Abstract
INTRODUCTION:
The previous researches aimed to evaluate the efficacy and safety of fecal microbiota transplantation (FMT) for ulcerative colitis (UC) in a short-term observation. The present study aimed to explore the optimum timing of FMT for maintaining the long-term clinical benefits and to target the gut microbiota that may help to predict the long-term success or failure of FMT in UC.
METHODS:
Two hundred two patients with UC were recruited from November 2012 to September 2018. The primary endpoint of this study was the maintaining time of the first and second courses of FMT. Relapse was defined as partial Mayo score ≥2 after achieving clinical remission and an increase of partial Mayo score ≥1 after achieving clinical response. The stool samples were analyzed by 16S rRNA gene sequencing.
RESULTS:
The median maintaining time of the efficacy was 120 days (IQR, 45–180) and 182.5 days (IQR, 105–311.25) from the first course and second course of FMT, respectively. No FMT-related serious adverse events were observed. The differences of the relative abundance in Eggerthella, Lactobacillus, and Ruminococcus between pre-FMT and 5 days post-FMT were remarkably correlated with the long-term clinical remission (P < 0.05).
DISCUSSION:
This study demonstrated that patients with UC should undergo the second course of FMT within 4 months after the first course of FMT for maintaining the long-term clinical benefits. The short-term alterations of microbiota after FMT may be conducive to predicting the long-term efficacy of FMT in UC (see Visual Abstract, Supplementary Digital Content, http://links.lww.com/CTG/A363).
INTRODUCTION
Ulcerative colitis (UC) is a chronic, refractory, and progressive inflammatory disease of the intestinal tract, which is characterized by persistent or relapsing symptoms including bloody diarrhea, anemia, and abdominal pain (1,2). Ample evidences support an essential role of the gut microbiota in contributing to intestinal inflammation (3–8). Inducing remission and maintaining the long-term remission are the imperative targets of various treatments in patients with UC. The efficacy of 5-ASAs, corticosteroids, and immunosuppressants is modest for the refractory patients in the long-term treatment (9,10). In addition, the possibilities of causing serious adverse events (AEs) and loss of response were the pressing problems of biologics in clinical practice (11,12). Therefore, more effective and safer treatment strategies are being evaluated for UC.
Increasing studies have demonstrated that fecal microbiota transplantation (FMT) could induce and maintain remission in patients with UC by remodeling the construction of gut microbiota in a short-term observation and has a low incidence of serious AEs (9,13–19). However, few studies touched on the frequency of FMT in UC for maintaining the long-term clinical benefits. Recently, our group reported that patients with Crohn's disease (CD) who achieved clinical remission from the first course of FMT should undergo the second course of FMT in less than 4 months after the initial course of FMT for better clinical outcomes (20). We hypothesized that the timing of the second course of FMT in UC and CD might be basically identical.
In addition, unfavorable alterations of the gut microbiota composition have been reported in UC over the past decade, including reduced diversity and a shift in bacterial taxa, especially a significant decrease of numerous short-chain fatty acid (SCFA)-producing bacteria (21–23). It is of great significance to search for microbiota that are related to the progression and recovery of disease as targets for treatment.
The present study aimed to explore the optimum timing of administering the second and third courses of FMT in UC and to target the microbiota that may help to predict the long-term success or failure of FMT. These results will be essential for the clinicians to consider sequential FMTs into clinical practice as a long-term treatment strategy for UC.
METHODS
Patients
This study as a part of a clinical trial (NCT01790061) was conducted at the Second Affiliated Hospital of Nanjing Medical University, Nanjing, China. Patients with UC who underwent FMT at our center from November 2012 to September 2018 were included in the study. The last follow-up was completed on January 1, 2019. Inclusion criteria were patients who were diagnosed as UC by a combination of typical clinical symptoms, endoscopic, and histological criteria for at least 6 months; patients with mild, moderate, and severe active UC (Mayo score from 3 to 12); patients who did not achieve satisfactory efficacy from the previous medications; and patients who provided informed consent. Patients were excluded if aged younger than 14 years, accompanied by other severe diseases, including other intestinal diseases, e.g., Clostridium difficile infection, malignant neoplasm, cardiopulmonary failure, serious liver and kidney disease, and follow-up less than 3 months. Patients who underwent the first course of FMT and did not have serious AEs were allowed to have the second course of FMT. In clinical practice, the clinicians suggested that patients who achieved clinical response from the first course of FMT should undergo the second course of FMT before disease relapse to maintain the long-term clinical benefits. The patients who did not achieve response from the initial FMT were suggested to undergo FMT again. However, when and whether to undergo the next course of FMT depend on patients' own decisions.
Study design
This is a prospective, observational, and cohort study of FMT in patients with UC. All eligible subjects provided written informed consent before participation in this study. The primary endpoint of this study was the maintaining time of the first and second courses of FMT. The secondary endpoint was the alterations of the gut microbiota. Corticosteroids were asked to be gradually cut down until stopped within at least 1 week before FMT. 5-Aminosalicylic acid was a sustained treatment before and after FMT if there was no allergic response to this medication. Other medications were asked to be stopped within 72 hours before FMT. The usage of probiotics was prohibited after FMT. The antibiotics were not advised to be used at random before conducting and communicating with the clinicians.
Donor screening and FMT procedure
Patients could have a choice to self-identify their relatives or friends as donors at the early stage of the study. The unrelated universal donors aged from 6 to 24 years old were selected from China fecal microbiota bank, and they were screened by strict exclusion criteria, which had been described in detail in our previous reports (9,13,24). After April 2014, the methodology of FMT was coined as washed microbiota transplantation (WMT), which is dependent on the automatic microbiota purification system (GenFMTer; FMT Medical, Nanjing, China) and washing process in a laboratory room with safety level 3 (25). The methodology of WMT has been released as a consensus statement in 2019 (26). From the process of feces defecation until the fresh bacteria infused into the in intestinal tract of patients should be done within 1 hour.
Single FMT through gastroscopy was performed from 2012 to 2014 in this population. With increasing needs of frequent FMTs (multiple FMTs), 2 types of transendoscopic enteral tubing (TET) (FMT Medical, Nanjing, China), including colonic TET and mid-gut TET, were used for delivering washed microbiota suspension in practice since 2014 (27,28). Owing to potential failure to hold the microbiota suspension in the colon for enough time, the suspension (fresh or frozen) is not permitted to be delivered through colonic endoscopy channel or enema in our protocol.
Maintaining time of the efficacy and safety assessment
The clinical efficacy of all patients was assessed at the point of week 1, week 4, week 12, and every 3 months after the initial FMT. Partial Mayo score that includes stool frequency, rectal bleeding, and physician's assessment was used to evaluate the clinical efficacy of FMT in UC. Clinical response in UC was defined as a decrease of partial Mayo score ≥3 and ≥20% from baseline plus a decrease in the rectal bleeding subscore of ≥1 or rectal bleeding subscore of ≤1, and clinical remission was defined as partial Mayo score ≤1 (29). Relapse was defined as partial Mayo score ≥2 after achieving clinical remission and an increase of partial Mayo score ≥1 after achieving clinical response (30). The median maintaining time of the efficacy from FMT was calculated by the survival curves analysis, which we considered as the optimum timing of the following course of FMT. AEs Common Terminology Criteria for Adverse Events (version 5.0) was applied to describe the intensity and relativity of AE with FMT. Only FMT-attributed AEs were reported in the study, including definitely, probably, and possibly related AEs.
Sample collection
A total of 61 stool samples for microbiota analysis were collected from 41 participants, including 22 patients with UC and 19 respective donors (3 samples degeneracy). Each donor provided a single stool sample. Forty-two UC samples were included: 22 samples at baseline and 20 at 5 days after the first course of FMT.
16S ribosomal RNA gene sequencing and analysis
Stool samples were collected by professional nursing staff working and stored at −80°C until shipping to the HRK-biotech laboratory for DNA extraction and sequencing. All microbial DNA was extracted from stool samples. Bacterial 16S ribosomal RNA (rRNA) gene sequences were PCR amplified that used bar-coded primers for the V4–V5 hypervariable region by the Phusion High-Fidelity PCR Master Mix with HF buffer (New England Biolabs, England). Products from each sample were mixed at equal molar ratios and then sequenced using the Illumina MiSeq platform (Illumina, Inc., San Diego, CA), which followed standard Illumina sequencing protocols.16S rRNA gene sequences were analyzed using a combination of software: mothur, UPARSE, and R. Operational taxonomic units were clustered at 97% similarity and filtered using the UPARSE pipeline.
Statistical analysis
The data were analyzed by SPSS Statistics (SPSS, Chicago, IL) or GraphPad (version 5; GraphPad Software, San Diego, CA). Normally distributed continuous data were expressed as mean ± SD. The Wilcoxon rank-sum tests and paired-samples t tests were used to analyze differences between 2 paired groups. Classification variable was analyzed by χ2 tests. More than 2 groups were analyzed by analysis of variance tests. A 2-tailed P value less than 0.05 was considered statistically significant.
RESULTS
Study population
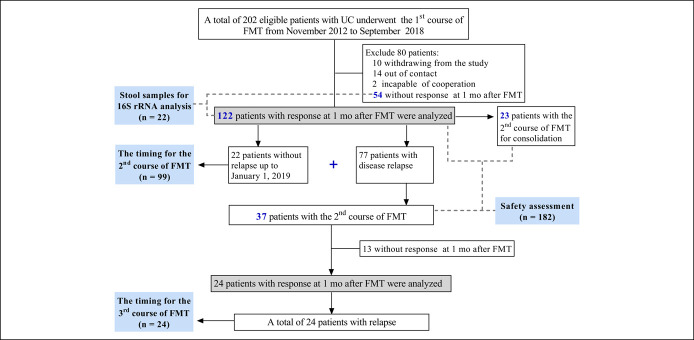
This single-center study included 202 eligible patients who underwent the first course of FMT from November 2012 to September 2018 (Figure 1). Twenty-six patients were lost to follow up. Clinical data of 176 patients was collected during the procedure of FMT and long-term follow-up.
Figure 1.
Flowchart of the study. FMT, fecal microbiota transplantation; UC, ulcerative colitis.
Of these, 122 patients who achieved clinical response at 1 month after the first course of FMT were included in the analysis for maintaining time of efficacy. Among 122 patients, 22 patients had sustained response until January 1, 2019 (the terminal point of follow-up), without undergoing the second course of FMT, 77 patients had disease relapse before the second course of FMT, and 23 patients underwent the second course of FMT before disease relapse for consolidation. We calculated the median maintaining time of efficacy from the first course of FMT in 99 patients, including 22 patients with sustained response and 77 patients with disease relapse.
There were 60 patients, including 23 patients without disease relapse and 37 patients with disease relapse before the second course of FMT, undergoing both first and second courses of FMT among 122 patients. We calculated the median maintaining time of efficacy from the second course of FMT in 24 patients who had response from FMT and had disease relapse before the third course of FMT.
Patient characteristics
The characteristics of all 122 patients who achieved response were shown in Table 1. The median follow-up was 25.5 months (IQR, 11.75–43). The median age of patients was 36 years (IQR, 29.0–49.25), and the median disease duration was 5.0 years (IQR, 2.0–8.0). 58.2% (71/122) of patients were men. 92.6% (113/122) of patients belong to moderate and severe. 89.3% (109/122) of patients had used mesalamine, 66.4% (81/122) had used corticosteroids, 29.5% (36/122) had used cyclosporine/azathioprine, and 6.6% (8/122) had used antitumor necrosis factor agents.
Table 1.
Characteristics of 122 patients with UC
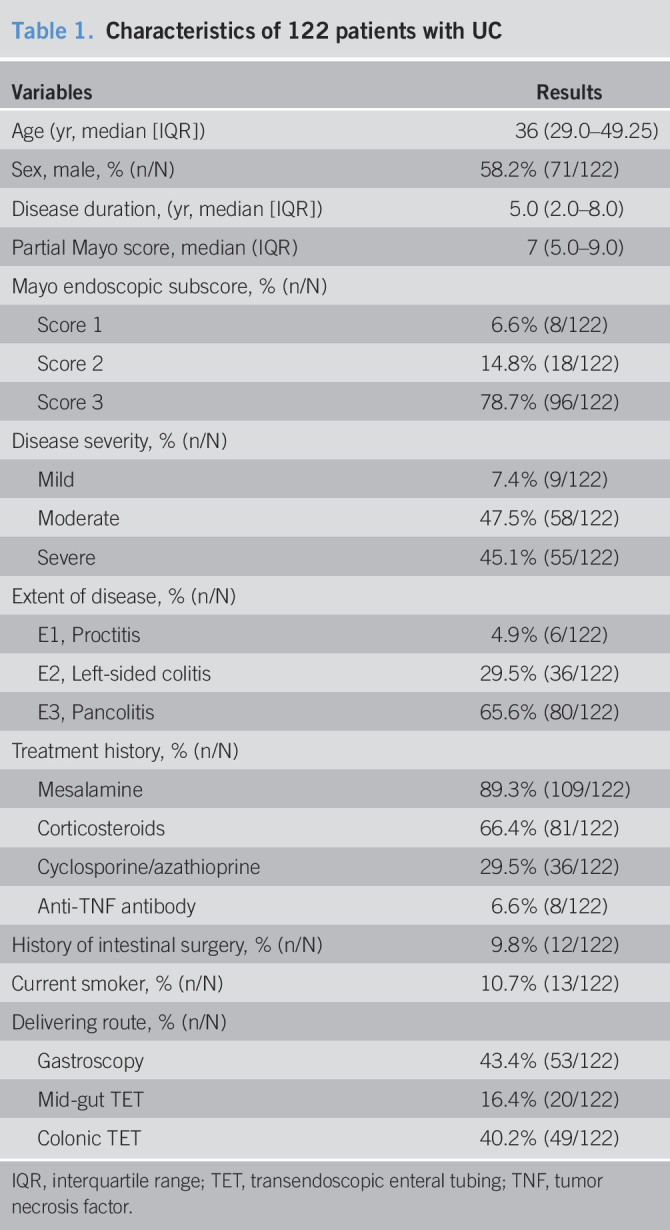
| Variables | Results |
| Age (yr, median [IQR]) | 36 (29.0–49.25) |
| Sex, male, % (n/N) | 58.2% (71/122) |
| Disease duration, (yr, median [IQR]) | 5.0 (2.0–8.0) |
| Partial Mayo score, median (IQR) | 7 (5.0–9.0) |
| Mayo endoscopic subscore, % (n/N) | |
| Score 1 | 6.6% (8/122) |
| Score 2 | 14.8% (18/122) |
| Score 3 | 78.7% (96/122) |
| Disease severity, % (n/N) | |
| Mild | 7.4% (9/122) |
| Moderate | 47.5% (58/122) |
| Severe | 45.1% (55/122) |
| Extent of disease, % (n/N) | |
| E1, Proctitis | 4.9% (6/122) |
| E2, Left-sided colitis | 29.5% (36/122) |
| E3, Pancolitis | 65.6% (80/122) |
| Treatment history, % (n/N) | |
| Mesalamine | 89.3% (109/122) |
| Corticosteroids | 66.4% (81/122) |
| Cyclosporine/azathioprine | 29.5% (36/122) |
| Anti-TNF antibody | 6.6% (8/122) |
| History of intestinal surgery, % (n/N) | 9.8% (12/122) |
| Current smoker, % (n/N) | 10.7% (13/122) |
| Delivering route, % (n/N) | |
| Gastroscopy | 43.4% (53/122) |
| Mid-gut TET | 16.4% (20/122) |
| Colonic TET | 40.2% (49/122) |
IQR, interquartile range; TET, transendoscopic enteral tubing; TNF, tumor necrosis factor.
Maintaining time of the efficacy and safety assessment
Among 176 patients, at 1 week, 1 month, 3 months, and 6 months after FMT, the rate of clinical response was 48.9%, 69.3%, 49.4%, and 32.7%, respectively, and the clinical remission rate was 25.0%, 34.1%, 33.0%, and 24.0%, respectively.
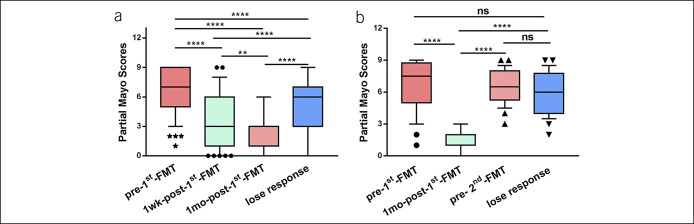
The median maintaining time of the efficacy from the first course of FMT in 99 patients was 120 days (IQR, 45–180) (Figure 2a). In addition, the median maintaining time of the second course of FMT in 23 patients for consolidation was 415 days (IQR, 255–780), which was significantly longer than the maintaining time of the 99 patients (P < 0.001) (Figure 2a). The median maintaining time of the second course of FMT in 24 patients was 182.5 days (IQR, 105–311.25) (Figure 2b). Compared with the maintaining time of the first course of FMT, the second course of FMT represented a trend of longer maintaining time (P = 0.067) (see Figure, Supplementary Digital Content 1, http://links.lww.com/CTG/A359). The partial Mayo scores of the 99 patients at various visits before and after the first course of FMT are shown in Figure 3a. A significant decrease of the partial Mayo scores at 1 week and 1 month after the first course of FMT was observed compared with the baseline (P < 0.0001). At the time of losing response, the partial Mayo scores increased remarkably compared with that at 1 week and 1 month after the first course of FMT (P < 0.0001). The 24 patients' partial Mayo scores at different visits before and after the second course of FMT are exhibited in Figure 3b. The partial Mayo scores increased significantly before the second course of FMT compared with that at 1 month after the first course of FMT (P < 0.0001).
Figure 2.
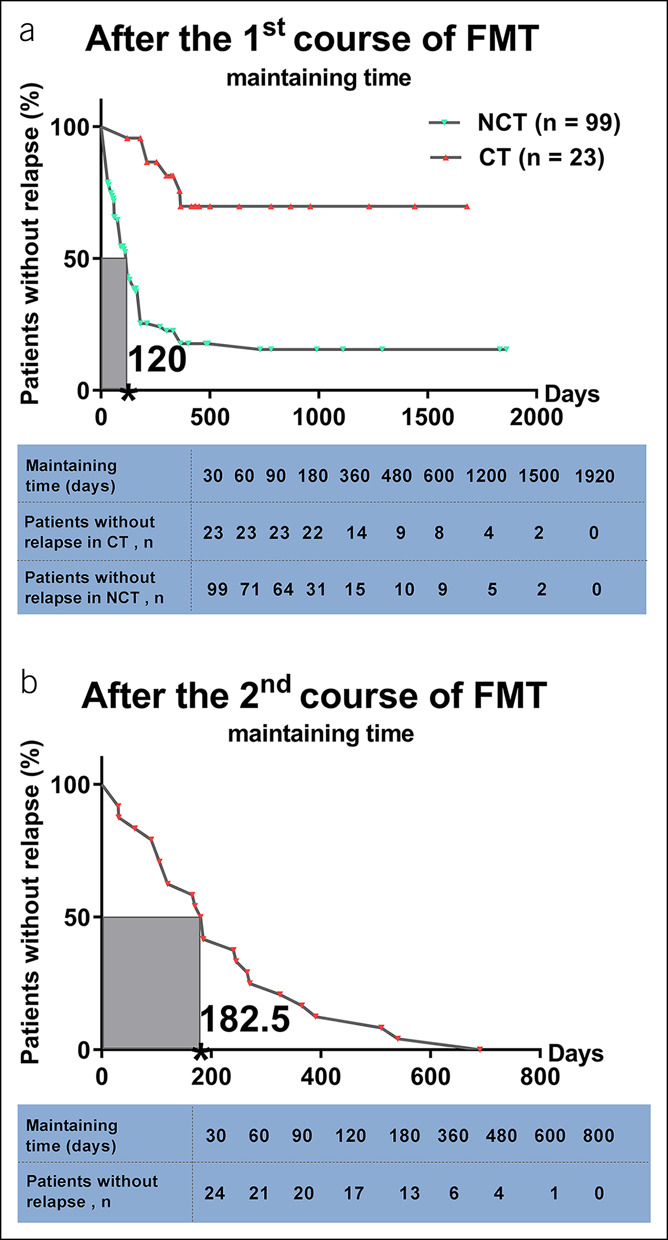
Maintaining time of the efficacy from FMT. (a) The maintaining time of the first course of FMT in 122 patients with UC. Compared with the maintaining time of the NCT group (n = 99), the CT group (n = 23) achieved longer maintaining time significantly (P < 0.001). The median maintaining time of the first course of FMT in the nonconsolidation group was 120 days (IQR, 45–180). (b) The maintaining time of the second course of FMT in 24 patients with UC. The median maintaining time of the second course of FMT was 182.5 days (IQR, 105–311.25). Kaplan–Meier survival curves were used to describe the time for relapse. P value less than 0.05 was considered statistically significant. CT, consolidation treatment; FMT, fecal microbiota transplantation; NCT, nonconsolidation treatment.
Figure 3.
The partial Mayo scores at various visits. (a) The partial Mayo scores of 99 patients before and after the first course of FMT. (b) The partial Mayo scores of 24 patients before and after the second course of FMT. More than 2 groups were analyzed by the ANOVA test. P value less than 0.05 was considered statistically significant. Significance levels: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. ANOVA, analysis of variance; FMT, fecal microbiota transplantation.
No serious AEs associated with FMT were observed during and after FMT among 182 courses of FMT. During the follow-up period, 43 AEs were observed in 33 patients with the rate of AEs to be 18.1% (33/182) (see Figure, Supplementary Digital Content 2, http://links.lww.com/CTG/A360). AEs from 90.9% (30/33) of patients occurred within 1 month after FMT. Increased frequency of defection, fever, rash, and abdominal pain showed a higher incidence among all AEs. Most AEs were self-recovered without medication use in the short term.
The dysbiosis of gut microbiota in UC
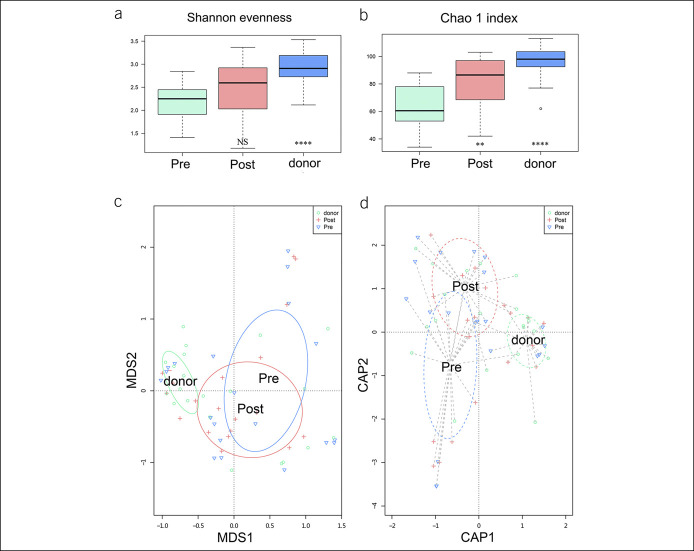
Analysis of alpha diversity revealed that both the diversity and the richness (calculated in the Shannon evenness, Chao 1 index) were significantly lower in patients with UC before FMT than the donors (P < 0.001) (Figure 4a, b). Analysis of the beta diversity calculated by multidimensional scaling, canonical analysis of principal coordinates (Figure 4c, d), and heat map (see Figure, Supplementary Digital Content 3, http://links.lww.com/CTG/A361) represented that gut microbiota community of pre-FMT samples was apart from that of donors. There was a trend that alpha and beta diversities of the post-FMT samples were analogous to the donors (Figure 4), which indicated that the dissimilarity between patients with UC and the donors lessened after FMT.
Figure 4.
The diversity of microbiota in donors and patients with UC before and after FMT. (a and b) Alpha diversity was calculated by Shannon evenness and Chao 1 index. The donors' and post-FMT patients' samples were compared with pre-FMT patients' samples. (c and d) Beta diversity was calculated by MDS and CAP coordinates. Each dot stands for 1 sample. P value less than 0.05 was considered statistically significant. Significance levels: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. The number of samples: donors (n = 19), patients with UC pre-FMT (n = 22), patients with UC post-FMT (n = 20). CAP, canonical analysis of principal; FMT, fecal microbiota transplantation; MDS, multidimensional scaling; UC, ulcerative colitis.
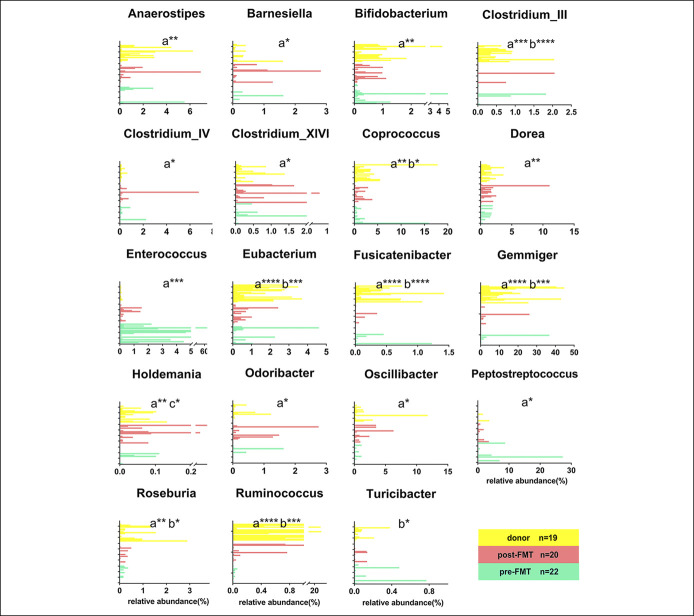
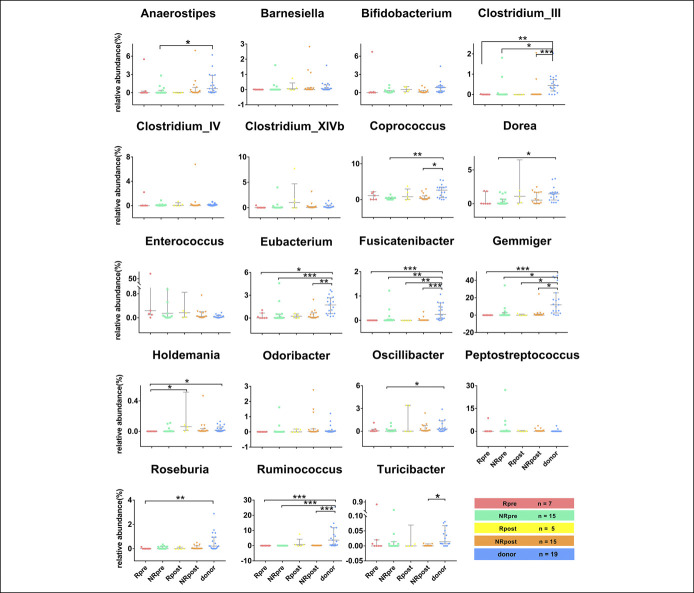
The relative abundance of Enterococcus was relatively higher in the pre-FMT samples compared with the donors (P < 0.05) (Figure 5). In particular, the genus Enterococcus was with an extremely low abundance in the donors, and it was undetectable in most of donors (Figure 5). The relative abundance of Anaerostipes, Bifidobacterium, Clostridium_IV, Coprococcus, Eubacterium, Odoribacter, Roseburia, and Ruminococcus, which were regarded as the SCFA-producing bacteria, were remarkably lower in patients with UC compared with the donors, suggesting that SCFA production was diminished in patients with UC (P < 0.05) (Figure 5). The relative abundance of Holdemania at 5 days after FMT increased significantly in patients with UC (P < 0.05). In addition, the relative abundance of Anaerostipes, Bifidobacterium, Clostridium_IV, and Odoribacter at 5 days after FMT was analogous to the donors. The detailed composition of gut microbiota in patients and donors was represented in Figure (see Supplementary Digital Content 4, http://links.lww.com/CTG/A362).
Figure 5.
Nineteen significant differential genera between patients with UC and donors. Differences were analyzed by the Kruskal-Wallis test. P value less than 0.05 was considered statistically significant. Significance levels: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. (a) Pre-FMT vs donor, (b) Post-FMT vs donor, and (c) Pre-FMT vs post-FMT. The number of samples: donors (n = 19), patients with UC pre-FMT (n = 22), patients with UC post-FMT (n = 20). FMT, fecal microbiota transplantation; UC, ulcerative colitis.
Gut microbiota predicted the efficacy of FMT in UC
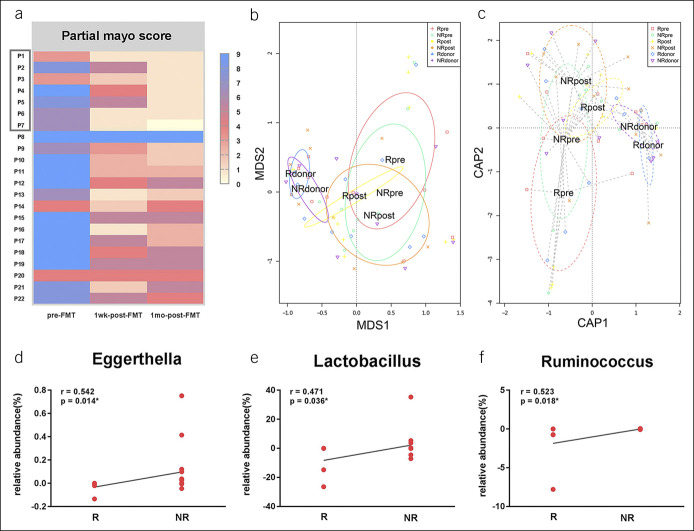
In the present study, the partial Mayo scores of the 22 patients who were chosen randomly from the 176 patients were shown in Figure 6a. 31.8% (7/22) of patients achieved clinical remission and experienced sustained response (at least 4 months) to FMT.
Figure 6.
Microbiota related to the long-term efficacy of FMT in patients with UC. (a) The partial Mayo score of the 22 patients at various visits (pre-FMT, 1 week post-FMT and 1 month post-FMT). The 7 patients (boxed) achieved clinical remission and maintaining time was at least 4 months. (b and c) Beta diversity was calculated by MDS and CAP coordinates in the remission (R) and nonremission (NR) groups. Each dot stands for 1 sample. (d–f) The relative abundance differences of genera Eggerthella (r = 0.542, P = 0.014), Lactobacillus (r = 0.471, P = 0.036), and Ruminococcus (r = 0.523, P = 0.018) between pre-FMT and 5 days post-FMT were positively correlated to the long-term efficacy (4 months after FMT) significantly. P value less than 0.05 was considered statistically significant. The number of samples: Rdonor (n = 7), NRdonor (n = 12), Rpre (n = 7), NRpre (n = 15), Rpost (n = 5), and NRpost (n = 15). CAP, canonical analysis of principal; FMT, fecal microbiota transplantation; NRpre, genera in the nonremission group before FMT; NRpost, genera in the nonremission group after FMT; Rdonor, genera in the remission group of donor; Rpre, relative abundance of genera in the remission group before FMT; Rpost, genera in the remission group after FMT; MDS, multidimensional scaling; NRdonor, genera in the nonremission group of donor; UC, ulcerative colitis.
As shown in Table (see Supplementary Digital Content 1, http://links.lww.com/CTG/A364), the clinical factors that may affect the efficacy at 4 months after FMT were analyzed by univariate analysis. The age, sex, disease duration, partial Mayo score, Mayo Endoscopic subscore, severity, and extent of disease in patients at baseline did not affect the efficacy of FMT. Furthermore, the method of microbiota preparation, the form of bacterial material and delivery route in FMT procedure had no remarkable effects on efficacy. In donors, age, sex, and genetic relationship with the respective patients did not influence the efficacy significantly.
We devoted to exploring whether the relative abundance of genera at baseline and 5 days post-FMT in patients and donors affected the efficacy at 4 months after FMT. Analysis of the beta diversity calculated by multidimensional scaling and canonical analysis of principal coordinates (Figure 6b, c) represented that gut microbiota community of patients and donors in the remission or nonremission group could not be differentiated clearly. In Figure 7, the most interesting finding is that the relative abundance of Eubacterium and Ruminococcus with remarkable differences both in the remission and nonremission groups (P < 0.001) before FMT compared with donors. Five days after FMT, the abundance of Eubacterium and Ruminococcus in the remission group increased and was close to the donors. However, a notable difference was still observed in the nonremission group compared with donors (P < 0.001) (Figure 7). The 2 genera could be the critical genera to identify the efficacy of FMT.
Figure 7.
Comparisons of the 19 genera relative abundance between UC patients with sustained remission and nonremission from FMT. NRpre, genera in the nonremission group before FMT; NRpost, genera in the nonremission group after FMT; Rpost, genera in the remission group after FMT; Rpre, relative abundance of genera in the remission group before FMT. P value less than 0.05 was considered statistically significant. Significance levels: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. The number of samples: donor (n = 19), Rpre (n = 7), NRpre (n = 15), Rpost (n = 5), and NRpost (n = 15).
To probe into whether the short-term changes of the genera abundance in patients after FMT were correlated to the long-term efficacy, the correlation was statistically analyzed by the Spearman rank correlation analysis. The differences of the relative abundance in genera Eggerthella, Lactobacillus, and Ruminococcus between pre-FMT and 5 days post-FMT in patients were positively correlated to efficacy (P < 0.05) (Figure 6d–f).
DISCUSSION
Several studies have demonstrated that FMT could effectively induce clinical response and improve clinical symptoms in patients with active UC in a short-term period (15,18,19). Limited literatures have evaluated the sustained long-term benefits achieved from FMT. Therefore, how to maintain the long-term benefits of FMT in UC should be an essential question. The current real-world study aimed to explore the optimum timing of performing the second and third courses of FMT for patients with UC and dedicated to figuring out the alteration of microbiota resulted from FMT, particularly for those with the predictive role for the long-term therapeutic success or failure.
Only the patients who achieved clinical efficacy would consult the clinicians about when they should undergo the next course of FMT for maintaining long-term benefits, so the 54 patients who did not have response after FMT were excluded for the calculation of maintaining time. Thus, in total, 122 patients were included in the analysis of the optimum timing of the second course of FMT. Twenty-three patients who underwent the second course of FMT before disease relapse were excluded for calculating the maintaining time of the first and second courses of FMT because we could not make clear that whether the maintaining time of efficacy in the 23 patients was owed to the only first or both courses of FMT.
In the present study, the median maintaining time of the efficacy from the first course of FMT was 120 days (IQR, 45–180) and the second course of FMT was 182.5 days (IQR, 105–311.25). The results showed that patients with UC should undergo the second course of FMT in less than 4 months after the initial course of FMT and be performed the third course of FMT in less than 6 months after the second FMT for the long-term clinical benefits. The estimated optimum timing of the next course of FMT could be used to instruct patients to undergo the following course of FMT before relapse to maintain the long-term clinical benefits. Importantly, it is not necessary to perform over-frequent FMTs to avoiding the waste of medical resources and social burden. Recently, Sood et al. (31) reported that FMT as a maintenance therapy in patients with UC who are in clinical remission after the first course of FMT may be conducive to maintaining clinical, endoscopic, and histological remission in the long term.
Our present study revealed that the genus Enterococcus was highly enriched in the stool samples of the patients with UC at baseline when compared with the donors. Several studies have shown that the 2 species of it including Enterococcus faecalis and Enterococcus faecium could induce UC in interleukin-10 knockout mice (32–35). The genus Enterococcus, which was considered as a trigger of UC, may be a potential therapeutic target. Further studies should aim to confirm it.
A study conducted by Paramsothy et al. demonstrated that the relative abundance of the SCFA-producing bacteria, such as Eubacterium hallii, Ruminococcus bromii, and Roseburia inulivorans, and the production of SCFAs significantly increased in the remission group after FMT compared with the patients with UC who did not achieve remission from FMT (18). Interestingly, we also found that after FMT, the abundance of Eubacterium and Ruminococcus in the remission group increased and was close to the donors. Perhaps an increase in the relative abundance of the genera Eubacterium and Ruminococcus could improve the efficacy of microbial intervention by improving the production of SCFAs, which needs to be confirmed by further studies.
This study further explored the role of characteristics of short-term changes of microbiota after FMT on predicting the long-term clinical benefits. Hyams et al. (36) found that the development of personalized clinical and biological signatures held the promise of informing UC therapeutic decisions. Besides, Paramsothy et al. (18) proposed that the species Eubacterium hallii, Ruminococcus bromii, and Roseburia inulivorans could be the predictors of FMT therapeutic success or failure in UC. In the present study, we observed that the differences in the relative abundance of genera Ruminococcus, Eggerthella, and Lactobacillus between pre-FMT and 5 days post-FMT were remarkably correlated with the long-term clinical remission. This indicated that the higher abundance of the genera was exhibited at 5 days post-FMT, the higher possibility of the long-term remission could be achieved.
In addition, the Eubacterium, Ruminococcus, and Lactobacillus were widely regarded as SCFA-producing bacteria, which presented a significant decrease of relative abundance in patients with UC or rCDI and which could be improved by FMT (37–39). Interestingly, Yilmaz et al. reported that an increasing relative abundance of Eggerthella was associated with the successful outcome of tumor necrosis factor-α inhibitor therapy in CD (3,40).
There were some limitations in the present study. The analysis of gut microbiota was only performed in limited patients and at the genus level. A scientific clinical predictive model should be established in further study. A larger sample size, randomized, and controlled study should be conducted to draw a more solid conclusion.
In conclusion, this study demonstrated that patients with UC could undergo the second course of FMT within 4 months after the initial course of FMT. These results will embark on the clinicians to consider sequential FMTs into practice as a long-term treatment strategy for UC. The relative abundance of Eubacterium, Ruminococcus, Eggerthella, and Lactobacillus of patients with UC may help to predict the long-term efficacy of FMT.
CONFLICTS OF INTEREST
Guarantor of the article: Faming Zhang, MD, PhD.
Specific author contributions: Qianqian Li, MD and Xiao Ding, MD, contributed equally to this work. F.Z., Q.L., and X.D. designed the study and edited the manuscript. Q.L., X.D., K.L., T.Z., L.X., B.C., P.L., and F.Z. were responsible for the recruitment and treatment of the patients. Q.L. and X.D. participated in the data analysis and wrote the manuscript. C.M. wrote and revised the manuscript. X.L., Y.L., and J.W. devoted to the visualization. J.B. provided scientific statistic analysis methods. All authors read and approved the final manuscript.
Funding support: This study was funded by the publicly donated Intestine Initiative Foundation; Primary Research & Development Plan of Jiangsu Province (BE2018751), Jiangsu Provincial Medical Innovation Team (Zhang F); National Natural Science Foundation of China (81873548, 81670495, 81600417); and the National Clinical Research Center for Digestive Diseases, Xi'an, China (2015BAI13B07).
Potential competing interests: None to report.
Ethics approval: This study was reviewed and approved by the Second Affiliated Hospital of Nanjing Medical University Institutional Review Board. All eligible subjects provided written informed consents before participation in this study.
Availability of data and materials: All data generated or analysed during this study are included in this published article (and its supplementary information files).
Trial registration: ClinicalTrials.gov NCT01790061.
Study Highlights.
WHAT IS KNOWN
✓ Increasing studies have demonstrated that FMT could induce and maintain remission in patients with UC in a short-term observation.
✓ Few studies touched on the frequency of FMT in UC for maintaining the long-term clinical benefits.
WHAT IS NEW HERE
✓ This study demonstrated that patients with UC could be performed the second course of FMT in less than 4 months after the initial course of FMT to maintain the long-term benefits.
✓ The relative abundance of Eubacterium, Ruminococcus, Eggerthella, and Lactobacillus in patients with UC may be conducive to predicting the long-term efficacy from FMT.
TRANSLATIONAL IMPACT
✓ These results will embark on the clinicians to consider sequential FMTs into practice as a long-term treatment strategy for UC.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to all participants of the study and appreciate the kindly help from Jie Zhang for providing data from China Microbiota Transplantation System (www.fmtbank.org).
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A359, http://links.lww.com/CTG/A360, http://links.lww.com/CTG/A361, http://links.lww.com/CTG/A362, http://links.lww.com/CTG/A363, http://links.lww.com/CTG/A364
References
- 1.Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017;389(10080):1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012;380(9853):1606–19. [DOI] [PubMed] [Google Scholar]
- 3.Caruso R, Lo BC, Nunez G. Host-microbiota interactions in inflammatory bowel disease. Nat Rev Immunol 2020;20(7):411–426. [DOI] [PubMed] [Google Scholar]
- 4.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448(7152):427–34. [DOI] [PubMed] [Google Scholar]
- 5.Garrett WS, Lord GM, Punit S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell 2007;131(1):33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dianda L, Hanby AM, Wright NA, et al. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol 1997;150(1):91–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Dalal SR, Chang EB. The microbial basis of inflammatory bowel diseases. J Clin Invest 2014;124(10):4190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortha A, Chudnovskiy A, Hashimoto D, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014;343(6178):1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui B, Li P, Xu L, et al. Step-up fecal microbiota transplantation strategy: A pilot study for steroid-dependent ulcerative colitis. J Transl Med 2015;13:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timmer A, Patton PH, Chande N, et al. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 2016;5:CD000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142(2):257–65 e251-253. [DOI] [PubMed] [Google Scholar]
- 12.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369(8):699–710. [DOI] [PubMed] [Google Scholar]
- 13.Ding X, Li Q, Li P, et al. Long-term safety and efficacy of fecal microbiota transplant in active ulcerative colitis. Drug Saf 2019;42(7):869–80. [DOI] [PubMed] [Google Scholar]
- 14.Costello SP, Hughes PA, Waters O, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: A randomized clinical trial. JAMA 2019;321(2):156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015;149(1):102–9.e106. [DOI] [PubMed] [Google Scholar]
- 16.Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015;149(1):110–8.e114. [DOI] [PubMed] [Google Scholar]
- 17.Nishida A, Imaeda H, Ohno M, et al. Efficacy and safety of single fecal microbiota transplantation for Japanese patients with mild to moderately active ulcerative colitis. J Gastroenterol 2017;52(4):476–82. [DOI] [PubMed] [Google Scholar]
- 18.Paramsothy S, Nielsen S, Kamm MA, et al. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology 2019;156(5):1440–54.e1442. [DOI] [PubMed] [Google Scholar]
- 19.Sood A, Mahajan R, Juyal G, et al. Efficacy of fecal microbiota therapy in steroid dependent ulcerative colitis: A real world intention-to-treat analysis. Intest Res 2019;17(1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li P, Zhang T, Xiao Y, et al. Timing for the second fecal microbiota transplantation to maintain the long-term benefit from the first treatment for Crohn's disease. Appl Microbiol Biotechnol 2019;103(1):349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schirmer M, Denson L, Vlamakis H, et al. Compositional and temporal changes in the gut microbiome of pediatric ulcerative colitis patients are linked to disease course. Cell Host Microbe 2018;24(4):600–10.e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maukonen J, Kolho KL, Paasela M, et al. Altered fecal microbiota in paediatric inflammatory bowel disease. J Crohns Colitis 2015;9(12):1088–95. [DOI] [PubMed] [Google Scholar]
- 23.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491(7422):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Z, Li P, Zhu J, et al. Multiple fresh fecal microbiota transplants induces and maintains clinical remission in Crohn's disease complicated with inflammatory mass. Sci Rep 2017;7(1):4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang T, Lu G, Zhao Z, et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: Clinical findings, animal studies and in vitro screening. Protein Cell 2020;11(4):251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fecal Microbiota Transplantation-standardization Study Group. Nanjing consensus on methodology of washed microbiota transplantation. Chin Med J 2020. [Epub ahead of print July 21, 2020.]. doi: 10.1097/CM9.0000000000000954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng Z, Xiang J, He Z, et al. Colonic transendoscopic enteral tubing: A novel way of transplanting fecal microbiota. Endosc Int Open 2016;4(6):E610–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long C, Yu Y, Cui B, et al. A novel quick transendoscopic enteral tubing in mid-gut: Technique and training with video. BMC Gastroenterol 2018;18(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice—A nationwide consecutive German cohort study. Aliment Pharmacol Ther 2016;43(10):1090–102. [DOI] [PubMed] [Google Scholar]
- 30.Aden K, Rehman A, Waschina S, et al. Metabolic functions of gut microbes associate with efficacy of tumor necrosis factor antagonists in patients with inflammatory bowel diseases. Gastroenterology 2019;157(5):1279–1292. [DOI] [PubMed] [Google Scholar]
- 31.Sood A, Mahajan R, Singh A, et al. Role of fecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: A pilot study. J Crohns Colitis 2019;13(10):1311–1317. [DOI] [PubMed] [Google Scholar]
- 32.Golinska E, Tomusiak A, Gosiewski T, et al. Virulence factors of Enterococcus strains isolated from patients with inflammatory bowel disease. World J Gastroenterol 2013;19(23):3562–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol 2002;160(6):2253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seishima J, Iida N, Kitamura K, et al. Gut-derived Enterococcus faecium from ulcerative colitis patients promotes colitis in a genetically susceptible mouse host. Genome Biol 2019;20(1):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kellingray L, Gall GL, Defernez M, et al. Microbial taxonomic and metabolic alterations during faecal microbiota transplantation to treat Clostridium difficile infection. J Infect 2018;77(2):107–18. [DOI] [PubMed] [Google Scholar]
- 36.Hyams JS, Davis Thomas S, Gotman N, et al. Clinical and biological predictors of response to standardised paediatric colitis therapy (PROTECT): A multicentre inception cohort study. Lancet 2019;393(10182):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cerqueira FM, Photenhauer AL, Pollet RM, et al. Starch digestion by gut bacteria: Crowdsourcing for carbs. Trends Microbiol 2020;28(2):95–108. [DOI] [PubMed] [Google Scholar]
- 38.Wopereis H, Sim K, Shaw A, et al. Intestinal microbiota in infants at high risk for allergy: Effects of prebiotics and role in eczema development. J Allergy Clin Immunol 2018;141(4):1334–42.e1335. [DOI] [PubMed] [Google Scholar]
- 39.Goh YJ, Barrangou R. Harnessing CRISPR-Cas systems for precision engineering of designer probiotic lactobacilli. Curr Opin Biotechnol 2019;56:163–71. [DOI] [PubMed] [Google Scholar]
- 40.Yilmaz B, Juillerat P, Øyås O, et al. Microbial network disturbances in relapsing refractory Crohn's disease. Nat Med 2019;25(2):323–336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.