Abstract
Background
Targeted muscle reinnervation is an emerging surgical technique to treat neuroma pain whereby sensory and mixed motor nerves are transferred to nearby redundant motor nerve branches. In a recent randomized controlled trial, targeted muscle reinnervation was recently shown to reduce postamputation pain relative to conventional neuroma excision and muscle burying.
Questions/purposes
(1) Does targeted muscle reinnervation improve residual limb pain and phantom limb pain in the period before surgery to 1 year after surgery? (2) Does targeted muscle reinnervation improve Patient-reported Outcome Measurement System (PROMIS) pain intensity and pain interference scores at 1 year after surgery? (3) After 1 year, does targeted muscle reinnervation improve functional outcome scores (Orthotics Prosthetics User Survey [OPUS] with Rasch conversion and Neuro-Quality of Life [Neuro-QOL])?
Methods
Data on patients who were ineligible for randomization or declined to be randomized and underwent targeted muscle reinnervation for pain were gathered for the present analysis. Data were collected prospectively from 2013 to 2017. Forty-three patients were enrolled in the study, 10 of whom lacked 1-year follow-up, leaving 33 patients for analysis. The primary outcomes measured were the difference in residual limb and phantom limb pain before and 1 year after surgery, assessed by an 11-point numerical rating scale (NRS). Secondary outcomes were change in PROMIS pain measures and change in limb function, assessed by the OPUS Rasch for upper limbs and Neuro-QOL for lower limbs before and 1 year after surgery.
Results
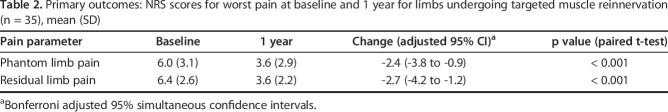
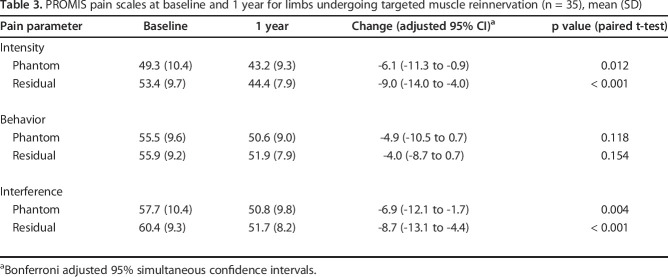
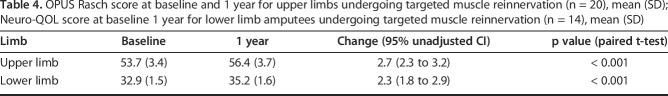
By 1 year after targeted muscle reinnervation, NRS scores for residual limb pain from 6.4 ± 2.6 to 3.6 ± 2.2 (mean difference -2.7 [95% CI -4.2 to -1.3]; p < 0.001) and phantom limb pain decreased from 6.0 ± 3.1 to 3.6 ± 2.9 (mean difference -2.4 [95% CI -3.8 to -0.9]; p < 0.001). PROMIS pain intensity and pain interference scores improved with respect to residual limb and phantom limb pain (residual limb pain intensity: 53.4 ± 9.7 to 44.4 ± 7.9, mean difference -9.0 [95% CI -14.0 to -4.0]; residual limb pain interference: 60.4 ± 9.3 to 51.7 ± 8.2, mean difference -8.7 [95% CI -13.1 to -4.4]; phantom limb pain intensity: 49.3 ± 10.4 to 43.2 ± 9.3, mean difference -6.1 [95% CI -11.3 to -0.9]; phantom limb pain interference: 57.7 ± 10.4 to 50.8 ± 9.8, mean difference -6.9 [95% CI -12.1 to -1.7]; p ≤ 0.012 for all comparisons). On functional assessment, OPUS Rasch scores improved from 53.7 ± 3.4 to 56.4 ± 3.7 (mean difference +2.7 [95% CI 2.3 to 3.2]; p < 0.001) and Neuro-QOL scores improved from 32.9 ± 1.5 to 35.2 ± 1.6 (mean difference +2.3 [95% CI 1.8 to 2.9]; p < 0.001).
Conclusions
Targeted muscle reinnervation demonstrates improvement in residual limb and phantom limb pain parameters in major limb amputees. It should be considered as a first-line surgical treatment option for chronic amputation-related pain in patients with major limb amputations. Additional investigation into the effect on function and quality of life should be performed.
Level of Evidence
Level IV, therapeutic study.
Introduction
Postamputation pain is a prevalent condition that is difficult to manage and remains an important detractor to the quality of life of patients who have undergone amputation. There are two major forms of amputation-related pain: residual limb pain, which is isolated to the residual limb “stump,” and phantom limb pain, which represents discomfort perceived in the lost limb [13]. These chronic pain conditions are common, with prevalence rates as high as 85% for phantom limb pain and 76% for residual limb pain reported in the evidence [10, 11, 13, 24]. Residual limb pain is thought to be due to symptomatic neuroma formation, and the current gold standard surgical treatment involves resection of the neuroma and “dunking” the freshly cut nerve ending into a more favorable microenvironment (bone, fat, vein, or healthy muscle) [3, 8, 14, 28, 30]. However, this solution does not prevent the nerve’s regenerative axon sprouting, ultimately leading to neuroma regeneration and potential recurrent pain. Targeted muscle reinnervation was initially developed to provide intuitive prosthesis control by transferring an amputated mixed motor and sensory nerve to a nearby recipient motor nerve. Serendipitously, reconfiguring the nerve-to-muscle relationship improved long-term chronic pain via nerve-to-nerve transfer, giving the sensory nerve “somewhere to go and something to do” [9, 25, 27].
In a randomized clinical trial (RCT), targeted muscle reinnervation was shown to reduce amputation-related chronic pain at 1 year postintervention when compared with the gold standard excision and muscle burying technique [9]. A subset of patients who were screened for the RCT but not enrolled, ultimately underwent targeted muscle reinnervation. We were interested to see if this subset was similar to cohort undergoing the RCT and whether they would have the same response. Additionally, we wished to obtain improved data regarding how targeted muscle reinnervation influenced functional outcomes.
Therefore, we asked: (1) Does targeted muscle reinnervation improve residual limb pain and phantom limb pain in the period before surgery to 1 year after surgery? (2) Does targeted muscle reinnervation improve Patient-reported Outcome Measurement System (PROMIS) pain intensity and pain interference scores at 1 year after surgery? (3) After 1 year, does targeted muscle reinnervation improve functional outcome scores (Orthotics Prosthetics User Survey [OPUS] with Rasch conversion and Neuro-Quality of Life [Neuro-QOL])?
Patients and Methods
Patient Selection
Between July 2013 and December 2017, adults with chronic pain who had undergone amputation of the major limbs were screened for an institutional review board-approved randomized controlled trial at Northwestern Memorial Hospital (Chicago, IL, USA), Walter Reed National Military Medical Center (Bethesda, MD, USA), and the University of Oklahoma (Oklahoma City, OK, USA) [9]. Patients who had amputations above the wrist or ankle and were older than 18 years were screened for randomization. Of the 85 patients who were screened, 43 patients underwent targeted muscle reinnervation for pain management but were not randomized because of prior surgical treatment for painful nerves, because they declined to participate in the clinical trial, or because they had a concomitant need for improved prosthetic control. Two patients underwent targeted muscle reinnervation on two limbs, and in these cases, the limb operated on first (determined by operative report) was selected for analysis. These patients are the focus of the present analysis.
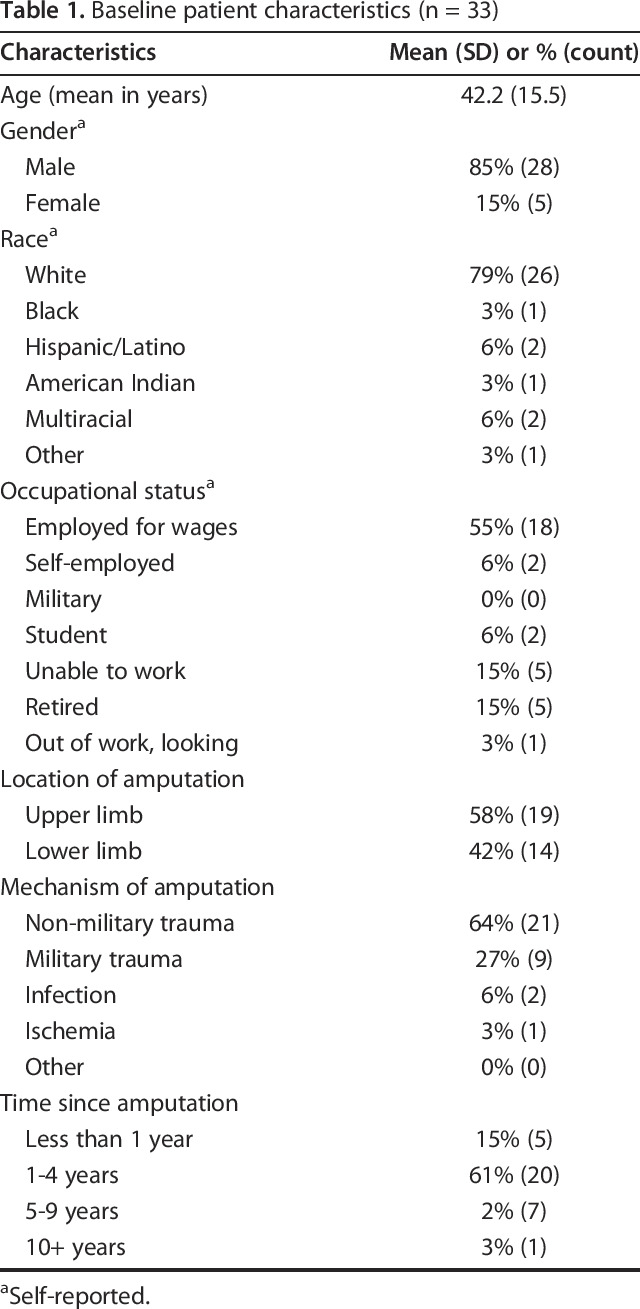
Baseline Characteristics
Ten of 43 patients did not have sufficient follow-up data. Our final cohort comprised 33 limbs from 33 patients who underwent targeted muscle reinnervation to treat a symptomatic neuroma (Table 1). Of the 33 patients, 85% (28 of 33) were men and 79% (26 of 33) were self-reported white. The mean age was 42.2 ± 15.5 years. More than half of the patients (18 of 33) were employed full-time for wages at the time of undergoing the surgery, 15% (5 of 33) were unable to work, 15% (5 of 33) were retired, and the remaining patients were self-employed (15%, 5 of 33), full-time students (6%, 2 of 33), or out of work (3%, 1 of 33). The cohort comprised 58% (19 of 33) upper extremity amputations and 42% (14 of 33) lower extremity amputations. The most common mechanism of amputation was non-military trauma (64%, 21 of 33), followed by military trauma (27%, 9 of 33), and infection (6%, 2 of 33). Fifteen percent (5 of 33) of patients underwent an amputation less than 1 year ago, 60% (20 of 33) between 1 and 4 years ago, 21% (7 of 33) between 5 and 9 years ago, and 3% (1 of 33) more than 10 years prior.
Table 1.
Baseline patient characteristics (n = 33)
Surgical Technique
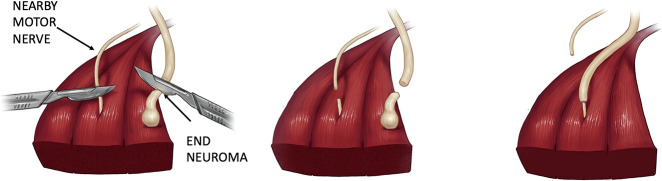
The details of the targeted muscle reinnervation surgical technique have been described in past reports [5, 15]. Briefly, the affected nerves are dissected and excised to healthy nerve fascicles, which are then coapted to the distal segment of a nearby surgically divided motor nerve (Fig. 1). All surgeons were trained by the senior author (GAD). One surgical detail was left to the discretion of the surgeon: whether the actual neuroma should be excised with the upstream neurotomy and targeted muscle reinnervation. Analysis of patients in this study showed whether the neuroma was removed or left in the surgical field after excision did not impact the outcome on pain measures; this is detailed in the results section.
Fig. 1.
This illustration depicts the surgical technique for targeted muscle reinnervation. (Published with permission from Sumanas W. Jordan MD, PhD).
Pain Measures
Change in patient-reported pain before and after targeted muscle reinnervation was assessed by an 11-point numerical rating scale (NRS) and three Patient-reported Outcome Measurement System (PROMIS) pain instruments for residual limb and phantom limb pain separately [26]. Residual limb and phantom limb pain were assessed preoperatively and again postoperatively at 3-month intervals for 1 year. For the NRS assessment, patients reported their worst and best pain levels on a 0- to 10-point pain scale (0 represents no pain and 10 represents the worst pain imaginable) in the past 24 hours and their current pain levels [12]. The primary outcomes were the change in scores for the worst NRS residual limb and phantom limb pain at 1 year after surgery. The evidence has shown that a change of 2 points on the NRS scale is the minimal clinically important difference (MCID) for patients with chronic pain and is associated with the highest degree of improvement on the patient’s global impression of change [23].
PROMIS is a well-validated outcomes tool that was developed with NIH funding. This instruments create t-scores ranging from zero to 100 with a mean of 50 and SD of 10. For the PROMIS assessment, three forms were used: pain behavior (Short Form 7a), pain intensity (Short Form 3a), and pain interference (Short Form 8a) [1, 7, 22]. For these parameters, higher scores infer worse outcomes. Changes in PROMIS pain measures were the secondary outcomes. MCID data for PROMIS parameters varies and is not yet published for all parameters; however, the evidence shows an estimated MCID ranging from 2 to 3 points for pain interference and 5 to 6 points for pain intensity [6, 20].
Functional Assessment
For patients who had undergone amputation of the upper limbs, the functional assessment was conducted using the 20-question Orthotics Prosthetics Users Survey (OPUS) Upper Extremity form, which was converted by a Rasch analysis to a standardized 0- to 100-point scale [4]. There is no evidence for the MCID for this exact survey in the amputee population; however, the MCID for OPUS lower extremity functional data in amputees is roughly 10 [21]. For those who had undergone lower limb amputation, the functional assessment was conducted using Neuro-Quality of Life (Neuro-QOL), which measures 17 functional domains and converts the raw score to a standard t-score, which ranges from 16.5 to 58.6 [18]. OPUS and Neuro-QOL both measure activities of daily living. The Neuro-QOL additionally focuses on mobility, such as difficulty sitting, standing, and walking under various conditions. Higher scores for both metrics indicate better function.
Statistical Analysis
Univariate analysis for all pain outcome measures included an assessment of skewness, kurtosis, and variance. Differences in outcomes from baseline (before surgery) to 1 year after targeted muscle reinnervation were assessed by a paired two-sample t test. Bonferroni’s adjustment was applied to control Type I errors for multiple comparisons of the primary outcomes [26]. Significance was defined as α = 0.05. The statistical analyses were conducted using SPSS software, version 23 (IBM Corp, Armonk, NY, USA).
Results
Residual Limb and Phantom Limb Pain
By 1 year after targeted muscle reinnervation, NRS scores for residual limb pain decreased from 6.4 ± 2.6 to 3.6 ± 2.2 (mean difference -2.7 [95% CI -4.2 to -1.3]; p < 0.001) and those for phantom limb pain decreased from 6.0 ± 3.1 to 3.6 ± 2.9 (mean difference -2.4 [95% CI -3.8 to -0.9]; p < 0.001) (Table 2). These findings are clinically important changes based on MCID data in the evidence as well [23]. The percentage of individuals experiencing severe residual limb pain (defined as an NRS score of 7 to 10) decreased from 58% (19 of 33) preoperatively to 6% (2 of 33) postoperatively. The percentage of individuals experiencing severe phantom limb pain (defined as an NRS score of 7 to 10) decreased from 52% (17 of 33) preoperatively to 15% (5 of 33) postoperatively.
Table 2.
Primary outcomes: NRS scores for worst pain at baseline and 1 year for limbs undergoing targeted muscle reinnervation (n = 35), mean (SD)
PROMIS Pain Scores
Patients had improvements in PROMIS residual limb pain intensity scores, which decreased from 53.4 ± 9.7 to 44.4 ± 7.9 (mean difference -9.0 [95% CI -14.0 to -4.0]; p < 0.001). Phantom limb pain intensity scores improved from 49.3 ± 10.4 to 43.2 ± 9.3 (mean difference -6.1 [95% CI -11.3 to -1.3]; p = 0.012) (Table 3). PROMIS residual limb pain interference scores decreased from 60.4 ± 9.3 to 51.7 ± 8.2 (mean difference -8.7 [95% CI -13.1 to -4.4]; p < 0.001) and phantom limb pain interference scores improved from an average of 57.7 ± 10.4 to 50.8 ± 9.8 (mean difference -6.9 [95% CI -12.1 to -1.7]; p < 0.001. The changes PROMIS intensity and interference scores can also be deemed clinically important based on MCID data published in the current evidence [6, 20]. There were non-important improvements in PROMIS behavior scores, with phantom limb pain behavior scores changing from 55.5 ± 9.6 to 50.6 ± 9.0 (mean difference -4.9 [95% CI -10.5 to 0.7]; p = 0.12) and residual limb pain behavior scores from 55.9 ± 9.2 to 51.9 ± 7.9 (mean difference -4.0 [95% CI -8.7 to 0.7]; p = 0.15).
Table 3.
PROMIS pain scales at baseline and 1 year for limbs undergoing targeted muscle reinnervation (n = 35), mean (SD)
Whether the neuroma was left in situ during the procedure did not affect patient-reported pain outcomes (see Appendix; Supplemental Digital Content 1, http://links.lww.com/CORR/A377). Patients with a neuroma left in-situ experienced similar reductions in NRS scores for residual limb pain and phantom limb pain as those whose neuromas were completely excised (residual limb: -2.3 ± 2.9 nonexcision versus -3.0 ± 3.0 excision [mean difference +0.6 {95% CI -2.5 to +3.7}; p = 0.57]; phantom limb: -2.4 ± 3.2 nonexcision versus -2.3 ± 2.7 excision [mean difference -0.1 {95% CI -3.1 to +3.0}; p = 0.94]). Likewise, there was no relationship between the neuroma being left in the surgical field after excision and PROMIS pain measure scores.
Functional Outcomes
An assessment of the upper and lower extremities before and after targeted muscle reinnnervation demonstrated functional improvement. OPUS Rasch scores increased from 53.7 ± 3.4 at baseline to 56.4 ± 3.7 at 1 year postoperatively (mean change 2.7 [95% CI 2.3 to 3.2]; p < 0.001) for patients undergoing upper extremity targeted muscle reinnnervation (Table 4). Neuro-QOL scores increased from 32.9 ± 1.5 to 35.2 ± 1.6 (mean change 2.3 [95% CI 1.8 to 2.9]; p < 0.001) for patients undergoing lower extremity targeted muscle reinnnervation (Table 4).
Table 4.
OPUS Rasch score at baseline and 1 year for upper limbs undergoing targeted muscle reinnervation (n = 20), mean (SD); Neuro-QOL score at baseline 1 year for lower limb amputees undergoing targeted muscle reinnervation (n = 14), mean (SD)
Discussion
Chronic residual limb and phantom limb pain, until recently, have been difficult to manage, and there have been numerous ineffective or inconsistent treatment strategies [3, 8, 14, 19, 20, 28, 29]. Targeted muscle reinnervation is a promising surgical intervention shown to improve amputation-related pain. With this study, we aimed to enlarge the patient sample size used to investigate the impact of targeted muscle reinnervation on residual limb and phantom limb pain and expand our knowledge on how this procedure may affect the functional status of amputees. Our investigation corroborates prior findings in which targeted muscle reinnervation led to clinically important improvement in chronic amputation-related pain 1 year out from intervention but does not reveal an improvement in functionality. Future studies should focus on the influence this procedure has on function and opioid use in this patient population.
The major limitation of this study is possible bias because of patient self-reporting and preconceptions of the efficacy of targeted muscle reinnervation. Some patients (n = 3) were enrolled in this study because they declined to be randomized, having heard through other sources (for example, the internet) that targeted muscle reinnervation was effective. Although the placebo effect may last beyond 1 year, the extended follow-up time (1 to 4 years postsurgery for most patients) and the use of multiple validated outcome assessments should reduce the impact of the placebo effect. The patients enrolled in this present study have similar reasons for neuroma formation as those in the RCT [9]. The breakdown of reasons for amputation are similar between this cohort and the RCT cohort. In the RCT, 85% of amputees had an amputation due to trauma and the remaining patients had an amputation because of infection. In this nonrandomized cohort, nearly 83% had an amputation due to trauma, with 11% having amputations because of infection and less than 6% caused by other reasons. Additionally, the results of our analysis are in line with those from the published RCT. With these findings and knowledge that most participants were excluded from the RCT because of prior surgery (n = 25), we feel that our current submission does not question the results of either study. An additional concern for bias is because the senior author (GAD) is the original developer of the targeted muscle reinnervation technique. However, in this surgical trial, the senior author performed only a minority of the procedures.
Targeted muscle reinnervation improved residual limb and phantom limb pain 1 year after intervention. Our mean difference in NRS data was more than the 2-point change deemed to be the MCID in published evidence. The clinically important change corresponds to patients’ ability to reduce pain medication for acute and chronic pain shown in a separate study [12]. Moreover, as mentioned, these findings are in line with the data from our randomized clinical trial. Specifically, when looking at the average change in NRS scores at 1 year, the randomized cohort had an average decrease in phantom limb pain of 3.2 and average decrease in residual limb pain of 2.9 [9].
PROMIS pain intensity and interference scores were similarly improved at 1 year after surgery. These clinically important findings strengthen the argument that targeted muscle reinnervation can improve amputation-related pain. Pain behavior, a measure of the external manifestations of pain (both deliberate and involuntary), did not lead to changes after targeted muscle reinnervation. The relatively smaller change in pain behavior measures may be attributed to the multifactorial nature of learned responses to pain. The pain behavior score tends be less sensitive to pain interventions than other PROMIS measures [2]. Whether the neuroma was left in situ did not influence the benefit of targeted muscle reinnervation on pain.
The present study also demonstrated improvement in global upper and lower extremity function scores 1 year after targeted muscle reinnervation. Most of the patients who underwent upper extremity surgery were excluded from the randomized controlled trial and were selected for this cohort because of the primary indication of improved prosthesis control. Thus, functional upper extremity improvement, consistent with prior reports of upper extremity targeted muscle reinnervation for advanced prosthesis control [16, 17], was as anticipated. However, as noted these were not clinically important findings. Lower extremity functional improvement, however, was not observed. We postulate that decreased pain would increase a patient’s ability to perform their activities of daily living and improve mobility. A continued analysis of a larger group of patients who have undergone lower extremity amputation is necessary. Additional investigation into (1) how functional these individuals are before surgical intervention and (2) how much they use a prosthesis overall is key to better understanding the functional impact this operation has on amputees.
Conclusions
Neuroma-related pain continues to be a challenging condition, affecting hundreds of thousands of patients. With this study, we document that targeted muscle reinnervation offers statistically and clinically important improvements to both residual limb and phantom limb pain for amputees. Future directions for research include a unified, cross-institutional patient outcome tool to facilitate further prospective, quantitative research for neuroma-related pain. Additional research focus can be placed on analyzing functional and quality of life parameters after targeted muscle reinnervation as we showed nominal but not clinically important improvements in function.
Footnotes
The institution of one or more of the authors (GAD, ILV) has received, during the study period, funding from the Office of the Assistant Secretary of Defense for Health Affairs, through the Peer Reviewed Orthopaedic Research Program under Award No. W81XWH-13-2-0100.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
References
- 1.Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, Lai JS. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askew RL, Cook KF, Revicki DA, Cella D, Amtmann D. Evidence from diverse clinical populations supported clinical validity of PROMIS pain interference and pain behavior. J Clin Epidemiol . 2016;73:103-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbera J, Albert-Pamplo R. Centrocentral anastomosis of the proximal nerve stump in the treatment of painful amputation neuromas of major nerves. J Neurosurg. 1993;79:331-334. [DOI] [PubMed] [Google Scholar]
- 4.Burger H, Franchignoni F, Heinemann AW, Kotnik S, Giordano A. Validation of the orthotics and prosthetics user survey upper extremity functional status module in people with unilateral upper limb amputation. J Rehabil Med . 2008;40:393-399. [DOI] [PubMed] [Google Scholar]
- 5.Cheesborough JE, Souza JM, Dumanian GA, Bueno RA., Jr Targeted muscle reinnervation in the initial management of traumatic upper extremity amputation injury. Hand (N Y) . 2014;9:253-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CX, Kroenke K, Stump TE, Kean J, Carpenter JS, Krebs EE, Bair MJ, Damush TM, Monahan PO. Estimating minimally important differences for the PROMIS pain interference scales: results from 3 randomized clinical trials. Pain. 2018;159:775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Revicki D, Amtmann D, Jensen M, Keefe F, Cella D. Development and analysis of PROMIS pain intensity scale. Quality of Life Research . 2012;20:18. [Google Scholar]
- 8.Dellon AL, Mackinnon SE, Pestronk A. Implantation of sensory nerve into muscle: preliminary clinical and experimental observations on neuroma formation. Ann Plast Surg. 1984;12:30-40. [DOI] [PubMed] [Google Scholar]
- 9.Dumanian GA, Potter BK, Mioton LM, Ko JH, Cheesborough JE, Souza JM, Ertl WJ, Tintle SM, Nanos GP, Valerio IL, Kuiken TA, Apkarian AV, Porter K, Jordan SW. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann Surg. 2019;270:238-246. [DOI] [PubMed] [Google Scholar]
- 10.Ehde DM, Czerniecki JM, Smith DG, Campbell KM, Edwards WT, Jensen MP, Robinson LR. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil . 2000;81:1039-1044. [DOI] [PubMed] [Google Scholar]
- 11.Ephraim PL, Wegener ST, MacKenzie EJ, Dillingham TR, Pezzin LE. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Arch Phys Med Rehabil. 2005;86:1910-1919. [DOI] [PubMed] [Google Scholar]
- 12.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149-158. [DOI] [PubMed] [Google Scholar]
- 13.Hsu E, Cohen SP. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res . 2013;6:121-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch H, Hubmer M, Welkerling H, Sandner-Kiesling A, Scharnagl E. The treatment of painful neuroma on the lower extremity by resection and nerve stump transplantation into a vein. Foot Ankle Int . 2004;25:476-481. [DOI] [PubMed] [Google Scholar]
- 15.Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int . 2004;28:245-253. [DOI] [PubMed] [Google Scholar]
- 16.Kuiken TA, Li G, Lock BA, Lipschutz RD, Miller LA, Stubblefield KA, Englehart KB. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA . 2009;301:619-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller LA, Stubblefield KA, Lipschutz RD, Lock BA, Kuiken TA. Improved myoelectric prosthesis control using targeted reinnervation surgery: a case series. IEEE Trans Neural Syst Rehabil Eng. 2008;16:46-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan SJ, Kelly VE, Amtmann D, Salem R, Hafner BJ. Self-reported cognitive concerns in people with lower limb loss. Arch Phys Med Rehabil. 2016;97:912-918.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poppler LH, Parikh RP, Bichanich MJ, Rebehn K, Bettlach CR, Mackinnon SE, Moore AM. Surgical interventions for the treatment of painful neuroma: a comparative meta-analysis. Pain . 2018;159:214-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purvis TE, Andreou E Neuman BJ Riley LH SKolasky RL. of PROMIS Health Domains Among Patients Presenting for Anterior Cervical Spine Surgery.Spine (Phila Pa 1976) . 2017;42:E1357-E1365. [DOI] [PubMed] [Google Scholar]
- 21.Resnik L, Borgia M. Reliability of outcome measures for people with lower-limb amputations: distinguishing true change from statistical error.Phys Ther . 2011;91:555-565. [DOI] [PubMed] [Google Scholar]
- 22.Revicki DA, Chen WH, Harnam N, Cook KF, Amtmann D, Callahan LF, Jensen MP, Keefe FJ. Development and psychometric analysis of the PROMIS pain behavior item bank. Pain. 2009;146:158-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8:283-291. [DOI] [PubMed] [Google Scholar]
- 24.Smith DG, Ehde DM, Legro MW, Reiber GE, del Aguila M, Boone DA. Phantom limb, residual limb, and back pain after lower extremity amputations. Clin Orthop Relat Res. 1999;361:29-38. [DOI] [PubMed] [Google Scholar]
- 25.Souza JM, Cheesborough JE, Ko JH, Cho MS, Kuiken TA, Dumanian GA. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop Relat Res. 2014;472:2984-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turk DC, Dworkin RH, McDermott MP, Bellamy N, Burke LB, Chandler JM, Cleeland CS, Cowan P, Dmitiova R, Farrar JT, Hertz S, Heyse S, Heyse JF, Iyengar S, Jadad AR, Jay GW, Jermano JA, Katz NP, Manning DC, Martin S, Max MB, McGrath P, McQuay HJ, Quessy S, Rappaport BA, Revicki DA, Rothman M, Stauffer JW, Svensson O, White RE, Witter J. Analyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations. Initiative on methods, measurement, and pain assessment in clinical trials. Pain . 2008;139:485-493. [DOI] [PubMed] [Google Scholar]
- 27.Valerio IL, Dumanian GA, Jordan SW, Mioton LM, Bowen JB, West JM, Porter K, Ko JH, Souza JM, Potter BK. Preemptive treatment of phantom and residual limb pain with targeted muscle reinnervation at the time of major limb amputation. J Am Coll Surg . 2019;228:217-226. [DOI] [PubMed] [Google Scholar]
- 28.Vernadakis AJ, Koch H, Mackinnon SE. Management of neuromas. Clin Plast Surg . 2003;30:247-268. [DOI] [PubMed] [Google Scholar]
- 29.Woo SL, Kung TA, Brown DL, Leonard JA, Kelly BM, Cederna PS. Regenerative peripheral nerve interfaces for the treatment of postamputation neuroma pain: a pilot study. Plast Reconstr Surg Glob Open. 2016;4:e1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood VE, Mudge MK. Treatment of neuromas about a major amputation stump. J Hand Surg Am. 1987;12:302-306. [DOI] [PubMed] [Google Scholar]