INTRODUCTION:
Hepatitis C virus (HCV) infection has been related to increased cardiovascular (CV) risk. The aim of this study was to analyze the impact of sustained virological response (SVR) on endothelial dysfunction and subclinical atherosclerosis in patients with hepatitis C virus treated with direct-acting antiviral agents.
METHODS:
A total of 114 patients were prospectively recruited and underwent CV risk assessment including (i) endothelial dysfunction determined through laser Doppler flowmetry and (ii) subclinical atherosclerosis, elucidated by the ankle-brachial index (ABI). Atherogenic lipid profile (total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides); markers of oxidative stress (oxidized low-density lipoprotein antibodies [OLAbs]), soluble markers of adhesion (vascular cell adhesion molecule [VCAM], e-selectin, and soluble markers of angiogenesis; and vascular endothelial growth factor, endothelial [EMPs] and platelet [PMPs] apoptotic microparticles, and cell-free DNA [cfDNA]) were measured. All determinations were performed at baseline, 12 weeks (SVR time), and 1 year after treatment.
RESULTS:
In patients with endothelial dysfunction, area of hyperemia improved after virus clearance (P = 0.013) and was related to significant decrease in VCAM, e-selectin (P < 0.001), and cfDNA (P = 0.017) and to increased OLAb levels (P = 0.001). In patients with subclinical atherosclerosis at baseline, a significantly improved ABI was seen after HCV clearance (P < 0.001). Levels of both EMPs and PMPs also decreased after SVR and at follow-up (P = 0.006 and P = 0.002, respectively).
DISCUSSION:
HCV clearance improved not only liver function but also endothelial dysfunction and subclinical atherosclerosis promoted by decrease in levels of VCAM, e-selectin, cfDNA, and PMPs and EMPs.
INTRODUCTION
Hepatitis C virus (HCV) has been associated with liver disease progression and proatherogenic conditions such as chronic subclinical inflammation, endothelial dysfunction, and, consequently, increased cardiovascular (CV) risk (1,2). The pathogenic mechanisms connecting infection and the CV risk has not been completely understood yet. HCV promoted steatosis in the liver, leading to an upregulation of inflammatory biomarkers that were able to generate oxidative stress, favor insulin resistance and liver damage, and promote an accumulation of fatty acids deposits that could thicken the artery wall and promote the formation of atheromatous plaques (3–5). Furthermore, HCV can colonize and replicate within carotid plaques, causing vascular inflammation generating damage in the vascular wall that might explain the increase of arterial stiffness observed in patients with HCV (6,7). Finally, HCV infection has been found to be an independent predictor factor for increased coronary atherosclerosis (8).
Endothelial dysfunction is the earliest event in the atherosclerosis process (9). Factors implicated in endothelial dysfunction include oxidative stress, inflammation, cell-free DNA (cfDNA) and adhesion molecules, and angiogenic factors. Area of hyperemia by Doppler flowmetry is a surrogate biomarker of degree of endothelial dysfunction (10). Subclinical atherosclerosis can be assessed by ankle-brachial index (ABI), a useful diagnostic tool strongly associated with increased risk of myocardial infarction and CV death when lower than 0.9 (11). Circulating microparticles (MPs) including platelet and endothelial-derived microparticles are also novel biomarkers associated with endothelial dysfunction and subclinical atherosclerosis (12,13); cfDNA is shown to be associated with endothelial damage (14) and has also been associated with CV risk among others (15,16). Finally, sustained virological response (SVR) in HCV reduces mortality rate, hepatic decompensation, and hepatocellular carcinoma appearance (17) together with improvement in metabolic disturbances such as insulin resistance (18) and type-2 diabetes mellitus (T2DM) (19–21). The aim of this study was to evaluate the effect of SVR with direct-acting antiviral agents (DAAs) on CV risk and its underlying mechanism in patients with HCV.
MATERIAL AND METHODS
Selection of patients
This was a multicentric and prospective study that included 114 patients with age range between 18 and 75 years treated for chronic HCV with DAA. CV risk factors must be in a stable situation during, at least, 3 months (including therapy for them). Liver fibrosis was determined by transient elastography. Exclusion criteria included (i) patients with HCV receiving interferon-based treatment; (ii) coinfection with HIV or hepatitis B virus infection or evidence of other liver disease (autoimmune, cholestatic, or metabolic disease); (iii) hepatocellular carcinoma; (iv) patients who received a liver transplant or were included in the waiting list before the enrolment; (v) symptomatic cryoglobulinemic vasculitis or vasculitis due to any other cause (cryoglobulins in the absence of clinical vasculitis were not exclusion criteria); (vi) end-stage renal failure; (vii) history of CV disease (stroke, peripheral artery disease, aortic aneurism, congestive heart failure, coronary artery disease, and nonfatal myocardial infarction). The study was conducted in accordance with the ethical guidelines described in the Declaration of Helsinki and the international conference on harmonization guidelines for good clinical practice. Each center that participated in the study received the approval of their Ethics Committee and ensured that participants understood the study and provided written informed consent. All data (including clinical, demographic, and analytical findings) were coded and deposited in a database.
Definition of the aims
The main aim of the study was to evaluate changes in CV risk after SVR in patients with HCV. To address a potential mechanism implicated on these changes, we evaluated the endothelial dysfunction determined through laser Doppler flowmetry and subclinical atherosclerosis, elucidated by the ABI.
Atherogenic lipid profile (total cholesterol [TC], low-density lipoprotein [LDL] cholesterol, high-density lipoprotein cholesterol, and triglycerides); markers of oxidative stress (oxidized low-density lipoprotein antibodies [OLAbs]), soluble markers of adhesion (vascular cell adhesion molecule [VCAM], e-selectin, soluble markers of angiogenesis; vascular endothelial growth factor [VEGF], endothelial [EMPs] and platelet [PMPs] apoptotic microparticles, and cfDNA) were also determined.
Clinical assessments
Clinical assessment was performed by structured questionnaires for vascular risk factors (hypertension, T2DM, dyslipidemia, alcohol consumption, and smoking status), physical activity, current medication, and viral infection history (duration of viral infection, previous antiviral treatment, viral load, and genotype). Metabolic syndrome was defined by the presence of at least 3 risk factors as previously described by the Adult Treatment Panel III Guidelines (22). SVR was defined as undetectable HCV-RNA levels 12 weeks after the end of therapy. Clinical characteristics, including age, sex, and other anthropometric measures along with biochemical measurements, were measured at baseline, 12 weeks, and 1 year after end of therapy. Fasting (overnight) blood samples were drawn from participants into tubes containing clot activator and separator gel (SST), K3-EDTA, and heparin-Vacutainer TM. The index of insulin resistance (Homeostatic Model Assessment for Insulin Resistance) was calculated using the formula: glucose (mg/dL) × insulin (μU/mL)/405, and all the biochemical parameters were determined in the central laboratory.
Assessment of endothelial dysfunction and subclinical atherosclerosis
Endothelial dysfunction.
A laser-Doppler linear Periflux System 5000 (Perimed SA, Järfälla, Sweden) was used to measure the flow-mediated dilatation, as previously described (10). The vascular response to 5-minute reactive hyperemia was used for the assessment of endothelium-dependent flow-mediated dilatation. Subjects with an area of hyperemia lower than 860 PU (arbitrary perfusion units) (82% sensibility/97% specificity) were considered pathological and suffering from endothelial dysfunction (10) (see Supplementary Methods, Supplementary Digital Content 1, http://links.lww.com/CTG/A334).
Subclinical atherosclerosis
The ABI was determined automatically (WatchBP Office, Microlife AG). Therefore, individuals with an ABI <0.90 in either leg were classified as having subclinical atherosclerosis based on the scientific statements from European society of Hypertension (23) (see Supplementary Methods, Supplementary Digital Content 1, http://links.lww.com/CTG/A334).
Soluble markers.
Blood samples were centrifuged at 3,000 rpm for 10 minutes within 2 hours from collection, and serum was immediately stored in aliquots at −80 °C until analysis as follows: (i) levels of VEGF were analyzed in serum using the VEGF ELISA Kit (R&D Systems, Minneapolis, MN); (ii) OLAb levels were determined using Human OLAb ELISA kit (MyBioSource, San Diego, CA); (iii) VCAM and e-selectin were assessed using VCAM and e-selectin ELISA kits, respectively (R&D Systems). Each determination was performed in duplicate, and the mean values were analyzed.
Microparticles were analyzed by flow cytometry in duplicate (BD LSRFortessa; BD Biosciences, San Diego, CA). Heparin-buffered blood samples were obtained and processed immediately at 13,000g for 20 minutes, and plasma was stored at −80 °C. Platelet-poor plasma (50 μL) was incubated for 20 min with monoclonal antibodies (CD31 FITC, CD41 Pacific Blue, and Annexin V PE). Data represent the mean of 2 independent experiments (see Supplementary Methods, Supplementary Digital Content 1, http://links.lww.com/CTG/A334). cfDNA levels were quantified in 400 μL of serum samples by quantitative PCR using a LightCycler 480 Real-Time PCR instrument (Roche Diagnostics, Basel, Switzerland) (see Supplementary Methods, Supplementary Digital Content 1, http://links.lww.com/CTG/A334).
Statistical analysis
Categorical variables are expressed as absolute frequencies and percentages (n, %). Noncategorical variables are presented using the mean plus their SEM or SD or the median and the interquartile range 25th–75th. The Kolmogorov-Smirnov tests were used to assess whether variables follow a normal distribution. The effect of variables with a normal distribution on viral eradication was performed using a 1-way ANOVA; Bonferroni tests were applied to confirm differences between groups when a significant main effect was found (P < 0.05). Friedman tests were used to compare variables with a nonnormal distribution. Variables associated with the dependent variables at the univariate analysis (P < 0.05) were included in the multivariate regression models. Binary logistic regression models were used to assess the relationship between: (i) pathological area of hyperemia, (ii) pathological ABI, and (ii) changes during the follow-up (Δ). Data were compared and analyzed with SPSS software (version 22.0; SPSS, Chicago, IL), and GraphPad Prism (version 6.0; GraphPad Software, La Jolla, CA) was used for graphics. For all analyses, P < 0.05 was considered statistically significant.
RESULTS
Baseline patient characteristics
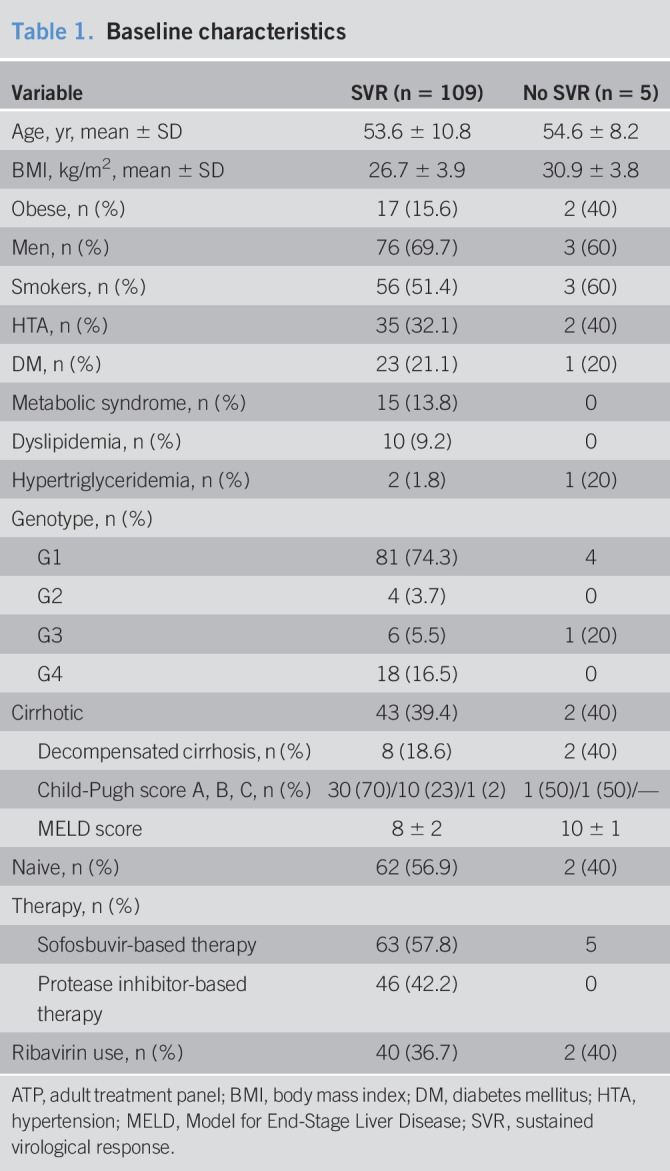
A total of 114 patients completed the study. Baseline demographic and clinical features of the overall cohort are presented in Table 1. Of the 114 patients, 109 (95.6%) patients achieved SVR after treatment, whereas viral relapse occurred in 5 patients. Ten of 45 patients with liver cirrhosis reported previous episodes of decompensation.
Table 1.
Baseline characteristics
In addition, 2 patients (1.83%) developed hepatocellular carcinoma during follow-up. No CV events were reported during the study period. Patients were classified according to baseline ABI, and 40% (n = 37) showed subclinical atherosclerosis; on the other side, 58 (50.8%) patients had endothelial dysfunction at baseline.
As shown in Table S1 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A334), several patients with CV risk factors were under treatment. No changes were reported regarding T2DM treatment, only 3 patients with hypertension changed treatment during the follow-up period, and patients with dyslipidemia discontinued statins therapy during antiarrhythmic drugs treatment according to the EASL recommendation on Treatment of Hepatitis C 2018 (24).
Lipid profile at baseline was compared between patients with compensated and decompensated cirrhosis, and no significant differences were found (see Table S2, Supplementary Digital Content 1, http://links.lww.com/CTG/A334).
Anthropometric data were analyzed at 3 time points: pretreatment, 12 weeks, and 1 year follow-up. There were no differences in the average of body mass index (P = 0.165), abdominal circumference (P = 0.152), and systolic (P = 0.458) and diastolic blood pressure (DBP; P = 0.878) levels (see Table S2, Supplementary Digital Content 1, http://links.lww.com/CTG/A334). According to alcohol consumption or smoking status, no changes were reported during the follow-up period. Transient elastography in kilopascals showed significant improvement in liver stiffness at 1-year follow-up (13.9 ± 9.5 vs 9.9 ± 5.8 kPa; P < 0.001).
Clinical assessments
Changes in biochemical profile are presented in Table 2. Liver function tests including aspartate aminotransferase, alanine aminotransferase, and γ-glutamyl transferase (GGT) normalized at 12 weeks after therapy and remained in the normal range during follow-up (P < 0.001). Platelet levels increased significantly (P < 0.001), and bilirubin levels decreased (P < 0.001). Creatinine levels slightly increased after viral eradication. Delta of creatinine increase was significantly higher in patients with cirrhosis (0.31 ± 0.85 vs 0.05 ± 0.12; P = 0.017). As expected, a significant increase was found in levels of TC, LDL-cholesterol (cLDL), lipoprotein A and apolipoprotein B (P < 0.001). In addition, high-density lipoprotein cholesterol (P = 0.082) and apolipoprotein A (P = 0.997) remained stable after viral eradication. Fasting glucose levels and hemoglobin A1 did not change over time but Homeostatic Model Assessment for Insulin Resistance decreased significantly (3.9 ± 2.6 vs 3.3 ± 2.3; P = 0.022).
Table 2.
Biochemical data of patients who achieved SVR (n = 109)
Endothelial dysfunction
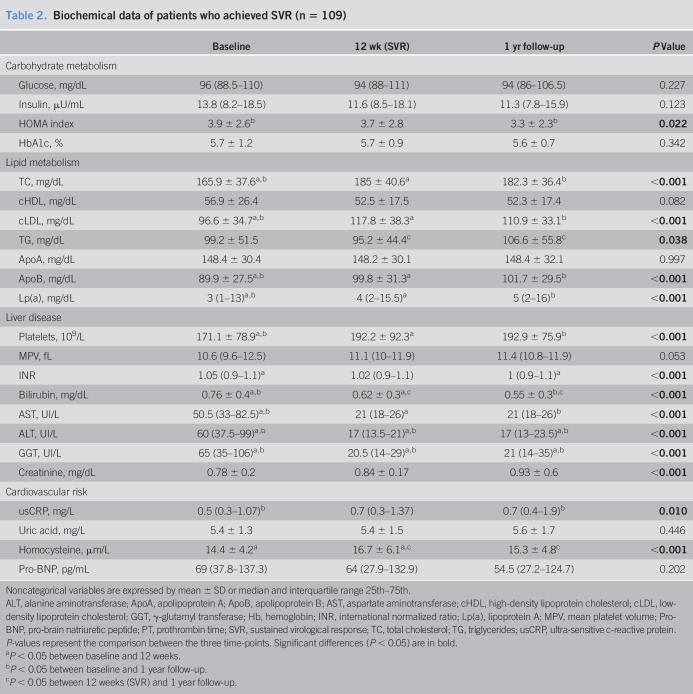
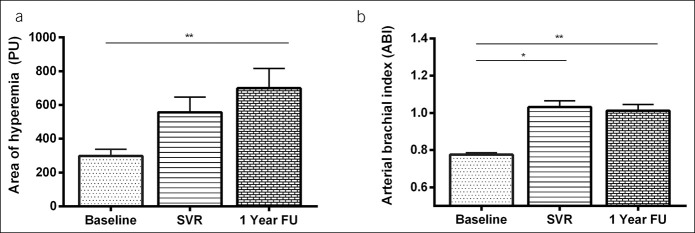
Area of hyperemia significantly improved in patients with SVR and endothelial dysfunction at baseline (n = 58) (297.7 ± 39.34, 556.8 ± 90.4, and 699.6 ± 116.4, P = 0.013) (Figure 1a). In univariate analysis, changes in systolic blood pressure, DBP, and GGT levels were found to be associated with an improvement of endothelial dysfunction (Table 3). In multivariate analysis, Δ DBP (0.932 [0.881–0.983; P = 0.010]) and Δ GGT (0.992 [0.985–0.998; P = 0.040]) remained independently linked to endothelial dysfunction improvement. In patients without endothelial dysfunction at baseline, the area of hyperemia significantly decreased (1786.5 ± 142.1 vs 1,206.1.8 ± 189.4 vs 1,398.1 ± 208.8 PU, P < 0.001); however, it remained at nonpathological levels during the follow-up. As shown in Figure 2, levels of endothelium-derived adhesion molecule markers, VCAM (Figure 2a) and e-selectin (Figure 2b), significantly decreased after SVR in the overall cohort and remained stable at 1-year follow-up (VCAM: 1763.1 ± 123.7 vs 1,215.5 ± 97.1 vs 1,141.2 ± 93.8 mg/mL; P < 0.001; e-selectin: 62.38 ± 2.27 vs 43.7 ± 1.64 vs 40.1 ± 1.6 pg/mL; P < 0.001). However, levels of VEGF did not change significantly (P = 0.079) after viral eradication (Figure 2c). As shown in Figure 2d, successful treatment promoted an increase in the concentration of antibody against oxidized LDL (379.5 ± 21.3 vs 415.6 ± 26.4 vs 483.9 ± 254, P = 0.01).
Figure 1.
Changes in area of hyperemia (a) and arterial brachial index (b) in patients who archived SVR, with pathological values at baseline. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. FU, follow-up; SVR, sustained virological response.
Table 3.
Univariable and multivariable analyses regarding improvement of endothelial dysfunction during follow-up period
Figure 2.
Levels of VCAM, e-selectin, VEGF, and OLAb in patients who achieved SVR before therapy (basal), at week 12 after treatment, and at 1 year follow-up. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. FU, follow-up; OLAb, oxidized low-density lipoprotein antibody; SVR, sustained virological response; VCAM, vascular cell adhesion molecule; VGEF, vascular endothelial growth factor.
Subclinical atherosclerosis
As shown in Figure 1b, in patients with pathological ABI at baseline (n = 37), SVR was associated with a significant increase in ABI at 12 weeks and 1-year follow-up (0.77 ± 0.01 vs 1.03 ± 0.03 vs 1.01 ± 0.03, P < 0.001), but no changes were seen in nonresponders (P = 0.165) or in patients without subclinical atherosclerosis (P = 0.949). Moreover, OLAb concentration increased at 1-year follow-up more in patients with subclinical atherosclerosis at baseline than in those without (Δ OLAb = 186.6 ± 50.1 vs 60.4 ± 34.7; P = 0.042) (Figure 3).
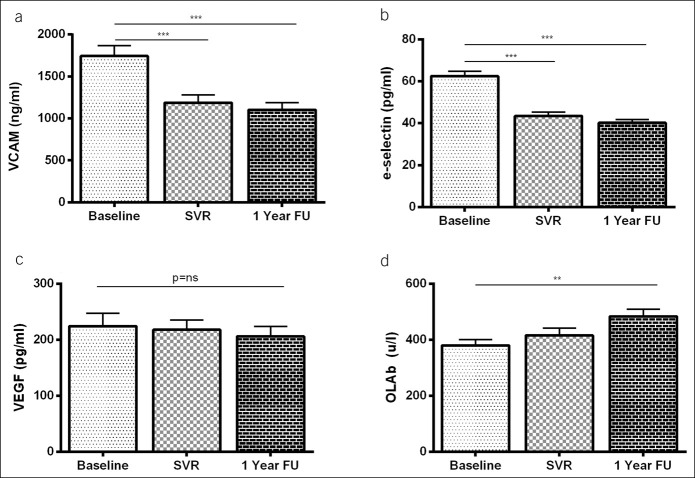
Figure 3.
Levels of EMPs and PMPs before therapy (baseline), at week 12 after treatment, and at 1 year follow-up in patients who achieved SVR. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01. EMP, endothelial apoptotic microparticle; FU, follow-up; PMP, platelet apoptotic microparticle; SVR, sustained virological response.
EMPs levels significantly decreased after viral eradication (524.9 ± 210.6 vs 255.3 ± 55.6 vs 247.2 ± 85.5 U/μL; P = 0.006), and PMPs decreased significantly in those patients with subclinical atherosclerosis (7,409.4 ± 3,320 vs 2,372.4 ± 732 vs 3,967.7 ± 1,486.2 U/μL; P = 0.007) (fold change in Figure 4c). Finally, in patients with endothelial dysfunction, levels of cfDNA decreased significantly at 1 year after therapy (567.5 ± 86.4 vs 395.1 ± 52.6 ng/mL; P = 0.017). No significant changes in biochemical, adhesion, oxidative stress and MPs were found in nonresponders (see Table S4, Supplementary Digital Content 1, http://links.lww.com/CTG/A334).
Figure 4.
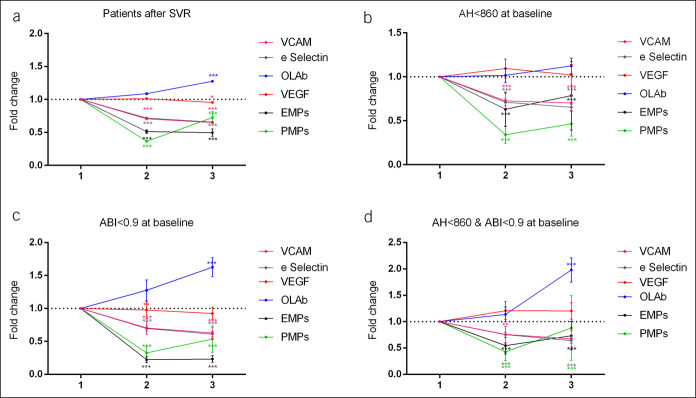
Fold change at 12 weeks and 1-year follow-up in soluble markers about baseline in all patients who achieved SVR (a), in patients with endothelial dysfunction at baseline (b), in patients with atherosclerosis at baseline (c), and both of them (d). Data are expressed as means ± SEM. *P < 0.05, **P < 0.01. ABI, arterial brachial index; AH, area of hyperemia; EMP, endothelial apoptotic microparticle; OLAb, oxidized low-density lipoprotein antibody; PMP, platelet apoptotic microparticle; SVR, sustained virological response; VCAM, vascular cell adhesion molecule; VGEF, vascular endothelial growth factor.
DISCUSSION
HCV clearance by DAAs improved endothelial dysfunction and subclinical atherosclerosis through a decrease of VCAM, e-selectin, cfDNA, and apoptotic MPs (EMPs and PMPs) in patients suffering from baseline endothelial dysfunction and/or subclinical atherosclerosis. The improvement of endothelial dysfunction and subclinical atherosclerosis was irrespective of fibrosis status, genotype, or DAAs regimen.
Interestingly, lipid levels changed to a more atherogenic profile with increase in TC, cLDL, apolipoprotein B, and lipoprotein-A, whereas endothelial dysfunction and subclinical atherosclerosis improved. This observation could be explained, at least, partly by: (i) lipid profiles that remain within the normal range; (ii) HCV ability to hijack lipid metabolism has given a “false” protective atherogenic profile that is restored after viral clearance; and (iii) the impact that HCV has on other CV risk factors such as insulin resistance (i.e., the improvement in the HOMA index) (25–29).
HCV has been associated with endothelial dysfunction and atherosclerosis, being an independent risk factor for stroke, coronary heart disease, and CV disease-related mortality, beyond liver-related outcomes (3,5). DAA-based therapy resulted in an increase of SVR rates improving overall and liver-related patients-reported outcomes (30). Indeed, the impact of SVR on endothelial dysfunction (31,32), subclinical vascular damage (28), and CV outcomes (33,34) after DAA-based therapy has been reported. We also observed a significant increase in the area of hyperemia after therapy related to decrease in GGT and DBP, 2 variables strongly related to metabolic syndrome and CV risk (35,36). Mechanisms by which HCV promote CV risk include chronic inflammation, endothelial dysfunction, and direct invasion of arterial wall (8). DAA viral clearance was also linked to an improvement in surrogate markers of endothelial dysfunction, including VCAM and e-selectin. In addition, we showed a decrease in cfDNA that was related to systemic inflammation status and subsequently, with endothelial dysfunction, and it was also associated with a worse CV risk profile (15,37). Indeed, cfDNA level changes correlated with an improvement in endothelial dysfunction (38).
EMPs are new biomarkers able to monitor endothelial dysfunction. EMPs modulate inflammation, coagulation leukocyte adhesion, and recruitment contributing to plaque growth with consecutive altered vasomotion (39). These data support the fact that the decrease in EMPs observed after DAA therapy might be directly involved in the improvement of inflammation and endothelial dysfunction (30). HCV has also been associated with subclinical atherosclerosis, with an increased risk of both carotid (40) and coronary (8) atherosclerosis. HCV eradication by DDAs improves carotid atherosclerosis in patients with advanced fibrosis/compensated cirrhosis (41), whereas other studies report opposed results (42). The association of an ABI < 0.9 with an increased rate of CV events and overall mortality is well established (43). Specifically, we found that SVR with DAA leads to an improvement in subclinical atherosclerosis in those patients with peripheral arterial disease, whereas no changes were observed in nonresponders or in patients without altered baseline ABI. These results are in agreement with previous studies that show a significant reduction in the prevalence of carotid thickening from baseline to end of follow-up after DAA treatment in patients with advanced fibrosis (39). A decrease in the concentration of OLAb was seen. Despite the controversial role of these antibodies, several studies have suggested an atheroprotective role of OLAb through the recognition of oxidized-specific epitopes (44,45). In this sense, these antibodies might be involved in binding and clearing proinflammatory OxLDL. Our data suggest that these changes in the OLAb concentration, which reflect a decrease of oxidative stress, could play a role in the improvement of endothelial dysfunction and arterial-brachial index seen in patients with subclinical atherosclerosis. Elevated concentration of PMPs participates in processes such as intercellular communication and atherosclerosis (46). In fact, an increase of PMPs has been observed in conditions associated with platelet activation such as hypertension (47), diabetes mellitus (48), and obesity (49), suggesting a correlation between PMPs levels and the severity of the vascular disease. In this study, we showed a significant decrease in PMPs levels after SVR that is more significant in those patients with a pathological ABI at baseline, supporting the mechanism by which the virus improves CV risk.
We analyzed changes on surrogate markers of portal hypertension (platelets count) and liver dysfunction (Model for End-Stage Liver Disease score) after DAA therapy in the cohort of patients with liver cirrhosis. Improvement of platelet count or Model for End-Stage Liver Disease score did not correlate with changes on MPs, cfDNA, adhesion molecules, or parameters of endothelial dysfunction and subclinical atherosclerosis (see Table S5, Supplementary Digital Content 1, http://links.lww.com/CTG/A334). Sofosbuvir-based regimen was associated with an increase in creatinine levels, although within normal range, with no related higher CV risk, which support the fact that viral eradication is more protective than its effect of increase creatinine levels or the atherogenic lipid profile.
Limitations of this study include a short length of follow-up that does not allow us to determine the impact that SVR has on the prevention of CV events and a low CV risk score that does not allow us to demonstrate whether SVR has an impact on CV events. Another limitation is the lack of an age- and body mass index-matched control group, but the rapidly evolving therapy against HCV in Spain did not allow us to include a control group arm.
In conclusions, successful DAA treatment in patients with HCV without history of CV disease, improves not only liver function but also CV risk through an improvement of endothelial dysfunction, which is stronger in those patients with worse state at baseline and independent of genotype and fibrosis stage of the disease.
CONFLICTS OF INTEREST
Guarantor of the article: Manuel Romero-Gómez, MD, PhD.
Specific author contributions: Study design: M.R.-G., J.A. Drafting the manuscript: M.R.-G. Statistical analyses and interpretation: R.M.-H., J.A., M.R.-G. Data acquisition and critical revision of the manuscript: R.M.-H., J.A., R.M., A.G.-G., A.R., H.C.M., R.G.-D., S.G., R.M.-V., D.M.-M., I.S.C., P.S., M.R.-G.
Financial support: Postdoctoral fellowship from the Spanish Government (Juan de la Cierva fellowship FJC1-2014-21675). Instituto de Salud Carlos III Project GLD17/00203.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ We searched PubMed for studies using the search terms hepatitis C virus (HCV) and “direct antiviral treatment” and “cardiovascular risk” or “endothelial dysfunction” or “atherosclerosis”. We found a previous study with 12667 patients with HCV under direct-acting antiviral agents therapy where they reported that patients who achieved sustained virological response (SVR) had lower risk of cardiovascular events.
✓ Endothelial dysfunction has been assessed in two different studies. One of them, which included 16 patients, showed a significant improvement in endothelial dysfunction 12 weeks after HCV eradication.
✓ The other one, conducted with 20 patients and a follow-up of 24 weeks, reached a similar conclusion along with a reduction of soluble adhesion molecules. Atherosclerosis improvement after SVR remains controversial.
✓ Although 1 study that included 182 patients with severe fibrosis with or without additional metabolic risk factors showed a significant improvement, 2 recent studies, one with 85 patients and another with 48, showed opposed results and concluded that low-density lipoprotein cholesterol and carotid intimamedia thickness were exacerbated after SVR.
WHAT IS NEW HERE
✓ To our knowledge, this is the first study where the estimation of cardiovascular risk includes, in addition to the evaluation of the standard lipid profile, measurements for endothelial dysfunction (laser Doppler flowmetry), atherosclerosis (arterial brachial index), soluble markers for adhesion, oxidative stress and angiogenesis, and novel biomarkers that have never been assessed in patients with HCV, such as cfDNA and apoptotic microparticles.
✓ Our study counts with a significant number of patients with different degrees of liver disease (114) and has evaluated all the previously described measurements at three different time points asfollows: baseline, 12 weeks, and 1-year follow-up.
✓ We found that successful direct-acting antiviral agents treatment in patients with HCV without a previous history of cardiovascular disease not only improves liver function but also cardiovascular risk through an improvement in adhesion markers, oxidative stress, and apoptotic microparticles, which leads to amelioration of endothelial dysfunction and atherosclerosis.
✓ The improvement was stronger in those patients with worse state at baseline and was independent of genotype and fibrosis stage disease.
TRANSLATIONAL IMPACT
✓ Cardiovascular risk is one of the worst outcomes for patients with HCV. New direct-acting antivirals agents clearly improve liver function, but their effect on cardiovascular risk remains controversial and needs to be further clarified.
✓ Our study, that evaluates cardiovascular risk through a wide spectrum of different determinations, concludes that both, endothelial dysfunction and atherosclerosis, ameliorate in patients with HCV, especially in those with worst values at baseline.
✓ Our study provides further evidence to support a positive effect of the treatment over cardiovascular-related morbimortality.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A334
REFERENCES
- 1.Moucari R, Asselah T, Cazals-Hatem D, et al. Insulin resistance in chronic hepatitis C: Association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology 2008;134:416–23. [DOI] [PubMed] [Google Scholar]
- 2.Ampuero J, Romero-Gomez M. Assessing cardiovascular risk in hepatitis C: An unmet need. World J Hepatol 2015;7:2214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roed T, Kristoffersen US, Knudsen A, et al. Increased prevalence of coronary artery disease risk markers in patients with chronic hepatitis C-a cross-sectional study. Vasc Health Risk Manag 2014;10:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramirez Alvarado MM, Sanchez Roitz C. Tumor necrosis factor-alpha, insulin resistance, the lipoprotein metabolism and obesity in humans [in Spanish]. Nutr Hosp 2012;27:1751–7. [DOI] [PubMed] [Google Scholar]
- 5.Rafieian-Kopaei M, Setorki M, Doudi M. Atherosclerosis: Process, indicators, risk factors and new hopes. Int J Prev Med 2014;5:927–46. [PMC free article] [PubMed] [Google Scholar]
- 6.Shizaka Y, Ishizaka N, Takahashi E, et al. Association between hepatitis C virus core protein and carotid atherosclerosis. Circ J 2003;67:26–30. [DOI] [PubMed] [Google Scholar]
- 7.Tomiyama H, Arai T, Hirose K, et al. Hepatitis C virus seropositivity, but not hepatitis B virus carrier or seropositivity, associated with increased pulse wave velocity. Atherosclerosis 2003;166:401–3. [DOI] [PubMed] [Google Scholar]
- 8.Alyan O, Kacmaz F, Ozdemir O, et al. Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score system. Cir J 2008;72:1960–5. [DOI] [PubMed] [Google Scholar]
- 9.Uonala M, Viikari JS, Laitinen T, et al. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: The cardiovascular risk in young Finns study. Circulation 2004;110:2918–23. [DOI] [PubMed] [Google Scholar]
- 10.Stiefel P, Moreno-Luna R, Vallejo-Vaz AJ, et al. Which parameter is better to define endothelial dysfunction in a test of postocclusive hyperemia measured by laser-Doppler flowmetry? Coron Artery Dis 2012;23:57–61. [DOI] [PubMed] [Google Scholar]
- 11.Wild SH, Byrne CD, Smith FB, et al. Low ankle-brachial pressure index predicts increased risk of cardiovascular disease independent of the metabolic syndrome and conventional cardiovascular risk factors in the Edinburgh Artery Study. Diabetes care 2006;29:637–42. [DOI] [PubMed] [Google Scholar]
- 12.Rautou PE, Vion AC, Amabile N, et al. Microparticles, vascular function, and atherothrombosis. Circ Res 2011;109:593–606. [DOI] [PubMed] [Google Scholar]
- 13.Lukasik M, Rozalski M, Luzak B, et al. Enhanced platelet-derived microparticle formation is associated with carotid atherosclerosis in convalescent stroke patients. Platelets 2013;24:63–70. [DOI] [PubMed] [Google Scholar]
- 14.Paunel-Gorgulu A, Wacker M, El Aita M, et al. cfDNA correlates with endothelial damage after cardiac surgery with prolonged cardiopulmonary bypass and amplifies NETosis in an intracellular TLR9-independent manner. Sci Rep 2017;7:17421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler A, Zangemeister-Wittke U, Stahel RA. Circulating DNA: A new diagnostic gold mine? Cancer Treat Rev 2002;28:255–71. [DOI] [PubMed] [Google Scholar]
- 16.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011;11:426–37. [DOI] [PubMed] [Google Scholar]
- 17.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012;308:2584–93. [DOI] [PubMed] [Google Scholar]
- 18.Romero-Gomez M, Del Mar Viloria M, Andrade RJ, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology 2005;128:636–41. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Gomez M, Fernandez-Rodriguez CM, Andrade RJ, et al. Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol 2008;48:721–7. [DOI] [PubMed] [Google Scholar]
- 20.Majumdar A, Kitson MT, Roberts SK. Systematic review: Current concepts and challenges for the direct-acting antiviral era in hepatitis C cirrhosis. Aliment Pharmacol Ther 2016;43:1276–92. [DOI] [PubMed] [Google Scholar]
- 21.Butt AA, Yan P, Shuaib A, et al. Direct-acting antiviral therapy for HCV infection is associated with a reduced risk of cardiovascular disease events. Gastroenterology 2019;156:987–96. [DOI] [PubMed] [Google Scholar]
- 22.Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97. [DOI] [PubMed] [Google Scholar]
- 23.Mansia G, De Backer G, Dominiczak A, et al. 2007 ESH-ESC guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European society of hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Press 2007;16:135–232. [DOI] [PubMed] [Google Scholar]
- 24.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018;69:461–511. [DOI] [PubMed] [Google Scholar]
- 25.Romero-Gomez M, Rojas A. Sofosbuvir modulates the intimate relationship between hepatitis C virus and lipids. Hepatology 2015;61:744–7. [DOI] [PubMed] [Google Scholar]
- 26.Lacerda GS, Medeiros T, Rosario NFD, et al. Exploring lipid and apolipoprotein levels in chronic hepatitis C patients according to their response to antiviral treatment. Clin Biochem 2018;60:17–23. [DOI] [PubMed] [Google Scholar]
- 27.Gitto S, Cicero AFG, Loggi E, et al. Worsening of serum lipid profile after direct acting antiviral treatment. Ann Hepatol 2018;17:64–75. [DOI] [PubMed] [Google Scholar]
- 28.Hum J, Jou JH, Green PK, et al. Improvement in glycemic control of type 2 diabetes after successful treatment of hepatitis C virus. Diabetes care 2017;40:1173–80. [DOI] [PubMed] [Google Scholar]
- 29.Novo G, Macaione F, Giannitrapani L, et al. Subclinical cardiovascular damage in patients with HCV cirrhosis before and after treatment with direct antiviral agents: A prospective study. Aliment Pharmacol Ther 2018;48:740–9. [DOI] [PubMed] [Google Scholar]
- 30.Tada T, Kumada T, Toyoda H, et al. Improvement of liver stiffness in patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. J Gastroenterol Hepatol 2017;32:1982–8. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt FP, Zimmermann T, Wenz T, et al. Interferon- and ribavirin-free therapy with new direct acting antivirals (DAA) for chronic hepatitis C improves vascular endothelial function. Int J Cardiol 2018;271:296–300. [DOI] [PubMed] [Google Scholar]
- 32.Di Minno MND, Ambrosino P, Buonomo AR, et al. Direct-acting antivirals improve endothelial function in patients with chronic hepatitis: A prospective cohort study. Intern Emerg Med 2020;15(2):263–71. [DOI] [PubMed] [Google Scholar]
- 33.Ahon P, Bourcier V, Layese R, et al. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology 2017;152:142–56.e2. [DOI] [PubMed] [Google Scholar]
- 34.Cacoub P. Hepatitis C virus infection, a new modifiable cardiovascular risk factor. Gastroenterology 2019;156:862–4. [DOI] [PubMed] [Google Scholar]
- 35.Singer AW, Osinusi A, Brainard DM, et al. Risk of cardiovascular and cerebrovascular events in hepatitis C patients following completion of direct-acting antiviral therapy: A retrospective cohort study [Abstract]. J Hepatol 2017;66:S282–3. [Google Scholar]
- 36.Yeboah J, Crouse JR, Hsu FC, et al. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 2007;115:2390–7. [DOI] [PubMed] [Google Scholar]
- 37.Jylhava J, Lehtimaki T, Jula A, et al. Circulating cell-free DNA is associated with cardiometabolic risk factors: The Health 2000 Survey. Atherosclerosis 2014;233:268–71. [DOI] [PubMed] [Google Scholar]
- 38.Munoz-Hernandez R, Vallejo-Vaz AJ, Sanchez Armengol A, et al. Obstructive sleep apnoea syndrome, endothelial function and markers of endothelialization. Changes after CPAP. PLoS One 2015;10:e0122091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loyer X, Vion AC, Tedgui A, et al. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ Res 2014;114:345–53. [DOI] [PubMed] [Google Scholar]
- 40.Petta S, Torres D, Fazio G, et al. Carotid atherosclerosis and chronic hepatitis C: A prospective study of risk associations. Hepatology 2012;55:1317–23. [DOI] [PubMed] [Google Scholar]
- 41.Petta S, Adinolfi LE, Fracanzani AL, et al. Hepatitis C virus eradication by direct-acting antiviral agents improves carotid atherosclerosis in patients with severe liver fibrosis. J Hepatol 2018;69:18–24. [DOI] [PubMed] [Google Scholar]
- 42.Ichikawa T, Miyaaki H, Miuma S, et al. Carotid intima-media thickness and small dense low-density lipoprotein cholesterol increase after one year of treatment with direct-acting antivirals in patients with hepatitis C virus infection. Intern Med 2019;58:1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992;326:381–6. [DOI] [PubMed] [Google Scholar]
- 44.Monaco C, Crea F, Niccoli G, et al. Autoantibodies against oxidized low density lipoproteins in patients with stable angina, unstable angina or peripheral vascular disease; pathophysiological implications. Eur Heart J 2001;22:1572–7. [DOI] [PubMed] [Google Scholar]
- 45.Shoji T, Nishizawa Y, Fukumoto M, et al. Inverse relationship between circulating oxidized low density lipoprotein (oxLDL) and anti-oxLDL antibody levels in healthy subjects. Atherosclerosis 2000;148:171–7. [DOI] [PubMed] [Google Scholar]
- 46.Barry OP, Pratico D, Lawson JA, et al. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Invest 1997;99:2118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Preston RA, Jy W, Jimenez JJ, et al. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 2003;41:211–7. [DOI] [PubMed] [Google Scholar]
- 48.Sabatier F, Darmon P, Hugel B, et al. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes 2002;51:2840–5. [DOI] [PubMed] [Google Scholar]
- 49.Stepanian A, Bourguignat L, Hennou S, et al. Microparticle increase in severe obesity: Not related to metabolic syndrome and unchanged after massive weight loss. Obesity 2013;21:2236–43. [DOI] [PubMed] [Google Scholar]