Abstract
INTRODUCTION:
Patients with functional gastrointestinal disorders (FGIDs) are classified based on their gastrointestinal (GI) symptoms, without considering their frequent extra-GI symptoms. This study defined subgroups of patients using both GI and extra-GI symptoms and examined underlying mechanisms with fructose and lactose breath tests.
METHODS:
Latent class analysis defined distinct clusters of patients with FGID based on their long-term GI and extra-GI symptoms. Sensory and breath gas responses after fructose and lactose ingestion were compared across symptom clusters to investigate differences in sensory function and fermentation by intestinal microbiota.
RESULTS:
Six symptom clusters were identified in 2,083 patients with FGID. Clusters were characterized mainly by GI fermentation-type (cluster 1), allergy-like (cluster 2), intense pain-accentuated GI symptoms (cluster 3), central nervous system (cluster 4), musculoskeletal (cluster 5), and generalized extra-GI (cluster 6) symptoms. In the 68% of patients with complete breath tests, the areas under the curve of GI and central nervous system symptoms after fructose and lactose ingestion differed across the clusters (P < 0.001). The clusters with extensive long-term extra-GI symptoms had greater symptoms after the sugars and were predominantly women, with family or childhood allergy histories. Importantly, the areas under the curves of hydrogen and methane breath concentrations were similar (P > 0.05) across all symptom clusters. Rome III criteria did not distinguish between the symptom clusters.
DISCUSSION:
Patients with FGID fall into clusters defined extensively by extra-GI symptoms. Greater extra-GI symptoms are associated with evidence of generalized sensory hypersensitivity to sugar ingestion, unrelated to intestinal gas production. Possible underlying mechanisms include metabolites originating from the intestinal microbiota and somatization.
INTRODUCTION
Patients with chronic gastrointestinal (GI) symptoms but without pathology identifiable by routine clinical diagnostics are classified as having functional gastrointestinal disorders (FGIDs). FGIDs are divided into several subgroups based on characteristic symptoms, currently defined by the Rome criteria (1). However, not only is the overlap between individual FGID extensive, but the extra-GI symptoms frequently experienced by patients with FGID also widely overlap with the symptoms of non-GI functional syndromes, such as chronic pelvic pain, chronic fatigue syndrome, or fibromyalgia (2–6). The current classifications by symptoms result in fragmented treatment approaches and present a barrier to the elucidation of underlying disease mechanisms (5–7). Recognition of the overlap in syndromes has led to the proposition of overarching functional diagnoses, such as the bodily distress syndrome or somatic symptom disorder and the biopsychosocial model of irritable bowel syndrome (IBS) and other functional disorders (8–10). Subsequently, as an extension of this model, there has been considerable research into the “brain–gut–microbiome axis” and psychoneuroimmunology (11,12). Despite much discussion it remains unclear, whether the functional syndromes constitute one syndrome with varying individual expression or whether different underlying pathologies are lumped together by similar phenotypes.
Several population- or disease-based studies using complex statistical modeling of symptoms associated with functional syndromes have distinguished oligo- and multisymptomatic patient subgroups (3,4,8). The subgroups with wide multiorgan symptoms have different demographic, cognitive, quality-of-life, and healthcare utilization characteristics than those with few- or single-organ symptoms (4,13). It would be of practical significance to understand, whether the distinction between the subgroups is explained by different underlying mechanisms. Although the intensity of GI symptoms might be related to visceral hypersensitivity in FGID, the link between the range of GI and extra-GI symptoms and underlying mechanisms remains unclear (14). Intestinal and generalized somatic hypersensitivity and sensitization might be related to inflammatory and cognitive causes, among others. Indeed, endogenous pain modulation by the central nervous system (CNS) is abnormal in IBS and functional dyspepsia (FD), providing a plausible explanation for modified extra-GI sensory, autonomic, and GI neuronal function in FGID (15–20). To further examine the relationships between GI and extra-GI symptoms in FGID and underlying sensory function, sugar breath tests were used, which have been shown to evoke GI and extra-GI symptoms in patients with FGID (21). The breath tests also allow assessment of the fermentation characteristics of the intestinal microbiota.
The aims of this large, single-center study were to define distinct subgroups of patients with FGID based on their long-term GI and extra-GI symptoms using cluster analysis and to compare their fermentation and sensory responses after fructose and lactose stimulation. Furthermore, the relationships between the subgroups defined by cluster analysis and by Rome III criteria were assessed. We hypothesized that extra-GI symptoms would distinguish between groups of patients with FGID appearing similar based on their GI symptoms and that patients with more extra-GI symptoms would have greater symptoms provoked during the sugar breath tests.
METHODS
Successive male and female patients older than 18 years referred to our GI practice for investigation of FGID and without evidence of organic GI disease were included. Study inclusion was based on a general medical evaluation, hematology, biochemistry, and stool testing for calprotectin and pancreas elastase. In addition, upper and lower endoscopies with biopsies were required in patients older than 40 years or in patients with diarrhea or fecal blood. Parasite and bacterial stool cultures and abdominal ultrasound were performed as clinically indicated. Patients with a documented history or evidence of organic extra-GI disease classified as clinically significant by the investigator were similarly excluded. Clinically significant disorders were defined as multiorgan diseases potentially explaining GI symptoms, such as diabetes, neurological and rheumatological disorders, generalized ischemic disorders, and systemic infectious disease. However, patients with functional non-GI disorders, such as fibromyalgia, chronic pelvic pain, chronic fatigue syndrome, or idiopathic urticaria, were not excluded.
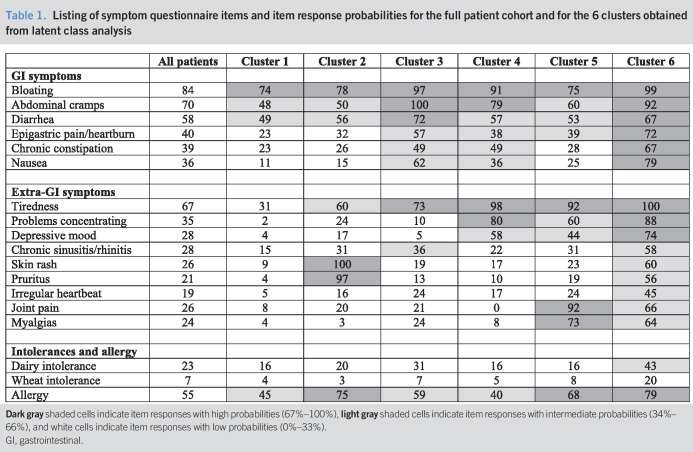
An experienced gastroenterologist (C.H.W.-S.) performed all the medical and dietary history taking and examinations. Patients completed a standardized questionnaire focusing on their long-term symptom profiles (Table 1), which included the specific Rome III questions for classification of GI symptoms into FGID groups and questions regarding childhood and family history, dietary intolerances or allergies, central nervous (mental fog, problems concentrating, fatigue, and depression), musculoskeletal (arthralgia and myalgia), dermatological (skin rash, pruritus, and urticaria), and cardiovascular system (arrhythmias, palpitations, and dizziness) symptoms (1,21–23). Some patients included in this study were part of a previous analysis with a different analytical focus, assessing predictive factors and the outcome of dietary modulation and the usefulness of breath tests (22,23).
Table 1.
Listing of symptom questionnaire items and item response probabilities for the full patient cohort and for the 6 clusters obtained from latent class analysis
Breath test protocol
All patients underwent fructose and lactose breath tests as previously described (21–23). No antibiotics, colonoscopy, or laxatives were permitted within 14 days, and patients consumed a defined low-saccharide diet for 24 hours before the tests. Patients arrived for testing in the morning after fasting overnight, without having smoked, chewed gum, or performed vigorous exercise for at least 4 hours. Breath tests were performed in a randomized sequence on 2 separate occasions at least 6 days apart. Breath samples were collected in sealed glass tubes (Quintron Instruments, Milwaukee, WI) before and hourly for 5 hours after ingestion of fructose 35 g or lactose 50 g dissolved in 300 mL tap water. These doses, along with the defining thresholds shown further, were chosen for consistency with previous studies (21–24). Hydrogen, methane, and CO2 concentrations were measured by BreathTracker SC (Quintron Instruments).
Malabsorption was defined as an increase >20 ppm in hydrogen or >10 ppm in methane concentrations over baseline (21,24). The following GI and extra-GI symptoms were scored hourly and rated for intensity (0 = none, 1 = mild, 2 = intense) concurrently with breath sampling: abdominal pain, arthralgia, bloating, borborygmi, diarrhea, diminished concentration, epigastric pain/heartburn, flatulence, fullness, headache, myalgia, nausea, and tiredness (21–23). Intolerance was defined as an increase of >2 over baseline in the aggregate GI symptom score, which is the sum of all 8 GI symptom intensities and has a maximum possible score of 16 (21–23).
Breath testing for evaluation of possible small intestinal bacterial overgrowth (SIBO) was not performed because of the inability of current breath tests to conclusively demonstrate SIBO. The study was performed in accordance with the tenets of the latest version of the Declaration of Helsinki. Cantonal Ethics Committee approval for anonymous data analysis was granted, and registration was retrospectively performed in ClinTrials.gov (NCT02085889).
Statistical analysis
The statistical analysis was performed by an author (S.S.O.) not involved in any clinical aspects of the study and in blinded fashion. Associations between numbers of GI and extra-GI symptoms were analyzed using Spearman correlation coefficient.
Cluster analysis and model development were performed as follows: long-term GI and extra-GI symptoms, allergies, and self-recognized food intolerances were assessed as being present or not. These binary data were subjected to latent class analysis to identify patient groups that had similar symptom profiles, with minimal within-group variation and maximum between-group variation. Latent class models were fitted consecutively from a 2-cluster to an 8-cluster solution (25). The optimal number of clusters to retain was determined by the following criteria: (i) goodness-of-fit statistics: Bayesian information criterion and Akaike Information Criterion; (ii) at least 5% of the total patient sample in each cluster; and (iii) face validity of the clusters for their clinical interpretability.
Patient characteristics and Rome III subgroups were compared across the latent class analysis-derived clusters using ANOVA and Fisher exact test. Aggregate GI and CNS symptoms (diminished concentration, headache, and tiredness) after sugar ingestion were grouped separately for association analysis with the long-term symptom clusters (21). The other extra-GI symptoms were not considered for association analysis with the long-term symptom clusters because they were shown to not be affected by sugar provocation (21). The areas under the curve (AUCs) of aggregate GI and CNS symptom scores and of the breath gas concentrations were compared between the patient clusters using Kruskal-Wallis and Dunn pairwise comparison tests with Bonferroni corrections for multiple comparisons. Multivariable logistic regression models were performed to assess the associations between Rome III subgroups and patient clusters adjusted for age and sex effects. These results were presented as odds ratios with 95% confidence intervals. A significance threshold of P < 0.05 was applied. The software package STATA version 15.1 (StataCorp LP, College Station, TX) was used.
RESULTS
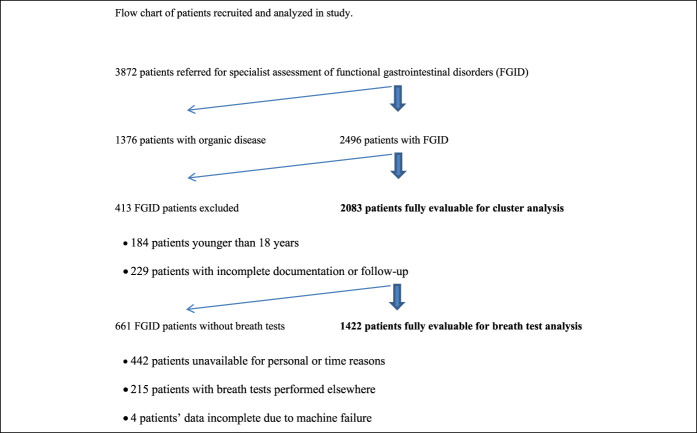
The recruitment flow of the 2,083 patients included is shown in Figure 1. Both fructose and lactose breath tests were performed and evaluable in 1,422 of patients (68% of complete cohort). Patients' characteristics are reported in Table 2.
Figure 1.
Flowchart of patients recruited and analyzed in study. FGID, functional gastrointestinal disorder.
Table 2.
Patient characteristics for the full patient cohort and the 6 clusters obtained from latent class analysis
Long-term symptom profiles
Prevalence estimates of the 18 long-term symptoms used for cluster analysis are reported in Table 1. Across all patients, the most prevalent GI symptoms were bloating (84%) and abdominal pain (70%), whereas the most frequent extra-GI symptoms were tiredness (67%) and concentration problems (35%); 55% of patients reported at least 1 allergy. The numbers of GI and extra-GI symptoms in individual patients were positively correlated (ρ = 0.35; P < 0.001).
Cluster description
The performance characteristics of the 7 different cluster solutions for the patients' long-term symptom profiles are shown in Table 1 (Supplementary Digital Content 3, http://links.lww.com/CTG/A323). The optimal model was a 6-cluster solution.
Item response probabilities for all patients and the 6 clusters are reported in Table 1. Face validity was apparent for all clusters, with most marked distinction in the distributions of extra-GI symptoms. GI fermentation-type symptoms, such as bloating, pain, stool changes, and epigastric pain/heartburn, were present across all 6 clusters, but almost exclusively characterized cluster 1 (35% of patients). Cluster 2 (6%) was distinguished by additional allergy-like symptoms, cluster 3 (19%) by intense pain-accentuated abdominal symptoms, cluster 4 (17%) by CNS symptoms, cluster 5 (10%) by musculoskeletal symptoms, and cluster 6 (14%) by generalized multiorgan symptoms. The prevalence of allergies was highest in clusters 2 (75%) and 6 (79%) and lowest in clusters 1 (45%) and 4 (40%). A radar plot of the main symptom and history groups across the clusters is shown Figure 2.
Figure 2.
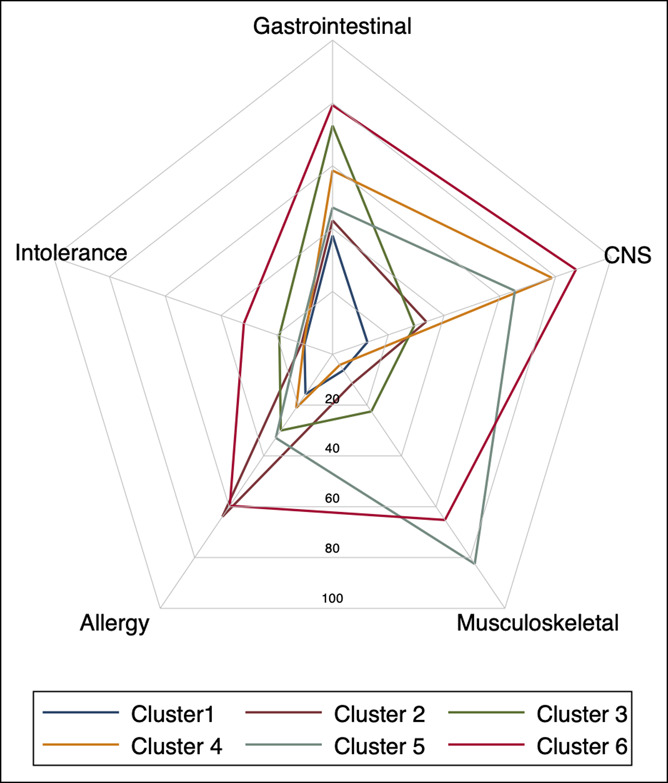
Radar plot of main symptom groups and self-diagnosed intolerances in the 6 long-term symptom clusters defined by latent class analysis. Data normalized to a 0–100 scale are shown. CNS, central nervous system.
Patient characteristics across clusters
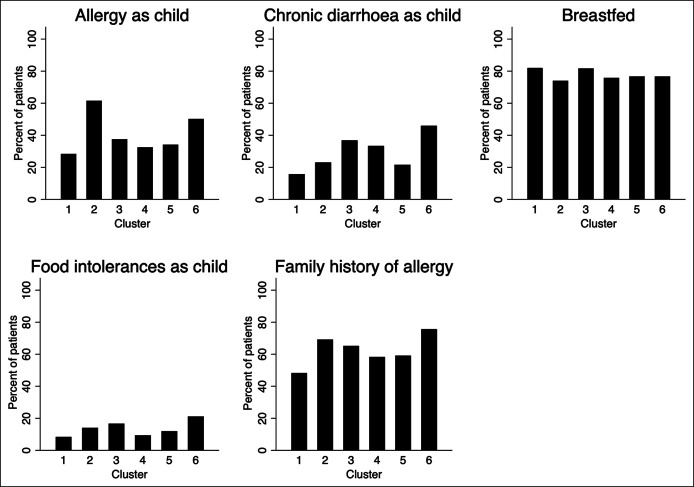
There were differences in age (P < 0.001) and sex (P < 0.001) distributions across the clusters (Table 2). Men constituted 42% of cluster 1 but only 15% of cluster 6. The distribution of ethnic subgroups was proportionate across clusters (P = 0.91). Childhood and family histories differed across the clusters. Childhood diarrhea, any food intolerance, and a family history of allergy were most common in cluster 6 (all P < 0.001), and a childhood history of any allergy was most common in clusters 2 (62%) and 6 (50%) and least common in cluster 1 (28%) (P < 0.001) (Figure 3). There were insignificant differences in the proportion of patients breast-fed across the clusters (P = 0.20).
Figure 3.
Pediatric and family histories in the 6 long-term symptom clusters defined by latent class analysis.
Associations of symptom clusters with Rome subgroups
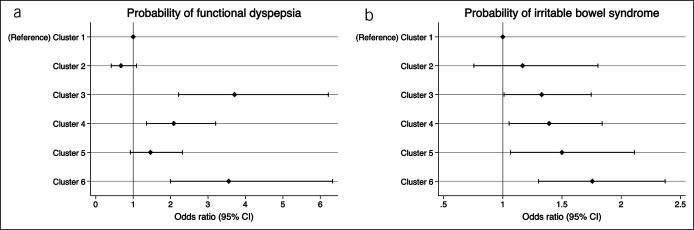
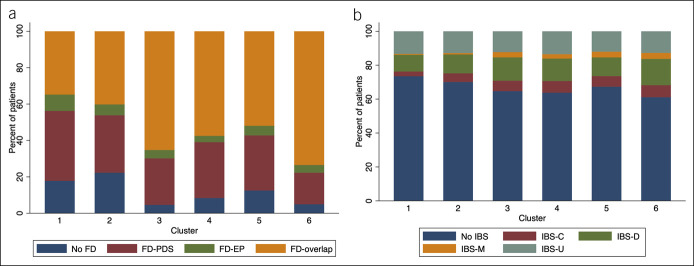
The prevalence of Rome III FGID subtypes differed numerically between the 6 symptom clusters (Table 2), ranging from 26% to 39% for IBS (P = 0.001) and from 78% to 95% for FD (P < 0.001). Multivariate analyses confirmed the significance and independence of these associations after adjustment for sex and age. Hence, a gradually increasing probability of IBS from cluster 1 (reference) to cluster 6 was observed (P < 0.001) (Figure 4). The associations with FD were less uniform and the highest probabilities of FD were observed for clusters 3 and 6 (P < 0.001) (Figure 4). The prevalence of the Rome subgroups of IBS and FD across the clusters is depicted in Figure 5. IBS-U (unsubtyped) and IBS-D (diarrhea) were similar and most common across all clusters. The FD-overlap subgroup was most common in clusters 3 to 6, whereas functional dyspepsia postprandial distress syndrome subtype was most common in cluster 1.
Figure 4.
Probabilities of being classified as having FD (upper panel) or IBS (lower panel) in the symptom clusters using Rome III criteria. Results of the age- and sex-adjusted analysis are shown. CI, confidence interval; FD, functional dyspepsia; IBS, irritable bowel syndrome; OR, odds ratio.
Figure 5.
Prevalence of FD (upper panel) and IBS (lower panel) Rome III subtypes across the 6 long-term symptom clusters defined by latent class analysis. FD-EP, functional dyspepsia epigastric pain subtype; FD-PDS, functional dyspepsia postprandial distress subtype; IBS, irritable bowel syndrome; IBS-C, irritable bowel syndrome constipation subtype; IBS-D, irritable bowel syndrome diarrhea subtype; IBS-M, irritable bowel syndrome mixed subtype; IBS-U, irritable bowel syndrome unsubtyped.
Associations between symptom clusters and sugar breath test responses
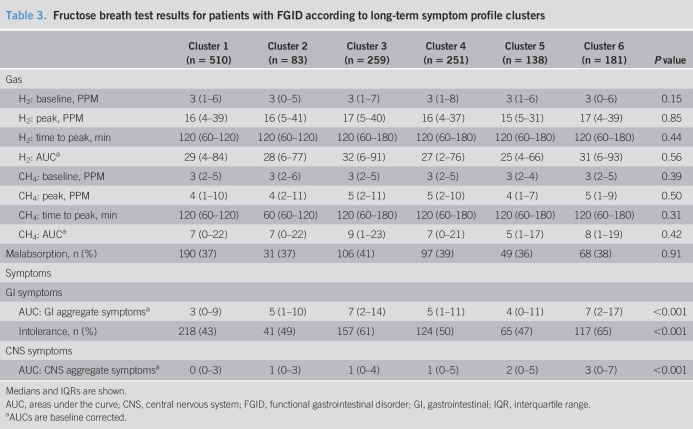
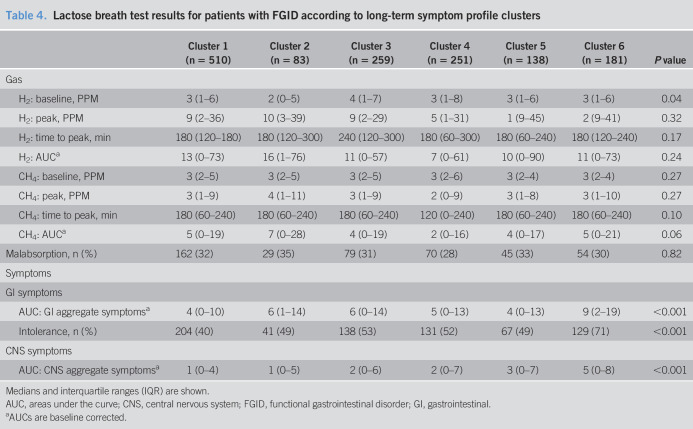
The AUCs of GI and CNS aggregate symptom scores after fructose and lactose differed across the 6 clusters (all P < 0.001). Symptom scores were consistently greatest in cluster 6 (Tables 3 and 4). The prevalence of intolerance increased from clusters 1 to 6 with fructose (43% and 65%, respectively, P < 0.001) and lactose (40% and 71%, respectively, P < 0.001). All 6 clusters showed similar hydrogen and methane breath concentration parameters and prevalence of malabsorption after fructose and lactose ingestion (all P > 0.05) (Tables 3 and 4). The radar plots of AUCs of the GI and CNS symptom and of hydrogen and methane breath concentrations are shown in Figure 1 (Supplementary Digital Content 1, http://links.lww.com/CTG/A321). Prevalence of malabsorption in the clusters ranged between 36% and 41% with fructose (P = 0.91) and between 28% and 35% with lactose (P = 0.82). Coexistence of intolerance and malabsorption decreased significantly from clusters 1 to 6 with fructose (64% and 48%, respectively, P < 0.001) and lactose (71% and 56%, respectively, P < 0.001) (see Figure 2 (Supplementary Digital Content 2, http://links.lww.com/CTG/A322).
Table 3.
Fructose breath test results for patients with FGID according to long-term symptom profile clusters
Table 4.
Lactose breath test results for patients with FGID according to long-term symptom profile clusters
DISCUSSION
Distinct clusters of long-term GI and extra-GI symptoms were defined in this large, single-center study of patients with FGID. The phenotypic clusters ranged from limited intestinal fermentation-like symptoms without (cluster 1) and with additional specific extra-GI (clusters 2, 4, and 5) or intense GI (cluster 3) symptoms, to whole-body symptoms (cluster 6). These clusters differed in their symptomatic responses to sugar ingestion and their demographic composition, but the exhaled gas concentrations during breath tests were similar across all clusters. The Rome III criteria did not distinguish reliably between the clusters of long-term symptoms, although the probabilities of IBS and FD varied numerically across the clusters.
Exploring symptom subgroups of functional GI disorders
Although GI symptoms are exclusively used in the current classification of subgroups of FGID, extra-GI symptoms are frequently present. Several patient clusters were distinguished by relatively organ-specific extra-GI symptoms and cluster 6 clearly presented as a broad multiorgan syndrome. Previous studies have demonstrated the importance of considering extra-GI symptoms in determining long-term outcomes in FGID (3–5,8). The evident overlap of FGID with other functional syndromes is the basis for the discussion on whether the various syndromes have 1 common cause or whether they represent an accumulation of different disease mechanisms in the same individual (3–8,26).
Sugar breath tests indicate different disease mechanisms
In this study, a well-known mechanistic approach was chosen to investigate the contributions of malabsorption, fermentation, and sensory hypersensitivity to the overlap of symptoms (23,24,27–29). We have recently shown that distinct GI and CNS symptoms are provoked during breath testing with fructose and lactose groups in patients with FGID (21). The intensity scores of the GI and CNS symptoms correlated significantly after ingestion of sugars, but only the severity of GI symptoms was correlated with breath hydrogen and methane concentrations (21). This suggests GI symptoms after the sugar ingestion are related to mechanical distension, whereas the CNS symptoms might be related to chemical sensory stimulation linked to fermentation products (21,30).
This study extended these results by examining the relationships between long-term symptoms and sensory and gas responses during breath tests. At the most basic level, a significant difference in the prevalence of fructose and lactose intolerance, as a marker of sensory response, was evident across the phenotypic clusters of FGID. Intolerance rates were greatest in the clusters with the most prominent long-term GI and extra-GI symptoms, corresponding to clusters 3 and 6. However, the prevalence of malabsorption after the sugars was similar across all symptom clusters. The increasing discordance between the gas and sensory responses from oligo-to multiorgan symptomatic clusters was confirmed using the more discriminatory analysis of the AUCs of gas and GI symptom responses. Therefore, although gas production and related intestinal distension might play a role in the background fermentation-like GI symptoms common to all symptom clusters, they do not explain the varying extra-GI symptoms or the additional GI symptoms peculiar to only some of the clusters.
Role of sensory sensitization
The greater sensory GI and CNS responses after sugar ingestion in the extensive multiorgan clusters are indicative of increased sensitivity, or sensitization, extending the evidence that mechanisms beyond malabsorption, osmotic, and fermentation effects underlie symptoms in FGID (21,30–33). Generalized sensitization, both inside and distant to the GI tract, might be related to neuronal stimulation by metabolites or cognitive amplification. Both mechanisms could be mediated through abnormal endogenous pain modulation, previously demonstrated to be abnormal in FGID with sensory testing and functional MRI studies (15,18–20). Intestinal hypersensitivity to distension and chemical stimulation as well as generalized hypersensitivity have been confirmed in subgroups of patients with IBS and FD (14,18,34–41). The data of this study suggest that sensitization to mechanical and chemical stimulation by gaseous or nongaseous pronociceptive metabolites of fructose and lactose are implicated in the wide spectrum of GI and extra-GI clinical symptoms in patients with FGID. The activity and composition of intestinal microbiota differ between IBS and controls, providing a potential mechanism for sensitization by the formation of proinflammatory and pronociceptive compounds, including monoamines, biogenic amines, short-chain fatty acids, neuropeptides, neurotransmitters, and especially advanced glycation end products (32,42,43). Several of the implicated pathways could explain multiorgan sensory hypersensitivity, including aberrant histaminergic and mast cell activation (31). Emerging data suggest changes in the intestinal microbiome also exist in FD (44).
Somatization is a further potential explanation for hypersensitivity in FGID (14,26,45,46). Somatization and sensory sensitization are associated, and distinction currently mainly relies on clinical judgment and questionnaires (47).
The relevance of early-life stressors and genetic factors in adult multiorgan symptoms in FGID is established and might explain the observed association with childhood chronic diarrhea, allergy or food intolerance, and a family history of allergy in cluster 6 compared with the fermentation-like cluster 1 (48).
Symptom cluster association with IBS, FD, and demographics
Symptom associations in FGID have mostly been studied in patients with IBS, which is largely defined by changes in bowel habit rather than a broad range of chronic GI symptoms. In this analysis, the clusters with a greater number of GI and extra-GI symptoms were more frequently associated with FD than with IBS. The Rome III FD-overlap subtype was most common in these clusters, reflecting both pain and postprandial aspects, rather than mainly postprandial fullness and early satiety. Exclusion of patients with FD from mechanistic studies of FGID, therefore, excludes patients with a greater degree of sensitization and constitutes a clear selection bias. There was no clear variation in Rome III IBS subtypes across the symptom clusters, implying that the Rome III criteria do not capture the underlying differences in patient clusters.
There was a difference in sex distribution between the symptom clusters, with more women in the multiorgan symptom clusters. Although the studied cohort was not population based, it is unlikely that this difference is random in such a large single-center study. This sex discrepancy against the background of a uniform prevalence of malabsorption across all symptom clusters indicates factors affecting sensory function or the metabolism of the intestinal microbiota are likely operative. Sex differences have been described for all of these factors, but their review is beyond the scope of this article (49,50).
Limitations
Specific psychological factors and further sensory tests were not additionally assessed in the mechanistic study of sensitization, omitting potentially relevant modulating factors in FGID. However, the influences of psychological factors and intestinal distension have been extensively reported in earlier studies (26,39,45). Furthermore, patients were not specifically questioned regarding recall of an acute infection at the onset of chronic symptoms. This would have been useful, even though such recall is often unreliable (51). SIBO is observed across a wide range of conditions and overlaps extensively with sugar intolerances. Its independent role in intolerances remains unclear, noninvasive testing is controversial, and consequently, we chose not to perform breath testing for SIBO in this study (52). Successive patients referred to our practice were enrolled in this study, conferring a selection bias, which would have been avoided with a population-based sample. However, the enriched recruitment seen in secondary- and tertiary-care patients enabled us to include a large number of male and female patients in the multiorgan symptom clusters, allowing a more balanced and in-depth analysis. This approach has been used successfully in previous studies (53). Furthermore, all patients were referred to and assessed by the same 2 staff in a single center, eliminating the substantial confounders inherent to the pooling of patients from different countries, centers, discontinuous recruitment periods, and using varying investigative protocols.
No separate discovery and validation cohorts were analyzed, raising the possibility of statistical overfit. Although validation in the same setting is not meaningful, application to a different setting and population might also lead to different results owing to the influence of endogenous and exogenous factors.
The questionnaire used in this study has not been externally validated but derives from the symptoms most commonly listed by our patients and quantified by a simple Likert scale. It has established high face validity across several studies and includes the standardized Rome classification questions (21–23). There is a clear need for a validated questionnaire for capture of extra-GI symptoms in patients with FGID.
CONCLUSIONS
Cluster analysis of long-term GI and extra-GI symptom profiles in more than 2,000 patients with FGID defined 6 distinct patient clusters. The cluster phenotypes varied from fermentation-like GI symptoms, to a combination of wider GI and focused extra-GI symptoms, to whole-body symptoms. Intestinal gas production or malabsorption of fructose or lactose measured by breath gas analysis did not provide an explanation for the different symptom clusters, in distinction to the sensory responses indicative of sensitization. Possible mechanisms for generalized hypersensitivity include metabolites originating from the intestinal microbiota and somatization. This study highlights the importance of the inclusion of extra-GI symptoms in the study and classifications of FGID.
CONFLICTS OF INTEREST
Guarantor of the article: Clive H. Wilder-Smith, MD.
Specific author contributions: Study design: C.H.W.-S.. Study execution: C.H.W.-S. and A.M.. Study analysis and interpretation: C.H.W.-S., A.M.D., and S.S.O., . Paper writing: C.H.W.-S., A.M.D., A.M., and S.S.O. All authors approved the final version of the manuscript.
Financial support: None to report.
Potential competing interests: None to report.
ClinTrials.gov: NCT02085889.
Study Highlights.
WHAT IS KNOWN
✓ Patients with FGIDs are classified according to their GI symptoms.
✓ Intestinal hypersensitivity exists in a substantial patient subset.
WHAT IS NEW HERE
✓ Patients with FGIDs fall into phenotypic clusters defined extensively by extra-GI symptoms.
✓ Patients with greater extra-GI symptoms have evidence of generalized sensory hypersensitivity to sugar ingestion unrelated to intestinal gas production.
TRANSLATIONAL IMPACT
✓ Extra-GI symptoms should be included in future studies and classifications of FGID.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at https://links.lww.com/CTG/A321, links.lww.com/CTG/A322, links.lww.com/CTG/A323
REFERENCES
- 1.Schmulson MJ, Drossman DA. What is new in Rome IV? J Neurogastroenterol Motil 2017;23:151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Wulffen M, Talley NJ, Hammer J, et al. Overlap of irritable bowel syndrome and functional dyspepsia in the clinical setting: Prevalence and risk factors. Dig Dis Sci 2019;64:480–6. [DOI] [PubMed] [Google Scholar]
- 3.Eliasen M, Schröder A, Fink P, et al. A step towards a new delimitation of functional somatic syndromes: A latent class analysis of symptoms in a population-based cohort study. J Psychosom Res 2018;108:102–17. [DOI] [PubMed] [Google Scholar]
- 4.Polster AV, Palsson OS, Törnblom H, et al. Subgroups of IBS patients are characterized by specific, reproducible profiles of GI and non-GI symptoms and report differences in healthcare utilization: A population-based study. Neurogastroenterol Motil 2019;31:e13483. [DOI] [PubMed] [Google Scholar]
- 5.Riedl A, Schmidtmann M, Stengel A, et al. Somatic comorbidities of irritable bowel syndrome: A systematic analysis. J Psychosom Res 2008;64:573–5. [DOI] [PubMed] [Google Scholar]
- 6.Sperber AD, Dekel R. Irritable bowel syndrome and co-morbid gastrointestinal and extra-gastrointestinal functional syndromes. J Neurogastroenterol Motil 2010;16:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology 2002;122:1140–56. [DOI] [PubMed] [Google Scholar]
- 8.Fink P, Schröder A. One single diagnosis, bodily distress syndrome, succeeded to capture 10 diagnostic categories of functional somatic syndromes and somatoform disorders. J Psychosom Res 2010;68:415–26. [DOI] [PubMed] [Google Scholar]
- 9.van der Feltz-Cornelis CM, Hoedeman R, Keuter EJ, et al. Presentation of the multidisciplinary guideline medically unexplained physical symptoms (MUPS) and somatoform disorder in The Netherlands: Disease management according to risk profiles. J Psychosom Res 2012;72:168–9. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka Y, Kanazawa M, Fukudo S, et al. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil 2011;17:131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am 2017;46:77–89. [DOI] [PubMed] [Google Scholar]
- 12.Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers 2016;2:16014–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandvik PO, Lydersen S, Farup PG. Prevalence, comorbidity and impact of irritable bowel syndrome in Norway. Scand J Gastroenterol 2006;41:650–6. [DOI] [PubMed] [Google Scholar]
- 14.Simren M, Törnblom H, Palsson OS, et al. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders. Gut 2018;67:255–62. [DOI] [PubMed] [Google Scholar]
- 15.Wilder-Smith CH. The balancing act: Endogenous modulation of pain in functional gastrointestinal disorders. Gut 2011;60:1589–99. [DOI] [PubMed] [Google Scholar]
- 16.Wilder-Smith CH, Li X, Shen L, et al. Dysfunctional endogenous pain modulation in patients with functional dyspepsia. Neurogastroenterol Motil 2014;26:489–98. [DOI] [PubMed] [Google Scholar]
- 17.Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroenterol 2007;13:3699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song GH, Venkatraman V, Ho KY, et al. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain 2006;126:79–90. [DOI] [PubMed] [Google Scholar]
- 19.Wilder-Smith CH, Schindler D, Lovblad K, et al. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 2004;53:1595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albusoda A, Ruffle JK, Friis KA, et al. Systematic review with meta-analysis: Conditioned pain modulation in patients with the irritable bowel syndrome. Aliment Pharmacol Ther 2018;48:797–806. [DOI] [PubMed] [Google Scholar]
- 21.Wilder-Smith CH, Olesen SS, Materna A, et al. Fermentable sugar ingestion, gas production, and gastrointestinal and central nervous system symptoms in patients with functional disorders. Gastroenterology 2018;155:1034–44. [DOI] [PubMed] [Google Scholar]
- 22.Wilder-Smith CH, Materna A, Wermelinger C, et al. Fructose and lactose intolerance and malabsorption testing: The relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther 2013;37:1074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilder-Smith CH, Olesen SS, Materna A, et al. Predictors of response to a low-FODMAP diet in patients with functional gastrointestinal disorders and lactose or fructose intolerance. Aliment Pharmacol Ther 2017;45:1094–106. [DOI] [PubMed] [Google Scholar]
- 24.Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: The North American Consensus. Am J Gastroenterol 2017;112:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stynes S, Konstantinou K, Ogollah R, et al. Novel approach to characterising individuals with low back-related leg pain: Cluster identification with latent class analysis and 12-month follow-up. Pain 2018;159:728–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Oudenhove L, Crowell MD, Drossman DA, et al. Biopsychosocial aspects of functional gastrointestinal disorders. Gastroenterology 2016;150:1355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao CK, Tuck CJ. The clinical value of breath hydrogen testing. J Gastroenterol Hepatol 2017;32(Suppl 1):20–2. [DOI] [PubMed] [Google Scholar]
- 28.Braden B. Methods and functions: Breath tests. Best Pract Res Clin Gastroenterol 2009;23:337–52. [DOI] [PubMed] [Google Scholar]
- 29.Wilder-Smith CH, Olesen SS, Materna A, et al. Repeatability and effect of blinding of fructose breath tests in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2019;31:e13497. [DOI] [PubMed] [Google Scholar]
- 30.Campbell AK, Matthews SB, Vassel N, et al. Bacterial metabolic “toxins”: A new mechanism for lactose and food intolerance, and irritable bowel syndrome. Toxicology 2010;278:268–76. [DOI] [PubMed] [Google Scholar]
- 31.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut 2016;65:155–68. [DOI] [PubMed] [Google Scholar]
- 32.Quigley EMM. The gut-brain axis and the microbiome: Clues to pathophysiology and opportunities for novel management strategies in irritable bowel syndrome (IBS). Clin Med 2018;7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enck P, Azpiroz F, Boeckxstaens G, et al. Functional dyspepsia. Nat Rev Dis Primers 2017;3:17081–8. [DOI] [PubMed] [Google Scholar]
- 34.Feng B, La JH, Schwartz ES, et al. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012;302:G1085–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammer J. Identification of individuals with functional dyspepsia with a simple, minimally invasive test: A single center cohort study of the oral capsaicin test. Am J Gastroenterol 2018;113:584–92. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Cao Y, Wong RK, et al. Visceral and somatic sensory function in functional dyspepsia. Neurogastroenterol Motil 2013;25:246–53. [DOI] [PubMed] [Google Scholar]
- 37.Bouin M, Plourde V, Boivin M, et al. Rectal distention testing in patients with irritable bowel syndrome: Sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology 2002;122:1771–7. [DOI] [PubMed] [Google Scholar]
- 38.Price DD, Craggs JG, Zhou Q, et al. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: Evidence from human psychophysics, animal models, and neuroimaging. Neuroimage 2009;47:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simrén M, Törnblom H, Palsson OS, et al. Cumulative effects of psychologic distress, visceral hypersensitivity, and abnormal transit on patient-reported outcomes in irritable bowel syndrome. Gastroenterology 2019;157:391–402. [DOI] [PubMed] [Google Scholar]
- 40.Stabell N, Stubhaug A, Flægstad T, et al. Increased pain sensitivity among adults reporting irritable bowel syndrome symptoms in a large population-based study. Pain 2013;154:385–92. [DOI] [PubMed] [Google Scholar]
- 41.Piché M, Arsenault M, Poitras P, et al. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain 2010;148:49–58. [DOI] [PubMed] [Google Scholar]
- 42.Rea K, O'Mahony SM, Dinan TG, et al. The role of the gastrointestinal microbiota in visceral pain. Handb Exp Pharmacol 2017;239:269–87. [DOI] [PubMed] [Google Scholar]
- 43.Shankar V, Homer D, Rigsbee L, et al. The networks of human gut microbe-metabolite associations are different between health and irritable bowel syndrome. ISME J 2015;9:1899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhong L, Shanahan ER, Raj A, et al. Dyspepsia and the microbiome: Time to focus on the small intestine. Gut 2017;66:1168–9. [DOI] [PubMed] [Google Scholar]
- 45.Grinsvall C, Törnblom H, Tack J, et al. Relationships between psychological state, abuse, somatization and visceral pain sensitivity in irritable bowel syndrome. United Eur Gastroenterol J 2018;6:300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clauwaert N, Jones MP, Holvoet L, et al. Associations between gastric sensorimotor function, depression, somatization, and symptom-based subgroups in functional gastroduodenal disorders: Are all symptoms equal? Neurogastroenterol Motil 2012;24:1088–e565. [DOI] [PubMed] [Google Scholar]
- 47.Croicu C, Chwastiak L, Katon W. Approach to the patient with multiple somatic symptoms. Med Clin North Am 2014;98:1079–95. [DOI] [PubMed] [Google Scholar]
- 48.Greenwood-Van Meerveld B, Johnson AC. Stress-induced chronic visceral pain of gastrointestinal origin. Front Syst Neurosci 2017;11:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, et al. Intestinal microbiota is influenced by gender and body mass index. PLoS One 2016;11:e0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Racine M, Tousignant-Laflamme Y, Kloda LA, et al. A systematic literature review of 10 years of research on sex/gender and experimental pain perception—Part 1 and part 2: Are there really differences between women and men? Pain 2012;153:602–35. [DOI] [PubMed] [Google Scholar]
- 51.Barbara G, Grover M, Bercik P, et al. Rome foundation working team report on post-infection irritable bowel syndrome. Gastroenterology 2019;156:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quigley EMM. The spectrum of small intestinal bacterial overgrowth (SIBO). Curr Gastroenterol Rep 2019;21:3–9. [DOI] [PubMed] [Google Scholar]
- 53.Van Oudenhove L, Holvoet L, Vandenberghe J, et al. Do we have an alternative for the Rome III gastroduodenal symptom-based subgroups in functional gastroduodenal disorders? A cluster analysis approach. Neurogastroenterol Motil 2011;23:730–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.