Abstract
INTRODUCTION:
With the emergence of multidrug-resistant organisms, the efficacy of antibiotic prophylaxis to prevent spontaneous bacterial peritonitis (SBP) has been debated. The aim of this study was to assess factors impacting effectiveness of SBP prophylaxis.
METHODS:
We searched PubMed, Embase, and the Cochrane Registry from inception to May 2019 to identify randomized controlled trials of patients with liver cirrhosis that assessed SBP occurrence/recurrence during antibiotic prophylaxis with the common antibiotic agents. Network meta-analysis was performed, pooling data with regard to incidence rate ratios (IRRs) of SBP, death, or extraperitoneal infections.
RESULTS:
Overall, 1,626 patients in 12 randomized controlled trials were included. During primary prophylaxis, the incidence rate of SBP and death in the norfloxacin-treated patients was 0.117 and 0.438 per patient-year, respectively, and IRRs of placebo vs norfloxacin were significantly higher (IRR 5.35, 95% confidence interval 1.99–14.38, P = 0.0009 for SBP and IRR 2.04, 95% confidence interval 1.20–3.44, P = 0.008 for death). The efficacy of norfloxacin to prevent SBP, but not death, decreased over time (annual percent change from 1992 to 2015 8.2%, P = 0.019), The positive treatment effect was lower in studies including patients with increased ascites protein (P = 0.021) or exceedingly high serum bilirubin (P = 0.012) levels. Norfloxacin was not superior to other antibiotics. The incidence rate of SBP was 2.5-fold higher in patients treated with norfloxacin as secondary compared with primary prophylaxis. No significant differences between treatment designs were observed in secondary prophylaxis.
DISCUSSION:
Norfloxacin remained superior to placebo in preventing SBP, yet the efficacy to prevent SBP, not death, decreased over time. Further studies to understand this phenomenon are urgently needed.
INTRODUCTION
Spontaneous bacterial peritonitis (SBP) constitutes the most frequent infection in patients with liver cirrhosis and is burdened with high morbidity and mortality (1–4). Causative bacterial organisms are believed to be enteric and to translocate through the intestinal barrier (5). Since the 1990s, antibiotic gut decontamination strategies have been advocated and proven to be effective to reduce the risk of de novo SBP, recurrence of SBP, and overall mortality (6–8). Current guidelines recommend primary antibiotic prophylaxis of SBP in cirrhotic patients with ascites and high-risk profiles and secondary antibiotic prophylaxis after resolved SBP (9,10).
Most interestingly, although earlier studies reported a significantly reduced occurrence or recurrence of SBP due to antibiotic prophylaxis (6,8), a more recently performed randomized controlled trial observed an increased survival in patients receiving norfloxacin prophylaxis only in those with low ascites fluid protein concentration (11).
Over the last decades, several studies observed a growing number of infections in patients with cirrhosis caused by Gram-positive bacteria and multidrug-resistant organisms (MDROs) (12–14). Most recently, the CANONIC study group reported a high and increasing prevalence of MDROs in culture-positive infections in patients with decompensated cirrhosis all over Europe (15). We have recently shown in a prospective cohort study that the effectiveness of SBP prophylaxis might be critically reduced in an environment of highly prevalent MDROs (16).
A recent Cochrane analysis including 23 studies could not find a significant benefit of antibiotic prophylaxis to prevent death or serious complications. However, many studies were of low quality, resulting in sparse data and high risk of bias (17). Thus, the type of antibiotic prophylaxis remains controversial (18).
Recent analyses reported on the safety and efficacy of rifaximin, with few randomized controlled trials available (19,20). Earlier reviews focused on studies with quinolone prophylaxis from the 1990s to the early 2000s (21,22). Yet, the development of prophylaxis efficacy over time and other factors influencing norfloxacin efficacy have not been investigated in detail. Therefore, with this systematic review and meta-analysis, we aimed to assess variables impacting the effectiveness of SBP prophylaxis and patient survival.
METHODS
This systematic review and meta-analysis was conducted applying standard methods according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (see Appendix Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A365) (23). The study and its protocol were registered in the PROSPERO register for systematic reviews (CRD42019140304).
Details on data sources and searches, study selection, inclusion/exclusion criteria, data extraction, and quality assessment can be found in Appendix Additional Methods Section, Supplementary Digital Content 1, http://links.lww.com/CTG/A365.
The primary outcome was to evaluate incidence rate ratios (IRRs) of SBP in patients with norfloxacin prophylaxis vs other prophylaxis or placebo and their change over time. In addition, IRRs of death and secondary extraperitoneal infections were evaluated.
Characteristics of individual studies were summarized as proportions for categorical variables and as averages for continuous variables. Data analysis used the R packages nlme, meta, and netmeta (R Foundation for Statistical Computing, Vienna, Austria; frequentist approach) for incidence rate meta-analysis and network meta-analysis of controlled clinical trials. Inverse variance and DerSimonian and Laird approaches were used. As follow-up intervals differed between studies, incidence rates per patient-year and IRRs of SBP, mortality, and extraperitoneal infections were calculated by using the given mean follow-up time and number of patients in each group. For comparison of treatments, norfloxacin, as the antibiotic of choice recommended in all guidelines at present, acted as reference treatment, and IRRs were compared with a fixed-effect network meta-analysis, whereas incidence rates were calculated using the random-effects model. Local consistency was checked by split network estimates to compare the contribution of direct and indirect evidence and to test for local inconsistency in network meta-analysis. Consistency analysis supported consistency between direct and indirect comparisons where available unless otherwise indicated. The network graph used nodes for the different treatments and illustrated the evidence of the direct treatment comparisons by thickness of the corresponding lines between the nodes. More detailed, the thickness of the lines is proportional to the inverse standard error of the fixed-effect models in direct comparison of the 2 treatments. Therefore, the line thickness does not only refer to the number of studies but also to the sample size of the respective studies.
Treatment effects were summarized as forest plot and a treatment-adjusted funnel plot to check for asymmetric treatment effects indicating publication bias. The influence of certain study characteristics such as middle year of population recruitment (e.g., trend over time), mean age, mean Child-Pugh score, mean bilirubin, mean albumin, mean creatinine, and mean ascites protein for different study groups was analyzed with a mixed-effect network meta-regression approach. All statistical tests were 2 sided and used a P value of α = 5%.
RESULTS
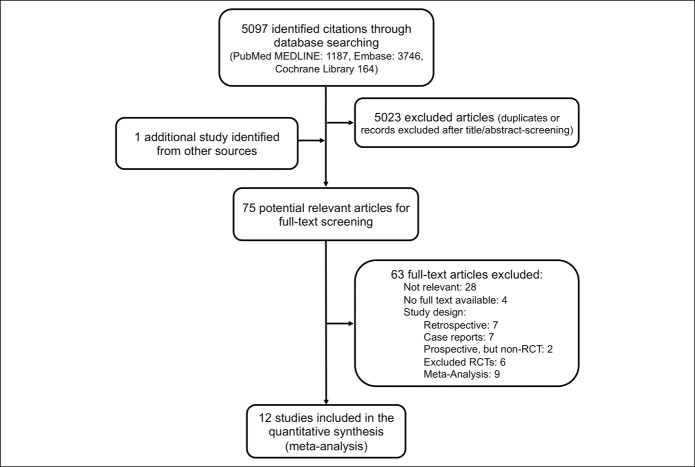
The systematic review identified 5,097 publications. One additional study was identified through reference search of relevant reviews and articles (11). After abstract screening and duplicate removal, 75 potential relevant articles were eligible for full-text screening, of those 20 studies were conducted prospectively, and 12 ultimately met our strict inclusion criteria and were included in the final synthesis (Figure 1).
Figure 1.
Summary of search results and study selection.
The characteristics of all prospective studies are summarized in Appendix Table 2, Supplementary Digital Content 1, http://links.lww.com/CTG/A365. Of those, 2 were not randomized (24,25), 3 did not provide separate data on patients with and without prior SBP (7,26,27), 1 did not provide data for patients while on prophylaxis (only 3 months after the end of prophylaxis) and no mean follow-up time was provided (28), and 3 studies did not use today's definition of SBP and data could not be retrieved otherwise (6,7,29). Detailed characteristics of the finally included 12 studies and their exclusion criteria are depicted in Appendix Tables 3 and 4, Supplementary Digital Content 1, http://links.lww.com/CTG/A365.
Overall, 1,626 patients (range 57–291 patients) were included in this study. Four studies were placebo controlled, and 1 tested an additional probiotic. All others compared 1 antibiotic against another antibiotic. Baseline patient's characteristics and prognostic factors were comparably distributed in the active and comparator groups across different trials.
Study quality question and study quality is reported in Appendix Tables 5 and 6, Supplementary Digital Content 1, http://links.lww.com/CTG/A365. All trials were rated as having good or fair quality.
Incidence of SBP in primary prophylaxis
Overall, 10 controlled clinical studies analyzing primary prophylaxis were included in this analysis. The network graph of the treatment comparisons is shown in Figure 2.
Figure 2.
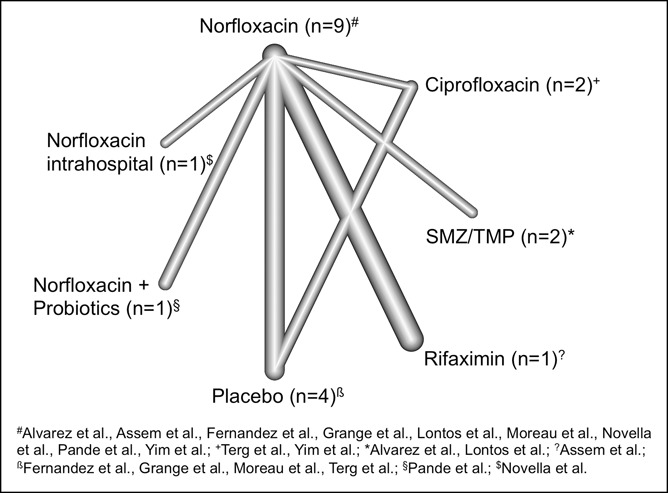
Network graph illustrating the direct treatment comparisons for primary prophylaxis. Evidence of the direct comparisons is illustrated by the thickness of the connecting lines. Note that evidence does not only refer to the number of studies but also to the sample size of the respective studies. SMZ/TMP, sulfamethoxazole/trimethoprim.
The incidence rate of SBP in the norfloxacin-treated patients was 0.117 per patient-year with a significant heterogeneity between the studies (I2 = 68.8%). The IRR of SBP between these prophylactic antibiotics was analyzed and illustrated in Figure 3a. Estimated heterogeneity was negligible between and within designs, and I2 was estimated nearly zero. Only the comparisons between placebo and norfloxacin (IRR 5.35, 95% confidence interval [CI] 1.99–14.38, P = 0.0009) and the comparisons between norfloxacin and exclusive in-hospital use of norfloxacin (IRR 16.14, 95% CI 2.11–123.37, P = 0.0074) were significant. IRR analysis favored a prophylaxis with rifaximin (IRR 0.55, 95% CI 0.23–1.32) or the combination of norfloxacin and probiotics (IRR 0.84, 95% CI 0.19–3.75) rather than a prophylaxis with norfloxacin, but the difference was not significant. Random-effects models revealed similar results in sensitivity analysis, and no asymmetry could be observed in the respective funnel plot (see Appendix Figure 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A365).
Figure 3.
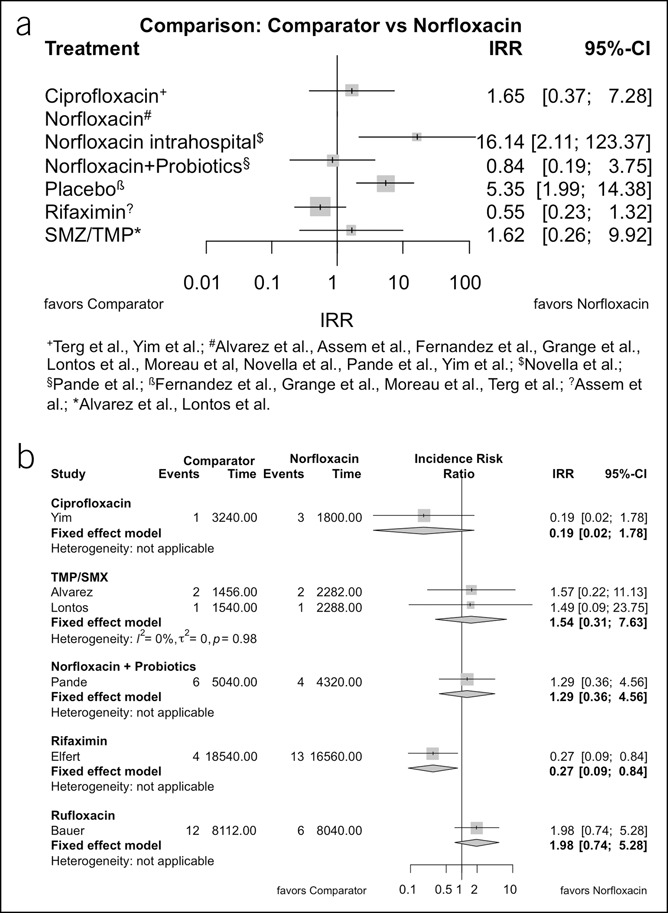
Forest plot illustrating estimated incidence rate ratios (IRRs) of norfloxacin with the respective antibiotic for spontaneous bacterial peritonitis prophylaxis from direct and indirect comparisons on a logarithmically scaled horizontal axis. (a) Results for primary prophylaxis. (b) Pairwise meta-analysis shows the results of a meta-analysis using subgroups for the different treatment comparators for secondary prophylaxis. CI, confidence interval. SMZ/TMP, sulfamethoxazole/trimethoprim.
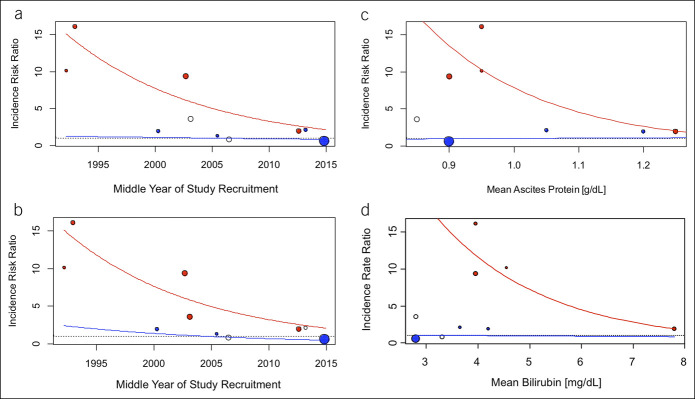
Network meta-regression was performed to analyze the effect of selected study characteristics on the IRR between norfloxacin and placebo or exclusive in-hospital norfloxacin. Here, IRRs for placebo vs norfloxacin significantly decreased over time from 15.35 in 1992 to 2.13 in 2015 (annual percent change 8.2%, P = 0.019), i.e., the positive treatment effect of norfloxacin decreased (Figure 4a, see Appendix Table 7, Supplementary Digital Content 1, http://links.lww.com/CTG/A365). Appendix Table 8, Supplementary Digital Content 1, http://links.lww.com/CTG/A365, depicts the IR of SBP in the 3 studies testing norfloxacin vs placebo, and Appendix Figure 2, Supplementary Digital Content 1, http://links.lww.com/CTG/A365, shows results for norfloxacin vs placebo and intrahospital norfloxacin vs placebo separately. Results showed a similar trend (P = 0.017) when time effect of treatment with fluoroquinolones was combined (placebo vs norfloxacin and ciprofloxacin, Figure 4b). Next, we analyzed additional factors influencing the IRR, i.e. norfloxacin prophylaxis efficacy: Here, the IRR decreased, i.e., the positive treatment effect of norfloxacin decreased, with increasing serum bilirubin (P = 0.012, Figure 4c) or increasing ascites protein (P = 0.021, Figure 4d), respectively. Interestingly, none of these clinical characteristics had a significant impact on IRRs between norfloxacin and other active treatments (excluding norfloxacin with probiotics and norfloxacin in-hospital treatment) or on IRRs between norfloxacin and other non-fluoroquinolones treatment (excluding also ciprofloxacin). Neither age, Child-Pugh score, albumin nor creatinine significantly impacted IRRs in a comparable analysis.
Figure 4.
Meta-regression plot of incidence rate ratios (IRRs) for spontaneous bacterial peritonitis in primary prophylaxis. Red points indicate the studies with estimated IRRs of placebo/norfloxacin intrahospital vs norfloxacin; blue points indicate the studies with estimated IRRs of other active treatments vs norfloxacin. Open circles indicate the studies with norfloxacin plus probiotics vs norfloxacin and placebo vs ciprofloxacin. The size of the circles corresponds to 1/standard error. Red lines show the trend in decreasing IRRs for placebo vs norfloxacin from direct and indirect comparisons, and the blue lines show the missing trend in other active treatments vs norfloxacin from direct and indirect comparisons in a network meta-regression model. (a, b) Results with respect to norfloxacin and all fluoroquinolones, respectively. (c, d) IRR with regard to mean ascites protein and mean serum bilirubin levels. Included studies: (a) and (b) included all studies with primary prophylaxis (see also Figure 1), (c) included all studies except Lontos et al. and Pande et al., and (d) included all studies except Lontos et al. due to insufficient data provided.
We also analyzed changes over time of all above-named investigated cofactors with meta-regression analysis. Here, a small but significant decrease of mean bilirubin (P = 0.013, see Appendix Figure 3a, Supplementary Digital Content 1, http://links.lww.com/CTG/A365) and mean albumin (P = 0.011, Appendix Figure 3b, Supplementary Digital Content 1, http://links.lww.com/CTG/A365) could be observed over time. Neither mean age, Child-Pugh score, creatinine nor ascites protein showed significant time trends in our analysis.
Incidence of death in primary prophylaxis
Overall, 7 controlled trials analyzing primary prophylaxis were included in this analysis. The network graph of the treatment comparisons is shown in Appendix Figure 4a, Supplementary Digital Content 1, http://links.lww.com/CTG/A365.
The incidence rate of death in the norfloxacin-treated patients was 0.438 per patient-year with a significant heterogeneity between the studies (I2 = 33.7%). Again, heterogeneity for the comparison of IRRs was small, and a forest plot for the fixed-effect network analysis is shown in Appendix Figure 5a, Supplementary Digital Content 1, http://links.lww.com/CTG/A365. Comparing with norfloxacin-treated patients, only the IRR for placebo was significant, showing again an advantage of norfloxacin treatment (IRR 2.04, 95% CI 1.20–3.44, P = 0.008).
When network meta-regression was performed, a slight increase of the IRR was observed in meta-regression analysis (i.e. the positive treatment effect slightly increased over time); however, it was only borderline significant (P = 0.051, Appendix Figure 6, Supplementary Digital Content 1, http://links.lww.com/CTG/A365). Neither age, Child-Pugh score, bilirubin, albumin, creatinine nor protein in ascites were significant in a comparable analysis.
Incidence of extraperitoneal infections in primary prophylaxis
Five controlled clinical studies analyzing primary prophylaxis were included in the analysis, and the network graph of the treatment comparisons is shown in Appendix Figure 4b, Supplementary Digital Content 1, http://links.lww.com/CTG/A365.
The incidence rate of extraperitoneal infections in norfloxacin-treated patients was 0.326 per patient-year with a significant heterogeneity between the studies (I2 = 68.4%). Again, heterogeneity for the comparison of IRRs was small, and a forest plot is shown in Appendix Figure 5b, Supplementary Digital Content 1, http://links.lww.com/CTG/A365. Compared with norfloxacin-treated patients, IRRs of extraperitoneal infections for the other treatments were not significant.
If possible, network meta-regression was performed to analyze the effect of selected study characteristics on the IRR for other infections. No significant associations with IRRs (norfloxacin vs placebo) were observed.
Incidence of SBP, death, and extraperitoneal infections in secondary prophylaxis
Overall, 6 controlled clinical studies analyzing secondary prophylaxis were included in this analysis. As all trials used norfloxacin, a subset analysis for direct comparisons was applied, and a network meta-analysis was not necessary.
The incidence rate of SBP in secondary prophylaxis in the norfloxacin-treated patients was 0.310 per patient-year with a homogeneous group (I2 = 0%). IRRs in favor of norfloxacin could be observed when compared with trimethoprim/sulfamethoxazole (IRR 1.54, 95% CI 0.31–7.63), with rufloxacin (IRR 1.98, 95% CI 0.74–5.28), and with norfloxacin in combination with probiotics (IRR 1.29, 95% CI 0.36–4.56; Figure 3b). IRRs were less favorable for norfloxacin when compared with ciprofloxacin and rifaximin (IRR 0.19, 95% CI 0.02–1.78, and IRR 0.27, 95% CI 0.09–0.84, respectively). Differences of IRRs between the different treatments were borderline significant (P = 0.0516); no asymmetry could be observed in the funnel plot.
The incidence rate of death in secondary prophylaxis in the norfloxacin-treated patients was 0.423 per patient-year, with I2 = 73.7%. Again, norfloxacin was less favorable in comparison to rifaximin (IRR 0.50, 95% CI 0.28–0.90), yet the model did not show overall significant differences between the 3 treatment designs (Appendix Figure 5c, Supplementary Digital Content 1, http://links.lww.com/CTG/A365). The incidence rate of other infections in secondary prophylaxis in the norfloxacin-treated patients was 0.417 per patient-year, with I2 = 19.2%. There were no significant differences between the 2 treatment designs (see Appendix Figure 5d, Supplementary Digital Content 1, http://links.lww.com/CTG/A365).
DISCUSSION
The findings of our systematic review and meta-analysis show the protective effect of norfloxacin as primary prophylaxis compared with placebo or the intrahospital administration of norfloxacin. Norfloxacin was not superior to other common antibiotic substances that could be analyzed. In primary prophylaxis, the efficacy of norfloxacin and quinolones—in general—to prevent SBP, but not death, decreased over time. The reduction of efficacy over time could not be directly associated with the increased severity of liver disease. The positive treatment effect of norfloxacin was lower in studies including patients with high bilirubin and high ascites protein levels.
Over the last decades, several studies have shown that infections caused by Gram-positive bacteria and MDROs constitute a prevalent and growing health care problem in patients with cirrhosis (12). The International Club of Ascites Global Study Group collected data from over 1,300 hospitalized patients with cirrhosis and bacterial or fungal infections at 46 centers worldwide and estimated the prevalence of MDRO to be 34%. Independent risk factors for MDRO infections were, among others, the use of antibiotics and prior health care exposure. Yet, the previous administration of an antibiotic prophylaxis for SBP was not found to be more frequent in patients with MDR infections (30). The CANONIC Study group recently reported an increase of culture-positive MDRO infections in patients with cirrhosis from 29% in 2011 to 38% in 2017/18 in Europe (15).
Lately, in a prospective single-center observational study, we assessed the prevalence of MDRO colonization and infections at baseline and while on SBP prophylaxis (16). We observed higher risks of SBP development in patients with any apparent MDRO, vancomycin-resistant enterococci, multidrug-resistant Gram-negative bacteria, or even quinolone-resistant Gram-negative bacteria while on quinolone prophylaxis. In competing risk analysis, quinolone-resistant Gram-negative bacteria were independently associated with prophylaxis failure.
In light of these observations, the type of antibiotic prophylaxis has been debated recently. In fact, in a large French multicenter trial for primary SBP prophylaxis in patients with Child-Pugh C cirrhosis, no survival benefit was shown (primary study end point) for norfloxacin prophylaxis in the whole patient collective (11). However, in a post hoc analysis, a survival benefit in patients with low ascites protein probably due to a reduced risk of Gram-negative infections was observed. Yet, survival was comparable if patients with previous infections were analyzed, possibly suggesting a reduced efficacy of antibiotic prophylaxis in patients with previous antibiotic treatment or health care exposure.
In this meta-analysis, we observed a significant benefit in overall survival and SBP prevention for patients receiving norfloxacin compared with placebo. However, SBP prevention was especially observed in older trials, and meta-regression analysis revealed a reduced efficacy over time. Results showed a similar trend when time effect of treatment with all fluoroquinolones vs placebo was combined (implicating a class effect) and when using the publication year as reference. Disease severity appeared to have a rather small influence when analyzing the time trend of available cofactors.
Because of missing data on MDRO prevalence in the analyzed trials, this meta-analysis cannot directly associate MDRO presence/occurrence with the decreasing efficacy of norfloxacin prophylaxis. Yet, the parallel increase of MDRO worldwide and our recent observations in the prospective MDRO screening study might possibly explain this phenomenon.
In our analysis, lower levels of protein in ascites were associated with an increasing positive treatment effect. This confirms the current understanding of low levels of protein in ascites as an established risk factor for SBP reflecting a good robustness and transferability of our model (9,11). Interestingly, we observed a decreasing treatment effect of norfloxacin in studies including patients with exceedingly high serum bilirubin levels. Of note, the European Association for the Study of the Liver and others recommend primary SBP prophylaxis in patients with elevated serum bilirubin levels (9,31). Indeed, most studies evaluated primary SBP prophylaxis in patients with a mean serum bilirubin >3 mg/dL; in some studies, patients were excluded if serum bilirubin was below this threshold. In our meta-regression approach, we observed high treatment efficacy in studies in which the mean serum bilirubin of the patient population was between 3 and 5 mg/dL, in line with current guidelines. There were no studies included that had mean serum bilirubin below 2.5 mg/dL; thus, there were insufficient data to estimate the efficacy for studies focusing on patients with low serum bilirubin. However, we found evidence that in studies with exceedingly high serum bilirubin levels, norfloxacin prophylaxis was less effective. This could be associated with the severity of liver disease or other unknown causes. However, except for serum bilirubin, other factors associated with disease severity (creatinine and albumin) or the Child-Pugh score itself were not associated with a reduced treatment effect in these patients.
In secondary prophylaxis, the incidence rate of SBP and secondary infections was more than 2.5-fold higher than in the primary prophylaxis group, whereas it remained comparable with regard to death. Elfert et al. (32) reported in the only trial testing rifaximin vs norfloxacin a reduced risk for SBP and death in the rifaximin group. So far, this is the only randomized controlled trial (RCT) showing superiority of rifaximin. In our analysis, we observed a tendency toward superiority for rifaximin compared with norfloxacin. Most interestingly, Elfert et al. included patients from Egypt, where high rates of MDRO have been reported (33). However, there are insufficient data to imply superiority of rifaximin over norfloxacin in patients with MDRO.
Methodically, our meta-analysis is the first to use IRRs to assess the risk of SBP or death. As SBP, death, and extraperitoneal infections are clearly time dependent and more incidences are observed the longer the observation period, this technique more adequately assesses the efficacy of SBP prophylaxis and allows study comparisons. By applying these methods, our results confirm data by Facciorusso et al. (34) with regard to primary prophylaxis who used a different approach without calculating IRRs.
Yet, our study has several limitations. First, only 10 and 6 trials could be included in our analysis for primary and secondary prophylaxis, reflecting that only a low number of high-quality RCTs are available, which investigate SBP prophylaxis in patients with cirrhosis and ascites. Thus, direct comparison between norfloxacin and other regimens often included only single studies, and no placebo-controlled trial evaluating secondary prophylaxis met our strict inclusion criteria; here, numbers of trials reporting on secondary outcomes were small. Thus, in these cases, validity was critically limited. However, strict study inclusion criteria with limitation to high-quality RCTs with reported follow-up intervals and the current definition of SBP resulted in a patient collective with low heterogeneity of IRRs and allowed comparison over time with regard to IRRs of SBP and extraperitoneal infections. A recent Cochrane analysis observed no treatment effect of antibiotics over placebo but also included several other trials of low quality with high risk of bias and sparse data, which—according to the authors—made the results unreliable with inconsistent differences and high confidence intervals (17). This emphasizes the importance to select only high-quality trials with strict inclusion and exclusion criteria and similar or at least comparable follow-up periods.
Yet, our meta-analysis could not completely adjust for all potential confounders, and meta-regression analysis of mortality over time, in particular, might be biased by changing treatment options and paradigms. If this might have translated in the observed trend toward better overall survival over time remains unclear (especially as the placebo group remained unaffected). As the efficacy of norfloxacin prophylaxis was significantly reduced and the IRRs of extraperitoneal infections were not significantly different, a potential other confounding factor—not yet identified—might be responsible. Finally, study characteristics and follow-up time were calculated as means given for the concerned study groups, and data were not available in all studies. Although time trends of different cofactors did not suggest an increased severity of liver disease of patients, methodically, the impact of liver disease could not be causatively analyzed. Thus, our model lacks the precision of an individual patient data-based meta-analysis.
Taken together, norfloxacin remained superior to placebo in preventing SBP over the last decades. For norfloxacin, positive treatment effects to prevent SBP, but not death, decreased over time, whereas an increased liver disease severity does not seem to completely explain this phenomenon. The positive treatment effect of norfloxacin was lower in studies including patients with high bilirubin and high ascites protein levels. In view of the growing MDRO prevalence among patients with cirrhosis, the type of prophylaxis and the choice of antibiotics may need revision in these patients. Randomized controlled trials on different antibiotics for SBP prophylaxis are needed to investigate this association and other possible risk factors and to seek out new prophylactic strategies. Until then, norfloxacin should still be used in clinical practice according to international guidelines.
CONFLICTS OF INTEREST
Guarantor of the article: Marcus M. Mücke, MD.
Specific author contributions: Christian M. Lange, MD, and Eva Herrmann, MD, contributed equally to the study/share senior authorship. The authors have contributed to the manuscript by planning the study (M.M.M., E.H., and C.M.L.), reviewing the literature (M.M.M., V.T.M., and C.M.L.), collecting the data (all authors), performing the meta-analyses (E.H. and M.M.M.), and assessment and interpretation of the data (all authors). M.M.M., E.H. and C.M.L. wrote the manuscript, and all authors read, revised, and approved the final version of the manuscript. All authors have seen and approved the final version of the manuscript, and all authors have significantly contributed to the work.
Financial support: None to report.
Potential competing interests: M.M.M. reports speaking fees from AbbVie, travel support from AbbVie, Gilead, and Intercept, and a research grant from Gilead, all unrelated to the submitted work. V.T.M. reports travel support from AbbVie and Gilead, all unrelated to the submitted work. C.G. reports travel support from Gilead, unrelated to the submitted work. K.M.S. reports travel support from AbbVie, unrelated to the submitted work. P.G.F. reports consultancy for SNIPR Biome, unrelated to the submitted work. J.F. reports speaking and/or consulting fees from Grifols and Merck/MSD, all unrelated to the submitted work. S.Z. reports speaking and/or consulting fees from AbbVie, Bristol-Myers Squibb, Falk, Gilead, Janssen, and Merck/MSD, all unrelated to the submitted work. J.T. reports speaking and/or consulting fees for Gore, Bayer, Alexion, MSD, Gilead, Intercept, Norgine, Grifols, Versantis, and Martin Pharmaceutical, all unrelated to the submitted work. C.M.L. reports speaker fees from AbbVie, Gilead, MSD, and Norgine and travel support from AbbVie and Gilead, all unrelated to the submitted work. E.H. reports no conflicts of interest related to this work.
Study Highlights.
WHAT IS KNOWN
✓ Spontaneous bacterial peritonitis (SBP) prophylaxis has been recognized to reduce the risk of SBP in patients with decompensated liver cirrhosis.
✓ SBP prophylaxis with norfloxacin is recommended by current guidelines.
WHAT IS NEW HERE
✓ The efficacy of norfloxacin, and fluoroquinolones in general, to prevent SBP significantly decreased over time.
✓ This phenomenon was not explained by an increased severity of liver disease.
✓ The positive treatment effect seems to be lower in patients with high ascites protein and those with exceedingly high serum bilirubin levels.
TRANSLATIONAL IMPACT
✓ In view of the growing multidrug-resistant organism prevalence among patients with cirrhosis, the type and choice of prophylaxis may need revision.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A365
REFERENCES
- 1.Arvaniti V, D'Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010;139(4):1246–5, 1256 e1-5. [DOI] [PubMed] [Google Scholar]
- 2.Bernard B, Grangé JD, Khac EN, et al. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: A meta-analysis. Hepatology 1999;29(6):1655–61. [DOI] [PubMed] [Google Scholar]
- 3.Tito L, Rimola A, Gines P, et al. Recurrence of spontaneous bacterial peritonitis in cirrhosis: Frequency and predictive factors. Hepatology 1988;8(1):27–31. [DOI] [PubMed] [Google Scholar]
- 4.Mücke MM, Rumyantseva T, Mücke VT, et al. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int 2018;38(4):645–53. [DOI] [PubMed] [Google Scholar]
- 5.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol 2014;60(1):197–209. [DOI] [PubMed] [Google Scholar]
- 6.Ginés P, Rimola A, Planas R, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: Results of a double-blind, placebo-controlled trial. Hepatology 1990;12(4 Pt 1):716–24. [DOI] [PubMed] [Google Scholar]
- 7.Rolachon A, Cordier L, Bacq Y, et al. Ciprofloxacin and long-term prevention of spontaneous bacterial peritonitis: Results of a prospective controlled trial. Hepatology 1995;22(4 Pt 1):1171–4. [DOI] [PubMed] [Google Scholar]
- 8.Grangé JD, Roulot D, Pelletier G, et al. Norfloxacin primary prophylaxis of bacterial infections in cirrhotic patients with ascites: A double-blind randomized trial. J Hepatol 1998;29(3):430–6. [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69(2):406–60. [DOI] [PubMed] [Google Scholar]
- 10.Gerbes AL, Gulberg V, Sauerbruch T, et al. German S 3-guideline “ascites, spontaneous bacterial peritonitis, hepatorenal syndrome”. Z Gastroenterol 2011;49(6):749–79. German. [DOI] [PubMed] [Google Scholar]
- 11.Moreau R, Elkrief L, Bureau C, et al. Effects of long-term norfloxacin therapy in patients with advanced cirrhosis. Gastroenterology 2018;155(6):1816–27.e9. [DOI] [PubMed] [Google Scholar]
- 12.Fernández J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: A prospective study. Hepatology 2012;55(5):1551–61. [DOI] [PubMed] [Google Scholar]
- 13.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med 2010;362(19):1804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weist K, Diaz Hogberg L. ECDC publishes 2013 surveillance data on antimicrobial resistance and antimicrobial consumption in Europe. Euro Surveill 2014;19(46):20962. [DOI] [PubMed] [Google Scholar]
- 15.Fernández J, Prado V, Trebicka J, et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol 2019;70(3):398–411. [DOI] [PubMed] [Google Scholar]
- 16.Mücke MM, Mayer A, Kessel J, et al. Quinolone- and multidrug-resistance predict failure of antibiotic prophylaxis of spontaneous bacterial peritonitis. Clin Infect Dis 2020;70(9):1916–24. [DOI] [PubMed] [Google Scholar]
- 17.Komolafe O, Roberts D, Freeman SC, et al. Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis in people with liver cirrhosis: A network meta-analysis. Cochrane Database Syst Rev 2020;1:CD013125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lombardi A, Zuccaro V, Fagiuoli S, et al. Prophylaxis of spontaneous bacterial peritonitis: Is there still room for quinolones? J Hepatol 2019;70(5):1027–8. [DOI] [PubMed] [Google Scholar]
- 19.Goel A, Rahim U, Nguyen LH, et al. Systematic review with meta-analysis: Rifaximin for the prophylaxis of spontaneous bacterial peritonitis. Aliment Pharmacol Ther 2017;46(11-12):1029–36. [DOI] [PubMed] [Google Scholar]
- 20.Kamal F, Khan MA, Khan Z, et al. Rifaximin for the prevention of spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhosis: A systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2017;29(10):1109–17. [DOI] [PubMed] [Google Scholar]
- 21.Cohen MJ, Sahar T, Benenson S, et al. Antibiotic prophylaxis for spontaneous bacterial peritonitis in cirrhotic patients with ascites, without gastro-intestinal bleeding. Cochrane Database Syst Rev 2009;2:CD004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernard B, Grangé JD, Khac EN, et al. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with ascites: A meta-analysis. Digestion 1998;59(Suppl 2):54–7. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 2009;151(4):264–9, W64. [DOI] [PubMed] [Google Scholar]
- 24.Danulescu RM, Ciobica A, Stanciu C, et al. The role of rifaximine in the prevention of the spontaneous bacterial peritonitis. Rev Med Chir Soc Med Nat Iasi 2013;117(2):315–20. [PubMed] [Google Scholar]
- 25.Shamseya MM, Madkour MA. Rifaximin: A reasonable alternative for norfloxacin in the prevention of spontaneous bacterial peritonitis in patients with HCV-related liver cirrhosis. Alexandria J Med 2016;52:219–26. [Google Scholar]
- 26.Sandhu BS, Gupta R, Sharma J, et al. Norfloxacin and cisapride combination decreases the incidence of spontaneous bacterial peritonitis in cirrhotic ascites. J Gastroenterol Hepatol 2005;20(4):599–605. [DOI] [PubMed] [Google Scholar]
- 27.Singh N, Gayowski T, Yu VL, et al. Trimethoprim-sulfamethoxazole for the prevention of spontaneous bacterial peritonitis in cirrhosis: A randomized trial. Ann Intern Med 1995;122(8):595–8. [DOI] [PubMed] [Google Scholar]
- 28.Mostafa T, Badra G, Abdallah M. The efficacy and the immunomodulatory effect of rifaximin in prophylaxis of spontaneous bacterial peritonitis in cirrhotic Egyptian patients. Turk J Gastroenterol 2015;26(2):163–9. [DOI] [PubMed] [Google Scholar]
- 29.Soriano G, Guarner C, Teixidó M, et al. Selective intestinal decontamination prevents spontaneous bacterial peritonitis. Gastroenterology 1991;100(2):477–81. [DOI] [PubMed] [Google Scholar]
- 30.Piano S, Singh V, Caraceni P, et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology 2019;156(5):1368–80.e10. [DOI] [PubMed] [Google Scholar]
- 31.Gerbes AL, Labenz J, Appenrodt B, et al. Updated S2k-guideline “complications of liver cirrhosis”. German Society of Gastroenterology (DGVS). Z Gastroenterol 2019;57(5):611–80. German. [DOI] [PubMed] [Google Scholar]
- 32.Elfert A, Abo Ali L, Soliman S, et al. Randomized-controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol 2016;28(12):1450–4. [DOI] [PubMed] [Google Scholar]
- 33.Fam NS, Defasque S, Bert F, et al. Faecal carriage of extended-spectrum beta-lactamase (ESBL)-producing enterobacteria in liver disease patients from two hospitals in Egypt and France: A comparative epidemiological study. Epidemiol Infect 2015;143(6):1247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Facciorusso A, Papagiouvanni I, Cela M, et al. Comparative efficacy of long-term antibiotic treatments in the primary prophylaxis of spontaneous bacterial peritonitis. Liver Int 2019;39(8):1448–58. [DOI] [PubMed] [Google Scholar]