 72 h to represent every 2 h post-feeding for 24 h.
5BCVFA, branched-chain VFA: isobutyrate + isovalerate + 2 methylbutyrate.
Effects of BSS and NPN source on digesta and microbial N flow are presented in Table 6. Similar to days 13 to 17, DMI and organic matter intake (OMI) were not affected (P > 0.05) by the addition NPN, NPN source, or BSS. Furthermore, flow of DM and OM to the omasum was also not impacted (P > 0.05). Nitric oxide, an intermediary of nitrate reduction to NH4, has been reported to cause relaxation of smooth muscle tissue in humans (Butler and Feelisch, 2008). Prior to the commencement of the current experiment, it was speculated that eCAN could reduce passage rate in ruminants by causing the smooth muscle in the rumen to relax, leading to less motility. The data do not support this hypothesis. Although there are very little data regarding the use of bismuth in ruminants, scientists have exhaustively examined its effects in human and mice models. In mice, bismuth ions have been reported to bind to gastrin compounds, inhibiting the effects of gastrin (Pannequin et al., 2004). Gastrin is a peptide hormone that is involved with the secretion of gastric juices and gut motility in monogastrics and also ruminants (Ozturk et al., 2013). There were numerical reductions in the flow to the omasum when BSS was provided; however, no significant differences were observed in the current experiment. OM truly digested in the rumen (OMTDR) was not affected by the addition of NPN, NPN source, or BSS (P > 0.05).
Table 6.
Effect of BSS and eCAN1 on intake, flow at the omasal canal, and microbial N flow and efficiency
| Treatment2 | P-value3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | NCTRL | U | NIT | UB | NITB | SEM | NPN | NS | B | B × N |
| DM | ||||||||||
| Intake, kg/d | 5.99 | 6.19 | 5.92 | 5.47 | 5.17 | 0.495 | 0.554 | 0.553 | 0.134 | 0.969 |
| Flow, kg/d | 5.64 | 5.91 | 5.48 | 5.19 | 4.86 | 0.714 | 0.666 | 0.536 | 0.295 | 0.937 |
| OM | ||||||||||
| Intake, kg/d | 5.58 | 5.77 | 5.52 | 5.09 | 4.80 | 0.473 | 0.556 | 0.551 | 0.134 | 0.969 |
| Flow, kg/d | 4.56 | 4.88 | 4.39 | 4.23 | 3.97 | 0.658 | 0.736 | 0.513 | 0.365 | 0.841 |
| OMTDR, kg/d | 1.28 | 1.17 | 1.67 | 1.14 | 0.61 | 0.364 | 0.685 | 0.965 | 0.099 | 0.116 |
| Microbial N flow, g/d | 20.41 | 22.43 | 20.19 | 23.85 | 22.71 | 3.227 | 0.514 | 0.545 | 0.493 | 0.845 |
| Microbial efficiency, g of MN4/ kg of OMTDR | 14.92 | 31.71 | 20.84 | 18.87 | 11.95 | 11.524 | 0.550 | 0.348 | 0.259 | 0.840 |
1eCAN = 5Ca(NO3)2-NH4NO3 – 65.1% nitrate DM basis.
2NCTRL, treatment 2.7g/kg BW of molasses; NIT, NCTRL plus 538 mg/kg BW of eCAN; NITB, treatment NIT plus 58.4 mg/kg BW of BSS; U, treatment NCTRL plus 182 mg/kg BW of urea; UB, treatment U plus 58.4 mg/kg BW of urea. Average ± SD calculated from 10 (NCTRL, U, NIT), 9 (UB) and 8 (NITB) animals per treatment. The largest SEM is provided.
3Observed significance levels for: TRT × T, treatment by time interaction; NPN, effect of NPN, NCTRL vs. the mean of U + NIT + UB + NITB; NS, effect of NPN source (excludes NCTRL); B, effect of BSS (excludes NCTRL); B × N, interaction of BSS and NPN source (excludes NCTRL).
4MN, microbial N.
Microbial N flow to the omasum was not impacted by the addition of NPN, NPN source, or BSS (P > 0.05). This is in agreement with Li et al. (2013) who provided Merino lambs with urea or CAN. The authors reported that when sheep were provided a barley-based diet, no differences in microbial N flow were observed. In fact, several research groups have reported that microbial N flows are similar for cattle and sheep consuming urea or nitrate as NPN sources (Nolan et al., 2010; Li et al., 2012; Olijhoek et al., 2016). It was unexpected that the addition of NPN did not increase microbial N flow out of the rumen. Researchers have reported that when CAN was provided to sheep consuming oaten chaff (4.1% CP), microbial N flow was increased by more than 160% (Nguyen et al., 2016). It is unclear why no changes in microbial N were observed in the current experiment. It has been reported that for maximal microbial growth, the ruminal concentration of NH3-N should be a minimum of 3.57 mM (Satter and Slyter, 1974). Although none of the treatments achieved this concentration of ruminal NH3-N when averaged over 24 h post-feeding (Table 4), NH3-N concentration was greater than this threshold for cattle receiving NPN for approximately 8 h post-feeding, whereas NCTRL never reached 3.57 mM (Figure 2). Therefore, it was expected that the animals consuming NPN would increase microbial protein synthesis compared with those subjected to NCTRL. The efficiency of microbial N (g/kg of OMTDR) was unchanged (P > 0.05) by treatment.
In conclusion, BSS did not have any effects on ruminal fermentation; however, rectal temperature was decreased with the addition of BSS. Further research should evaluate BSS on ruminal fermentation of cattle consuming high-sulfur, concentrate-based diets. The addition of eCAN had negative effects on total VFA concentration in the rumen of cattle consuming bahiagrass hay and molasses. The data indicate that eCAN may not be a viable NPN source for cattle consuming poor-quality hay-based diets. Future research should inquire about the differences in the effect of nitrate on differing sources and conservation methods of forages.
Acknowledgments
This material is based upon work that is supported by the Foundational Program (Grant no. 2016-08402) and the Agriculture and Food Research Initiative—Food, Natural Resources and Human Sciences Education and Literacy Initiative (Award no. 2017-67011-26063) from the United States Department of Agriculture National Institute of Food and Agriculture. We wish to thank Quality Liquid Feeds for donating the molasses used in this experiment, Westway Feed Products for donating the urea used in this experiment, and GRASP Ind. & Com. LTDA for donating the encapsulated calcium ammonium nitrate. Furthermore, we also wish to acknowledge D. Thomas, D. Wood, M. Foran, D. Jones, and O. Helms for their assistance in collecting the data.
Glossary
Abbreviations
- A:P
acetate to propionate ratio
- ADF
acid detergent fiber
- BSS
bismuth subsalicylate
- BUN
blood urea nitrogen
- BW
body weight
- CP
crude protein
- DM
dry matter
- DMI
DM intake
- eCAN
encapsulated calcium ammonium nitrate
- NDF
neutral detergent fiber
- NPN
non-protein nitrogen
- NSAID
nonsteroidal anti-inflammatory drug
- OM
organic matter
- OMTDR
OM truly digested in the rumen
- TDN
Total digestible nutrients;
- VFA
volatile fatty acid
Conflict of interest statement
R.C.A. reports that he is the R&D manager for GRASP Ind. & Com. LTDA, the company which donated calcium ammonium nitrate. The other authors report no conflicts of interest.
Literature Cited
- Ahvenjärvi, S, Vanhatalo A, Huhtanen P, and Varvikko T. . 2000. Determination of reticulo-rumen and whole-stomach digestion in lactating cows by omasal canal or duodenal sampling. Br. J. Nutr. 83:67–77. doi: 10.1017/S0007114500000106 [DOI] [PubMed] [Google Scholar]
- Arthington, J D, Rechcigl J E, Yost G P, McDowell L R, and Fanning M D. . 2002. Effect of ammonium sulfate fertilization on bahiagrass quality and copper metabolism in grazing beef cattle. J. Anim. Sci. 80:2507–2512. doi: 10.2527/2002.80102507x [DOI] [PubMed] [Google Scholar]
- Asanuma, N, Yokoyama S, and Hino T. . 2015. Effects of nitrate addition to a diet on fermentation and microbial populations in the rumen of goats, with special reference to Selenomonas ruminantium having the ability to reduce nitrate and nitrite. Anim. Sci. J. 86:378–384. doi: 10.1111/asj.12307 [DOI] [PubMed] [Google Scholar]
- Binnerts, W T, Van′t Klooster A T, and Frens A M. . 1968. Soluble chromium indicator measured by atomic absorption in digestion experiments. Vet. Rec. 82:470. [Google Scholar]
- Bland, M V, Ismail S, Heinemann J A, and Keenan J I. . 2004. The action of bismuth against Helicobacter pylori mimics but is not caused by intracellular iron deprivation. Antimicrob. Agents Chemother. 48:1983–1988. doi: 10.1128/AAC.48.6.1983-1988.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick, G A, and Kang J H. . 1980. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 63:64–75. doi: 10.3168/jds.S0022-0302(80)82888-8 [DOI] [PubMed] [Google Scholar]
- Butler, A R, and Feelisch M. . 2008. Therapeutic uses of inorganic nitrite and nitrate: from the past to the future. Circulation 117:2151–2159. doi: 10.1161/CIRCULATIONAHA.107.753814 [DOI] [PubMed] [Google Scholar]
- Cashman, J N. 1996. The mechanisms of action of NSAIDs in analgesia. Drugs 52 (Suppl5):13–23. doi: 10.2165/00003495-199600525-00004 [DOI] [PubMed] [Google Scholar]
- Ciriaco, F M, Henry D D, Mercadante V R, Schulmeister T M, Ruiz-Moreno M, Lamb G C, and DiLorenzo N. . 2016. Effects of molasses and crude glycerol combined in a liquid supplement on ruminal fermentation in beef steers consuming bermudagrass hay. J. Anim. Sci. 94:3851–3863. doi: 10.2527/jas.2016-0491 [DOI] [PubMed] [Google Scholar]
- Cole, N A, McCuistion K, Greene L W, and McCollum F T. . 2011. Effects of concentration and source of wet distillers grains on digestibility of steam-flaked corn-based diets fed to finishing steers1. Prof. Anim. Sci. 27:302–311. doi: 10.15232/S1080-7446(15)30493-9 [DOI] [Google Scholar]
- Duin, E C, Wagner T, Shima S, Prakash D, Cronin B, Yáñez-Ruiz D R, Duval S, Rümbeli R, Stemmler R T, Thauer R K, . et al. 2016. Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3-nitrooxypropanol. Proc. Natl. Acad. Sci. U. S. A. 113:6172–6177. doi: 10.1073/pnas.1600298113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Zaiat, H M, Araujo R C, Soltan Y A, Morsy A S, Louvandini H, Pires A V, Patino H O, Correa P S, and Abdalla A L. . 2014. Encapsulated nitrate and cashew nut shell liquid on blood and rumen constituents, methane emission, and growth performance of lambs. J. Anim. Sci. 92:2214–2224. doi: 10.2527/jas.2013-7084 [DOI] [PubMed] [Google Scholar]
- Gould, D H. 1998. Polioencephalomalacia. J. Anim. Sci. 76:309–314. doi: 10.2527/1998.761309x [DOI] [PubMed] [Google Scholar]
- Gozho, G N, McKinnon J J, Christensen D A, Racz V, and Mutsvangwa T. . 2009. Effects of type of canola protein supplement on ruminal fermentation and nutrient flow to the duodenum in beef heifers. J. Anim. Sci. 87:3363–3371. doi: 10.2527/jas.2009-1841 [DOI] [PubMed] [Google Scholar]
- Gregorini, P, Gunter S A, and Beck P A. . 2008. Matching plant and animal processes to alter nutrient supply in strip-grazed cattle: timing of herbage and fasting allocation. J. Anim. Sci. 86:1006–1020. doi: 10.2527/jas.2007-0432 [DOI] [PubMed] [Google Scholar]
- Guyader, J, Tavendale M, Martin C, and Muetzel S. . 2016. Dose-response effect of nitrate on hydrogen distribution between rumen fermentation end products: an in vitro approach. Anim. Prod. Sci. 56:224–230. doi: 10.1071/AN15526 [DOI] [Google Scholar]
- Hawksworth, E L, Andrews P C, Lie W, Lai B, and Dillon C T. . 2014. Biological evaluation of bismuth non-steroidal anti-inflammatory drugs (BiNSAIDs): stability, toxicity and uptake in HCT-8 colon cancer cells. J. Inorg. Biochem. 135:28–39. doi: 10.1016/j.jinorgbio.2014.02.012 [DOI] [PubMed] [Google Scholar]
- Henry, D D, Ruiz-Moreno M, Ciriaco F M, Kohmann M, Mercadante V R, Lamb G C, and DiLorenzo N. . 2015. Effects of chitosan on nutrient digestibility, methane emissions, and in vitro fermentation in beef cattle. J. Anim. Sci. 93:3539–3550. doi: 10.2527/jas.2014-8844 [DOI] [PubMed] [Google Scholar]
- Ipharraguerre, I R, Reynal S M, Liñeiro M, Broderick G A, and Clark J H. . 2007. A comparison of sampling sites, digesta and microbial markers, and microbial references for assessing the postruminal supply of nutrients in dairy cows. J. Dairy Sci. 90:1904–1919. doi: 10.3168/jds.2006-159 [DOI] [PubMed] [Google Scholar]
- Janssen, P H. 2010. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed Sci. Technol. 160:1–22. doi: 10.1016/j.anifeedsci.2010.07.002 [DOI] [Google Scholar]
- Krizsan, S J, and Huhtanen P. . 2013. Effect of diet composition and incubation time on feed indigestible neutral detergent fiber concentration in dairy cows. J. Dairy Sci. 96:1715–1726. doi: 10.3168/jds.2012-5752 [DOI] [PubMed] [Google Scholar]
- Latham, E A, Anderson R C, Pinchak W E, and Nisbet D J. . 2016. Insights on alterations to the rumen ecosystem by nitrate and nitrocompounds. Front. Microbiol. 7:228. doi: 10.3389/fmicb.2016.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C, Araujo R C, Koenig K M, and Beauchemin K A. . 2015. Effects of encapsulated nitrate on eating behavior, rumen fermentation, and blood profile of beef heifers fed restrictively or ad libitum. J. Anim. Sci. 93:2405–2418. doi: 10.2527/jas2014-8851 [DOI] [PubMed] [Google Scholar]
- Lee, C, Araujo R C, Koenig K M, and Beauchemin K A. . 2017a. Effects of encapsulated nitrate on growth performance, carcass characteristics, nitrate residues in tissues, and enteric methane emissions in beef steers: finishing phase. J. Anim. Sci. 95:3712–3726. doi: 10.2527/jas.2017.1461 [DOI] [PubMed] [Google Scholar]
- Lee, C, Araujo R C, Koenig K M, and Beauchemin K A. . 2017b. Effects of encapsulated nitrate on growth performance, nitrate toxicity, and enteric methane emissions in beef steers: backgrounding phase. J. Anim. Sci. 95:3700–3711. doi: 10.2527/jas2014-8845 [DOI] [PubMed] [Google Scholar]
- Lee, C, Araujo R C, Koenig K M, and Beauchemin K A. . 2017c. In situ and in vitro evaluations of a slow release form of nitrate for ruminants: nitrate release rate, rumen nitrate metabolism and the production of methane, hydrogen, and nitrous oxide. Anim. Feed Sci. Technol. 231:97–106. doi: 10.1016/j.anifeedsci.2017.07.005 [DOI] [Google Scholar]
- Leng, R A. 2008. The potential of feeding nitrate to reduce enteric methane production in ruminants. A Report to Department of Climate Change Commonwealth Government of Australia. Canberra [accessed August 4, 2013]. Available from: http://www.penambulbooks.com
- Levitt, M D, Springfield J, Furne J, Koenig T, and Suarez F L. . 2002. Physiology of sulfide in the rat colon: use of bismuth to assess colonic sulfide production. J. Appl. Physiol. (1985). 92:1655–1660. doi: 10.1152/japplphysiol.00907.2001 [DOI] [PubMed] [Google Scholar]
- Li, L, Davis J, Nolan J, and Hegarty R. . 2012. An initial investigation on rumen fermentation pattern and methane emission of sheep offered diets containing urea or nitrate as the nitrogen source. Anim. Prod. Sci. 52:653–658. doi: 10.1071/AN11254 [DOI] [Google Scholar]
- Li, L, Silveira C I, Nolan J V, Godwin I R, Leng R A, and Hegarty R S. . 2013. Effect of added dietary nitrate and elemental sulfur on wool growth and methane emission of Merino lambs. Anim. Prod. Sci. 53:1195–1201. doi: 10.1071/AN13222 [DOI] [Google Scholar]
- Linden, D R, Titgemeyer E C, Olson K C, and Anderson D E. . 2014. Effects of gestation and lactation on forage intake, digestion, and passage rates of primiparous beef heifers and multiparous beef cows. J. Anim. Sci. 92:2141–2151. doi: 10.2527/jas.2013-6813 [DOI] [PubMed] [Google Scholar]
- Linneen, S K, Harding A R, Smallwood M T, Horn G W, Jennings J S, Goad C L, and Lalman D L. . 2015. In vivo ruminal degradation characteristics and apparent digestibility of low-quality prairie hay for steers consuming monensin and optimase. J. Anim. Sci. 93:3941–3949. doi: 10.2527/jas.2014-8772 [DOI] [PubMed] [Google Scholar]
- Manhart, M D. 1990. In vitro antimicrobial activity of bismuth subsalicylate and other bismuth salts. Rev. Infect. Dis. 12 (Suppl 1):S11–S15. doi: 10.1093/clinids/12.supplement_1.s11 [DOI] [PubMed] [Google Scholar]
- Martínez, M E, Ranilla M J, Ramos S, Tejido M L, Saro C, and Carro M D. . 2009. Evaluation of procedures for detaching particle-associated microbes from forage and concentrate incubated in Rusitec fermenters: efficiency of recovery and representativeness of microbial isolates. J. Anim. Sci. 87:2064–2072. doi: 10.2527/jas.2008-1634 [DOI] [PubMed] [Google Scholar]
- Newbold, J R, van Zijderveld S M, Hulshof R B, Fokkink W B, Leng R A, Terencio P, Powers W J, van Adrichem P S, Paton N D, and Perdok H B. . 2014. The effect of incremental levels of dietary nitrate on methane emissions in Holstein steers and performance in Nelore bulls. J. Anim. Sci. 92:5032–5040. doi: 10.2527/jas.2014-7677 [DOI] [PubMed] [Google Scholar]
- Nguyen, S H, Barnett M C, and Hegarty R S. . 2016. Use of dietary nitrate to increase productivity and reduce methane production of defaunated and faunated lambs consuming protein-deficient chaff. Anim. Prod. Sci. 56:290–297. doi: 10.1071/AN15525 [DOI] [Google Scholar]
- Nolan, J V, Hegarty R S, Hegarty J, Godwin I R, and Woodgate R. . 2010. Effects of dietary nitrate on fermentation, methane production and digesta kinetics in sheep. Anim. Prod. Sci. 50:801–806. doi: 10.1071/AN09211 [DOI] [Google Scholar]
- Olijhoek, D W, Hellwing A L F, Brask M, Weisbjerg M R, Højberg O, Larsen M K, Dijkstra J, Erlandsen E J, and Lund P. . 2016. Effect of dietary nitrate level on enteric methane production, hydrogen emission, rumen fermentation, and nutrient digestibility in dairy cows. J. Dairy Sci. 99:6191–6205. doi: 10.3168/jds.2015-10691 [DOI] [PubMed] [Google Scholar]
- Ozturk, A S, Guzel M, Askar T K, and Aytekin I. . 2013. Evaluation of the hormones responsible for the gastrointestinal motility in cattle with displacement of the abomasum; ghrelin, motilin and gastrin. Vet. Rec. 172:636. doi: 10.1136/vr.101322 [DOI] [PubMed] [Google Scholar]
- Pannequin, J, Kovac S, Tantiongco J P, Norton R S, Shulkes A, Barnham K J, and Baldwin G S. . 2004. A novel effect of bismuth ions: selective inhibition of the biological activity of glycine-extended gastrin. J. Biol. Chem. 279:2453–2460. doi: 10.1074/jbc.M309806200 [DOI] [PubMed] [Google Scholar]
- Pogge, D J, Drewnoski M E, and Hansen S L. . 2014. High dietary sulfur decreases the retention of copper, manganese, and zinc in steers. J. Anim. Sci. 92:2182–2191. doi: 10.2527/jas2013-7481 [DOI] [PubMed] [Google Scholar]
- de Raphélis-Soissan, V, Li L, Godwin I R, Barnett M C, Perdok H B, and Hegarty R S. . 2014. Use of nitrate and Propionibacterium acidipropionici to reduce methane emissions and increase wool growth of Merino sheep. Anim. Prod. Sci. 54:1860–1866. doi: 10.1071/AN14329 [DOI] [Google Scholar]
- Romero, J J, Zarate M A, Queiroz O C, Han J H, Shin J H, Staples C R, Brown W F, and Adesogan A T. . 2013. Fibrolytic enzyme and ammonia application effects on the nutritive value, intake, and digestion kinetics of bermudagrass hay in beef cattle. J. Anim. Sci. 91:4345–4356. doi: 10.2527/jas.2013-6261 [DOI] [PubMed] [Google Scholar]
- Ruiz-Moreno, M, Binversie E, Fessenden S W, and Stern M D. . 2015. Mitigation of in vitro hydrogen sulfide production using bismuth subsalicylate with and without monensin in beef feedlot diets. J. Anim. Sci. 93:5346–5354. doi: 10.2527/jas.2015-9392 [DOI] [PubMed] [Google Scholar]
- Satter, L D, and Slyter L L. . 1974. Effect of ammonia concentration of rumen microbial protein production in vitro. Br. J. Nutr. 32:199–208. doi: 10.1079/bjn19740073 [DOI] [PubMed] [Google Scholar]
- Smith, D R, Dilorenzo N, Leibovich J, May M L, Quinn M J, Homm J W, and Galyean M L. . 2010. Effects of sulfur and monensin concentrations on in vitro dry matter disappearance, hydrogen sulfide production, and volatile fatty acid concentrations in batch culture ruminal fermentations. J. Anim. Sci. 88:1503–1512. doi: 10.2527/jas.2009-2498 [DOI] [PubMed] [Google Scholar]
- Suarez, F L, Furne J K, Springfield J, and Levitt M D. . 1998. Bismuth subsalicylate markedly decreases hydrogen sulfide release in the human colon. Gastroenterology 114:923–929. doi: 10.1016/s0016-5085(98)70311-7 [DOI] [PubMed] [Google Scholar]
- Ungerfeld, E M, and Kohn R A. . 2006. The role of thermodynamics in the control of ruminal fermentation. In: Sejrsen, K, Hvelplund T, and Nielsen M O, editors. Ruminant physiology: digestion, metabolism and impact of nutrition on gene expression, immunology and stress. Wageningen (Netherlands):Wageningen Academic Publishers; p. 55–85. [Google Scholar]
- Van Soest, P J, Robertson J B, and Lewis B A. . 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Veneman, J B, Muetzel S, Hart K J, Faulkner C L, Moorby J M, Perdok H B, and Newbold C J. . 2015. Does dietary mitigation of enteric methane production affect rumen function and animal productivity in dairy cows? PLoS One. 10:e0140282. doi: 10.1371/journal.pone.0140282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakoob, J, Abbas Z, Usman M W, Awan S, Naz S, Jafri F, Hamid S, and Jafri W. . 2013. Comparison of antimicrobial activity of zinc chloride and bismuth subsalicylate against clinical isolates of Helicobacter pylori. Microb. Drug Resist. 20:305–309. doi: 10.1089/mdr.2013.0086 [DOI] [PubMed] [Google Scholar]
- Zhao, L, Meng Q, Ren L, Liu W, Zhang X, Huo Y, and Zhou Z. . 2015. Effects of nitrate addition on rumen fermentation, bacterial biodiversity and abundance. Asian-Australas. J. Anim. Sci. 28:1433–1441. doi: 10.5713/ajas.15.0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Z, Yu Z, and Meng Q. . 2012. Effects of nitrate on methane production, fermentation, and microbial populations in in vitro ruminal cultures. Bioresour. Technol. 103:173–179. doi: 10.1016/j.biortech.2011.10.013 [DOI] [PubMed] [Google Scholar]
- van Zijderveld, S M, Gerrits W J, Apajalahti J A, Newbold J R, Dijkstra J, Leng R A, and Perdok H B. . 2010. Nitrate and sulfate: effective alternative hydrogen sinks for mitigation of ruminal methane production in sheep. J. Dairy Sci. 93:5856–5866. doi: 10.3168/jds.2010-3281 [DOI] [PubMed] [Google Scholar]
- van Zijderveld, S M, Gerrits W J, Dijkstra J, Newbold J R, Hulshof R B, and Perdok H B. . 2011. Persistency of methane mitigation by dietary nitrate supplementation in dairy cows. J. Dairy Sci. 94:4028–4038. doi: 10.3168/jds.2011-4236 [DOI] [PubMed] [Google Scholar]
- Zinn, R A, and Owens F N. . 1986. A rapid procedure for purine measurement and its use for estimating net ruminal protein synthesis. Can. J. Anim. Sci. 66:157–166. doi: 10.4141/cjas86-017 [DOI] [Google Scholar]