Abstract
Background
Several kinds of cutting guides, including patient-specific instrumentation, navigation, standard cutting guides, accelerometer-based navigation, and robotic guidance, are available to restore a planned alignment during TKA. No previous study has simultaneously compared all of these devices; a network meta-analysis is an especially appealing method because it allows comparisons across approaches that were not compared head-to-head in individual randomized controlled trials.
Questions/purposes
We performed a network meta-analysis to determine whether novel approaches to achieving implant alignment, such as patient-specific instrumentation, navigation, accelerometer-based navigation, and robotic guidance, provide any advantage over standard cutting guides in terms of: (1) hip-knee-ankle (HKA) alignment outliers greater than ± 3°, (2) outcome scores (1989 - Knee Society Score and WOMAC score) measured 6 months after surgery, or (3) femoral and tibial implant malalignment (greater than ± 3°), taken separately, in the frontal and sagittal plane, as well as other secondary outcomes including validated outcome scores 1 and 2 years after surgery.
Methods
In our network meta-analysis, we included randomized controlled trials comparing the different cutting guides by using at least one of the previously specified criteria, without limitation on language or date of publication. We searched electronic databases, major orthopaedic journals, proceedings of major orthopaedic meetings, ClinicalTrials.gov, and the World Health Organization’s International Clinical Trials Registry Platform until October 1, 2018. This led to the inclusion of 90 randomized controlled trials involving 9389 patients (mean age 68.8 years) with 10,336 TKAs. Two reviewers independently selected trials and extracted data. The primary outcomes were the proportion patients with malalignment of the HKA angle (defined as HKA > 3° from neutral) and the Knee Society Score and WOMAC scores at 6 months postoperatively. We combined direct and indirect comparisons using a Bayesian network meta-analysis framework to assess and compare the effect of different cutting guides on outcomes. Bayesian estimates are based on the posterior distribution of an endpoint and are called credible intervals. Usually the 95% credible interval, corresponding to a posterior probability of 0.95 that the endpoint lies in the interval, is computed. Unlike the frequentist approach, the Bayesian approach does not allow the calculation of the p value.
Results
The proportion of HKA outliers was lower with navigation than with patient-specific instrumentation (risk ratio 0.46 [95% credible interval (CI) 0.34 to 0.63]) and standard cutting guides (risk ratio 0.45 [95% CI 0.37 to 0.53]); however, this corresponded to an actual difference of only 12% of patients for navigation versus 21% of patients for patient-specific instrumentation, and 12% of patients for navigation versus 25% for standard cutting guides. We found no differences for other comparisons between different cutting guides, including robotics and the accelerometer. We found no differences in the Knee Society Score or WOMAC score between the different cutting guides at 6 months. Regarding secondary outcomes, navigation reduced the risk of frontal and sagittal malalignments for femoral and tibial components compared with the standard cutting guides, but none of the other cutting guides showed superiority for the other secondary outcomes.
Conclusions
Navigation resulted in approximately 10% fewer patients having HKA outliers of more than 3°, without any corresponding improvement in validated outcomes scores. It is unknown whether this incremental reduction in the proportion of patients who have alignment outside a window that itself has been called into question will justify the increased costs and surgical time associated with the approach. We believe that until or unless these new approaches either (1) convincingly demonstrate superior survivorship, or (2) convincingly demonstrate superior outcomes, surgeons and hospitals should not use these approaches since they add cost, have a learning curve (during which some patients may be harmed), and have the risks associated with uncertainty of novel surgical approaches.
Level of Evidence
Level I, therapeutic study.
Introduction
Achieving accurate alignment during TKA is important because of concerns for premature loosening [19]. In addition, function may be impaired in the setting of marked malalignment [39]. Nevertheless, the importance of precise alignment remains controversial. Some historical series found an association between a neutral alignment and aseptic loosening [3, 19, 39, 41]. Conversely, more recent long-term series did not find this association [36, 47].
Five types of cutting guides are currently available. Standard cutting guides are the oldest and the most commonly used option. These guides rely on manual alignment relative to the intramedullary canal or landmarks palpated at the time of surgery (on the tibia) and angles determined in preoperative planning (on the femur). Introduced in 1997, navigation allows surgeons to have real-time information on alignment during surgery, using a system that includes trackers attached to bone followed by the use of an infrared camera and software to interpret the data [6, 42]. An accelerometer is a simplified navigation system because it allows the surgeon to determine the knee’s frontal alignment without implantation of trackers in the patient’s limb [15]. Developed during the 2010s, patient-specific instrumentation uses custom-fit cutting guides manufactured from a three-dimensional (3-D) model of the patient’s knee determined with MRI or CT images obtained before surgery [12, 23, 32]. Finally, there was a resurgence of interest in modern robotic guidance in the last decade. It is the most recently developed technique, in which bony cuts are performed by a robotic arm that stops once it reaches the targeted area, according to the surgeon's preoperative planning [1, 18].
However, new approaches like patient-specific instrumentation, navigation, standard cutting guides, accelerometer-based navigation, and robotic guidance are expensive [5]. They also add surgical time [2], can have substantial learning curves, and have been associated with unique (and sometimes serious) complications [2]. In addition, several randomized trials have suggested that they may not improve alignment [49] (or not improve it enough to justify the added time or cost associated with their use). Long-term studies, when they have been available, have found no survivorship advantages to these techniques, including clinical function. However, these approaches have intuitive appeal, and some other studies have indeed demonstrated alignment advantages [21, 2]. The challenge when synthesizing an evidence base consisting of dozens of randomized trials is that not all of the techniques of interest have been compared head-to-head in individual trials. Moreover, many head-to-head randomized controlled trials (RCTs) or two-by-two meta-analyses have been conducted to compare these different cutting guides, but they have shown contradictory results [9, 16, 20, 22, 29, 30, 33, 35, 38, 44]. No study that we know of has simultaneously compared the different available techniques. In this setting, network meta-analysis is potentially useful [24].
This approach allows the comparison of approaches that were not evaluated directly in individual trials. That is, if approach A was compared with approach B in several randomized trials, and approach B was compared with approach C in others, a network meta-analysis allows the comparison of approach A to approach C; as such, it is a powerful means to answer important clinical questions.
We therefore performed a network meta-analysis to determine whether novel approaches to achieving implant alignment, such as patient-specific instrumentation, navigation, accelerometer-based navigation, and robotic guidance, provide any advantage over standard cutting guides in terms of: (1) hip-knee-ankle (HKA) alignment outliers greater than ± 3° (a more reasonable cutoff of 5° would be preferable to a cutoff of 3° but the 5° cutoff was too rarely reported), (2) outcome scores (1989 Knee Society Score and WOMAC score) measured 6 months after surgery, or (3) femoral and tibial implant malalignment (greater than ± 3°), taken separately in the frontal and sagittal plane, as well as other secondary outcomes including validated outcome scores 1 and 2 years after surgery.
Materials and Methods
This systematic review and network meta-analysis was registered in PROSPERO (Number CRD42018091913) and is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Search Strategy
We searched electronic databases (Medline, CENTRAL and Embase) starting at the inception date up to October 1, 2018 using dedicated search equations adapted to each database (see Appendix 1; Supplemental Digital Content 1, http://links.lww.com/CORR/A362). We manually searched three major orthopaedic journals (the Journal of Bone and Joint Surgery American Volume, the Journal of Arthroplasty, and Clinical Orthopaedics and Related Research®) and conference proceedings of orthopaedic meetings (the American Academy of Orthopaedic Surgeons, the American Association of Hip and Knee Surgeons, and the Société Française de Chirurgie Orthopédique et Traumatologie) from January 1, 2005 to October 1, 2018. We also searched ClinicalTrials.gov and the World Health Organization’s International Clinical Trials Registry Platform. Finally, we systematically screened reference lists of systematic reviews and meta-analyses for any additional references.
Study Eligibility and Selection Process
All RCTs that compared at least two types of cutting guides and reported at least one of our predefined outcomes were included. No restriction on language or date of publication was applied. Exclusion criteria were TKA for an indication other than osteoarthritis, TKA after knee osteotomy or unicompartmental knee arthroplasty on the same knee, and TKA revision.
Two main types of outcomes were assessed in this review: the proportion of HKA outliers, defined as an HKA angle outside the 177° to 183° range within the first 6 months postoperatively and functional outcomes at 6 months postoperatively, evaluated with either the 1989 Knee Society Score (KSS) or the WOMAC score (see Appendix 2; Supplemental Digital Content 2, http://links.lww.com/CORR/A363).
The secondary outcomes were malalignment of tibial and femoral implants in the frontal, sagittal, and axial planes, defined as an angle more or less than 3° from the intended value within the first 6 months postoperatively (see Appendix 2; Supplemental Digital Content 2, http://links.lww.com/CORR/A363); functional outcomes (1989 KSS and WOMAC score) at 1 and 2 years postoperatively; the mean operative time, in minutes, from skin incision to closure; and the incidence of postoperative complications.
Two reviewers (PAB, SC) independently screened titles, summaries, and full texts whenever necessary to assess the eligibility of each study. Disagreements were resolved by a third reviewer (MRR) and by contacting the corresponding author when doubt persisted.
Selection and General Characteristics
Our search initially retrieved 1364 records. Finally, 90 RCTs with 9389 patients and 10,336 knees were included (Fig. 1). The most frequent comparison was navigation versus standard cutting guides in 58% (52 of 90) of the trials. Standard cutting guides were compared with every other type of cutting guide. Three studies directly compared patient-specific instrumentation and navigation (Fig. 2). One trial had three arms (navigation, patient-specific instrumentation, and standard cutting guides) (see Appendix 3; Supplemental Digital Content 3, http://links.lww.com/CORR/A364). The risk of bias of each study remains heterogeneous notably concerning the “sequence generation” and “allocation concealment” (see Appendix 4; Supplemental Digital Content 4, http://links.lww.com/CORR/A365).
Fig. 1.
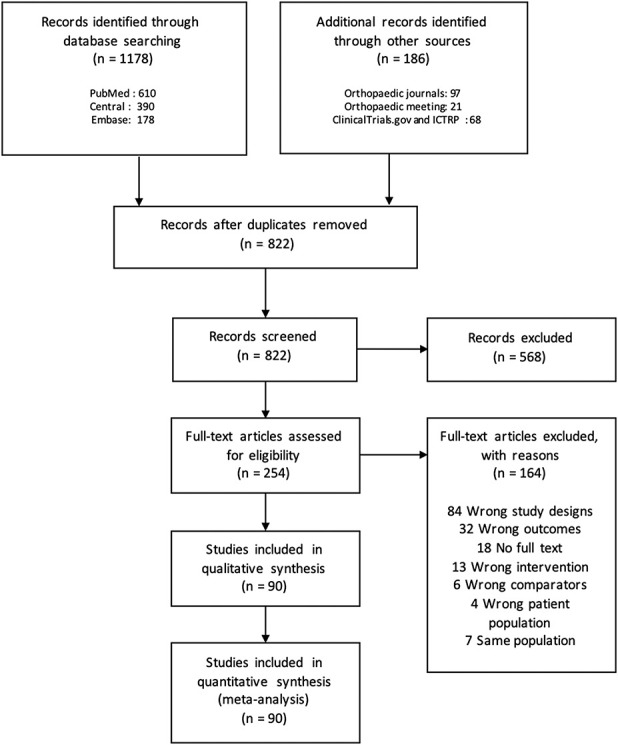
This PRISMA flow chart shows the selection process of the network meta-analysis comparing the efficacy of different cutting guides in TKA; ICTRP = International Clinical Trials Registry Platform.
Fig. 2.
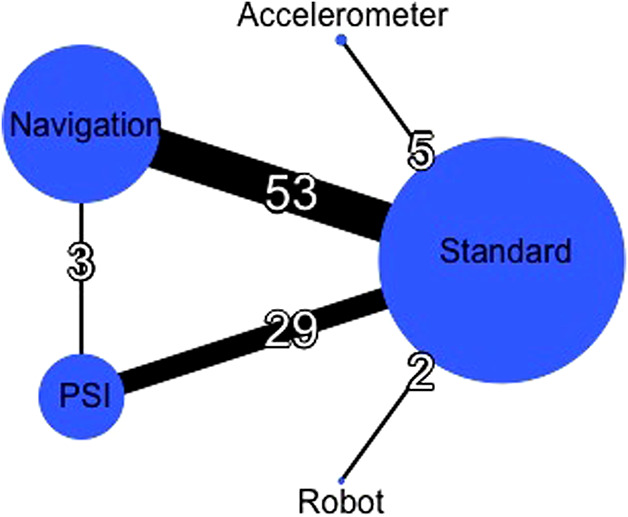
In this global network diagram, the nodes (circles) represent different cutting blocks, and their sizes were proportional to the sample size of each respective intervention. The edges (lines) indicate direct comparisons, and their thicknesses were proportional to the number of studies available.
Data Extraction and Risk of Bias Assessment
The same reviewers (PAB, SC) independently extracted the data and evaluated the risk of bias using the Cochrane risk-of-bias tool [13] in a dedicated data extraction form that was tested on 10 random records. When there was disagreement, a third reviewer (MRR) was involved to reach consensus.
We collected the following data: general study information: title, date of publication, authors, country, contact details of the corresponding author, and funding sources; characteristics of the study: design, inclusion and exclusion criteria, and follow-up duration; population characteristics: mean age, proportion of men, mean or median values for size, weight, and BMI; cutting guide used: standard cutting guides, navigation, patient-specific instrumentation, accelerometer-based navigation, or robotic guidance; prothesis characteristics: manufacturer, product name, type of tibial bearing, type of fixation, and conservation of the posterior cruciate ligament with a posterior-stabilized system; surgical characteristics: unilateral or bilateral simultaneous arthroplasty, use of a tourniquet, surgical approach, or patellar resurfacing; and results for outcomes of interest: frequencies of binary outcomes, mean and SD for quantitative variables, and the number of patients included in each group. Regarding continuous outcomes, when the mean or SD was not available, we estimated them from the median and the first and third quartiles and/or ranges using approximation formulas [14, 48] (see Appendix 5; Supplemental Digital Content 5, http://links.lww.com/CORR/A366). The corresponding authors were contacted if there were missing data.
Patient Involvement
Patients were not involved in any aspect of the study design, conduct, or development of the research question or outcome measures. This study is a systematic review and network meta-analysis of existing published research; therefore, there was no active patient recruitment for data collection.
Data Synthesis and Analysis
The unit of analysis was the knee. Binary outcomes were compared between cutting guides using risk ratios, and continuous outcomes were compared using mean differences with 95% credible intervals (CIs). For direct comparisons, we conducted a conventional meta-analysis to synthesize the results, using random-effects models and fixed-effects models as sensitivity analyses. We combined direct and indirect comparisons via a network meta-analysis using the hierarchical model of Lu and Ades [28] with a Bayesian approach. In a Bayesian framework, estimates are based on the posterior distribution of the endpoint and are called credible intervals (Cis). Usually, the 95% CI corresponding to the interval has a posterior probability of 95% of the endpoint that lies within it. Unlike the frequentist approach, the Bayesian approach does not allow the calculation of the p value. Conclusions are drawn from whether or not 1 (which corresponds to a no-difference relative risk) or 0 (which corresponds to no mean differences) belongs to the credible interval. We used Markov Chain Monte Carlo to implement the model. Binary non-informative prior distribution was used for dichotomous outcomes and normal non-informative prior distribution was used for continuous outcomes [46]. The model’s convergence was assessed using Brooks-Gelman-Rubin plots [4]. We evaluated the fit via residual deviance [8]. We checked the assumption of transitivity by considering similarity in the distribution of age, sex, and BMI across the different pairwise comparisons. A network diagram was used for each outcome to present direct comparisons between interventions. We obtained a treatment hierarchy using the surface under the cumulative ranking curve and mean ranks. To evaluate the assumption of inconsistency, we used a node-splitting approach [7], and we compared the fit between the consistency and inconsistency models. We statistically assessed the presence of heterogeneity within each network comparison using the I2 statistic. Subgroup analyses were planned for the main outcomes according to the surgical approach and risk of bias. We conducted sensitivity analyses for the main results using network meta-regression analyses adjusted successively for the mean age, mean BMI, percentage of women, and number of TKAs per study. A comparison-adjusted funnel plot of older treatment versus newer treatment was used to assess small study effects within each pairwise comparison. Cutting guides were ranked in order from oldest to newest: standard cutting guides, navigation, patient-specific instrumentation, accelerometer-based navigation, and robotic guidance. We used the GEMTC R-package (version 0.8-2) for analyses and netmeta R-package (version 0.9-8) for network diagrams in R version 3.5.2 software (R foundation for statistical Computing, Vienna, Austria). The quality of evidence for the main outcomes was evaluated using the GRADE framework [40].
Results
HKA Angle Outliers Greater Than +/- 3°
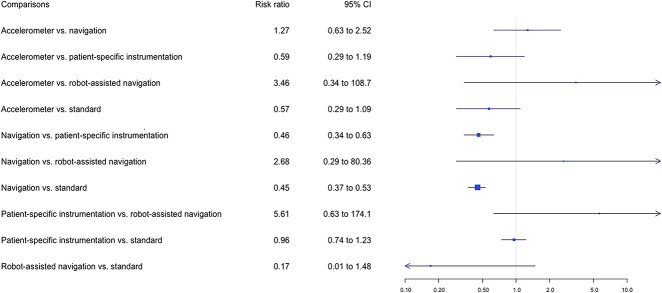
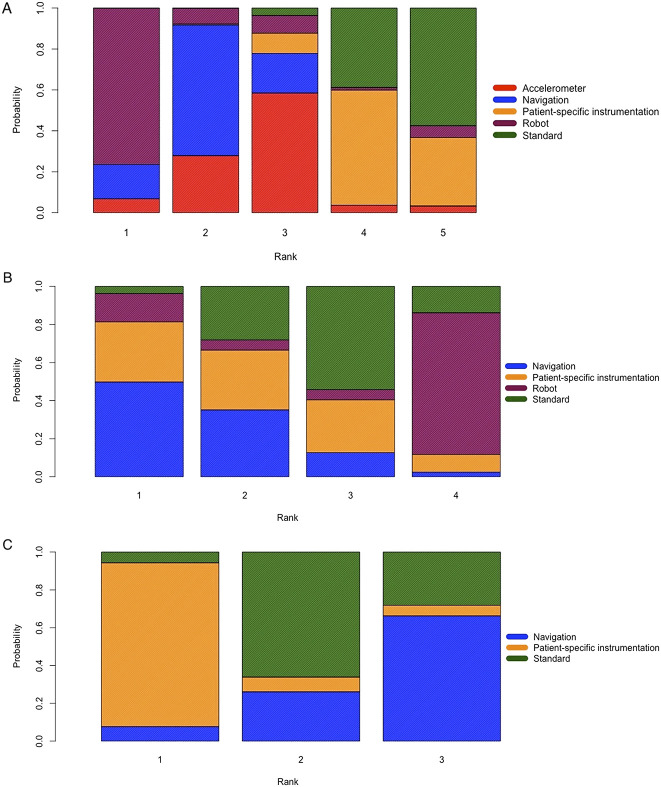
The proportion of HKA angle outliers greater than +/- 3° was lower in the navigation group than in the patient-specific instrumentation group (12% versus 21%, risk ratio 0.46 [95% CI 0.34 to 0.63]) and in the standard cutting guide group (12% versus 25%, risk ratio 0.45 [95% CI 0.37 to 0.53]), with I2 = 19% (Fig. 3). Sixty-eight percent (61 of 90) of studies reported malalignment of the HKA angle. TKA using standard cutting guides was the most used control procedure (Fig. 4). The results of pairwise comparisons confirmed that navigation against the standard cutting guide reduced the risk of malalignment of the HKA angle (RR 0.43 [95% CI 0.36 to 0.53]; I2 = 51.2%) (see Appendix 6; Supplemental Digital Content 6, http://links.lww.com/CORR/A367). However, this corresponded to a relatively small proportion of the patients being influenced by the difference in accuracy. Robot-assisted surgery had the highest probability of having the lowest proportion of HKA angle outliers greater than +/- 3° but without any significant difference (Fig. 5).
Fig. 3.
Forest plots in the network meta-analysis of the relative risks of HKA misalignments between the different cutting guides are shown. The data are presented as risk ratios with 95% credibility intervals, which were obtained using random effects models. The I2 of the network meta-analysis was 19%.
Fig. 4 A-C.
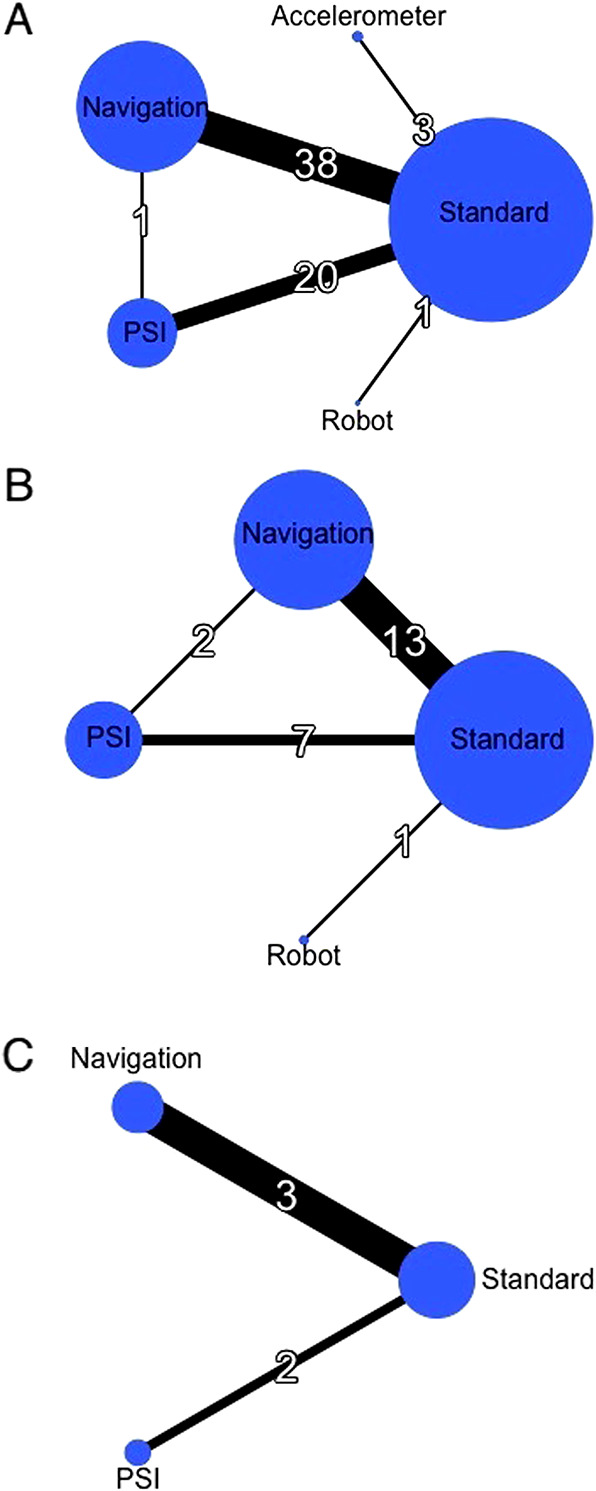
These network diagrams of the main outcomes show (A) the HKA malalignment, (B) KSS at the first 6 months, and (C) WOMAC scores at the first 6 months.
Fig. 5 A-C.
These images show rank probabilities of (A) the HKA malalignment angle, (B) delta KSS at the first 6 months postoperatively, and (C) delta WOMAC scores at the first 6 months postoperatively for each cutting guide. The x axis represents the ranking. The y axis represents the probability of rank: 0% to 100%. Each color represents one cutting guide: red for accelerometer-based navigation, blue for navigation, yellow for patient-specific instrumentation, purple for robot navigation, and green for the standard cutting guide; PSI = patient-specific instrumentation.
Functional Outcomes
We found no differences between the different cutting guides either on KSS or WOMAC at 6 months.
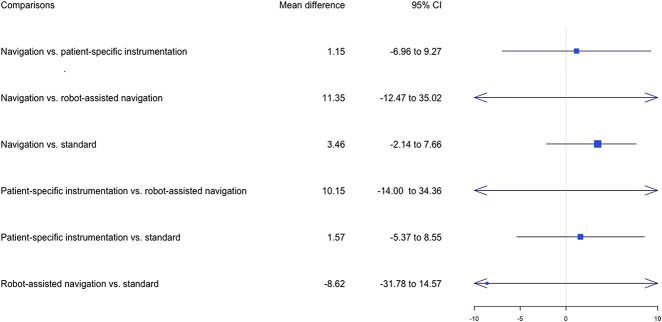
Concerning the KSS at 6 months, the results of the pairwise comparison confirmed those results and did not indicate any differences in functional outcomes (see Appendix 6; Supplemental Digital Content 6, http://links.lww.com/CORR/A367). Twenty-one studies (23%) reported the KSS at 6 months postoperatively (Fig. 4). Navigation had the highest probability of leading to the highest KSS mean values (Fig. 5), although no differences were found between the different cutting guides (Fig. 6).
Fig. 6.
Forest plots in the network meta-analysis of the mean difference of KSS between preoperatively and 6 months postoperatively are shown The data are presented as the relative mean differences with 95% credibility intervals, which were obtained using random effects models. The I2 of the network meta-analysis was 3%.
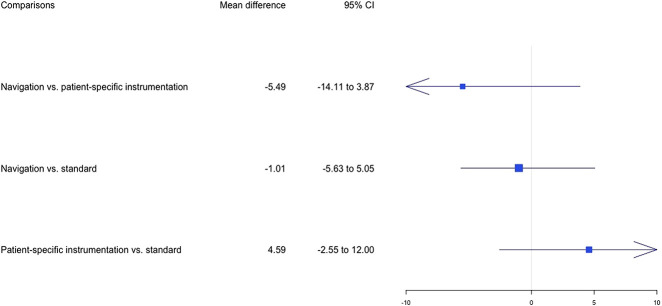
Concerning the WOMAC at 6 months, the results of the pairwise comparison did not indicate any differences (see Appendix 6; Supplemental Digital Content 6, http://links.lww.com/CORR/A367). WOMAC scores at 6 months postoperatively were available in five studies (5%) (Fig. 4). Patient-specific instrumentation had the highest probability of leading to the highest WOMAC mean values (Fig. 5), although no differences were found between the different cutting guides (Fig. 7).
Fig. 7.
Forest plots in the network meta-analysis of the mean difference of WOMAC scores between preoperatively and 6 months postoperatively are shown The data are presented as the relative mean differences with 95% credibility intervals, which were obtained using random effects models. The I2 of the network meta-analysis was 1%.
Secondary Outcomes
In general, all tested alternatives provided some improvement in overall alignment. Concerning frontal femoral outliers +/-3°, navigation reduced their proportion compared with standard cutting guides (5% versus 14%; RR 0.46 [95% CI 0.31 to 0.69]). The proportion of outliers for frontal femoral component alignment was lower in the accelerometer group than in the patient-specific instrumentation group (1% versus 11%; RR 0.23 [95% CI 0.07 to 0.69]) and in the standard cutting guide group (1% versus 14%; RR 0.18 [95% CI 0.06 to 0.49]). Concerning frontal tibial outliers +/-3°, navigation reduced their proportion compared with standard cutting guides (5% versus 10%; RR 0.55 [95% CI 0.36 to 0.81]) and compared with the patient-specific instrumentation group (5% versus 10%; RR 0.40 [95% CI 0.21 to 0.74]).
Regarding femoral sagittal outliers +/-3°, navigation reduced their proportion compared with standard cutting guides (14% versus 30%; RR 0.54 [95% CI 0.38 to 0.74]) and compared with patient-specific instrumentation (14% versus 35%; RR 0.49 [95% CI 0.30 to 0.76]). As for tibial sagittal outliers +/-3°, navigation reduced their proportion compared with standard cutting guides (15% versus 24%; RR 0.56 [95% CI 0.37 to 0.84]) and compared with patient-specific instrumentation (15% versus 30%; RR 0.34 [95% CI 0.18 to 0.59]). Accelerometer and standard cutting guides reduced the proportion of outliers against patient-specific instrumentation (2% versus 30%; RR 0.35 [95% CI 0.11 to 0.99] and 24% versus 30%; RR 0.68 [95% CI 0.56 to 0.89]). We found no differences between groups regarding the rotation of femoral and tibial implants (see Appendix 7; Supplemental Digital Content 7, http://links.lww.com/CORR/A368). For robotic guidance in sagittal, frontal and axial alignment, we did not find any direct comparison in the evidence.
As for secondary clinical outcomes, we observed no differences in the KSS and WOMAC scores at 1 and 2 years postoperatively between the different cutting guides (see Appendix 8; Supplemental Digital Content 8, http://links.lww.com/CORR/A369).
Surgical procedures using navigation were longer than those using patient-specific instrumentation (95 minutes versus 70 minutes, mean difference 20.19 [95% CI 14.08 to 26.28] and standard cutting guides (95 minutes versus 78 minutes, mean difference 17.03 [95% CI 13.03 to 21.06]) (see Appendix 8; Supplemental Digital Content 8, http://links.lww.com/CORR/A369). We did not find any difference concerning accelerometer and robotic guidance in surgical time. Direct comparisons did not suggest any differences in postoperative complications (see Appendix 9; Supplemental Digital Content 9, http://links.lww.com/CORR/A370).
Grade Evaluation
We found no differences regarding distribution of age, sex, number of participants and BMI in each comparison of the network. An adequate random sequence generation and an adequate treatment allocation concealment were specified in 62% (56 of 90) and 50% trials (45 of 90) respectively. Outcome assessors and patients were blinded in 54% (49 of 90) and in 20% studies (18 of 90) respectively. Overall, for the items incomplete outcome data regarding radiological, clinical, and perioperative outcomes, risk was estimated low. In 60% (54 of 90) of studies, the lead final analysis was intention to treat. Finally, heterogeneity for different outcomes was low: 19% for HKA outliers, 3% for KSS at 6 months, and 1% for WOMAC. Overall, studies were well conducted and data for our extraction was available.
We performed the GRADE evaluation for the two main outcomes. For HKA outliers +/-3°, it suggests that the evidence is moderate for navigation/ patient-specific instrumentation, navigation/standard, patient-specific instrumentation /standard comparison and accelerometer, and very low for robot/standard comparison (see Appendix 10; Supplemental Digital Content 10, http://links.lww.com/CORR/A371).
For KSS at the 6 months post-surgery, it suggests that the evidence is moderate for
navigation/ patient-specific instrumentation, low for navigation/standard and patient-specific instrumentation /standard comparison, and very low for robot/standard comparison (see Appendix 10; Supplemental Digital Content 10, http://links.lww.com/CORR/A371). For WOMAC at the 6 months post-surgery, it suggests that the evidence is high for navigation/standard and moderate for patient-specific instrumentation/standard comparison (see Appendix 10; Supplemental Digital Content 10, http://links.lww.com/CORR/A371).
Discussion
Although some studies have suggested that small differences in alignment matter in terms of TKA implant survivorship [3, 19] and knee function [39], others have not [36, 47]. In the interest of making alignment as consistent as possible, surgeons therefore have explored a variety of approaches, including navigation, accelerometers, patient-specific instrumentation, and robotics. It is important to determine the degree to which these approaches achieve those goals without losing sight of the fact that these approaches also have potential disadvantages, including increased cost, increased surgical time, a difficult learning curve, and unique—and sometimes severe—complications [2, 5]. A network meta-analysis is a useful approach to synthesize a large amount of data in this setting because this approach allows the comparison of approaches that were not compared directly in individual trials. We found an incremental reduction in the risk of small (± 3°) HKA outliers when navigation was used compared with the other approaches, but no associated improvements in validated outcomes scores. The small alignment benefit came with a large increase in surgical time. Until or unless future studies can demonstrate a benefit in terms of survivorship, knee pain, cost effectiveness, or function of navigation for these other newer approaches, based on our findings we recommend against their widespread use.
Limitations
However, this study had several limitations. Few trials evaluated more recent techniques, such as the use of an accelerometer [11, 17, 21, 34, 43] or robot [25, 26], making it difficult to assess the benefits of these two techniques in relation to their cost. Unfortunately, we were unable to collect clinical outcomes after 2 years postoperatively due to the lack of information in published studies, even though joint prostheses are expected to have a longevity of 15 years or more. Finally, the ranking results were based on point estimate probabilities, and the results do not fully reflect the uncertainty of the ranking as outlined by Trinquart et al. [45]. As a consequence, we must consider ranking results as trends rather than definitive conclusions.
We found that many trials did not evaluate clinical outcomes; only 24 trials reported clinical outcomes. Only three trials simultaneously evaluated our three primary outcomes, illustrating the high level of heterogeneity regarding the outcomes planned and reported. In addition, the reporting of clinical outcomes was notably heterogeneous across trials. The WOMAC was reported with different numeric scales, whereas the KSS was more homogeneously used and reported in the included RCTs [27]. Such heterogeneity in evaluated outcomes and their definitions makes it difficult to compare results between trials and combinations by meta-analysis. This highlights the need to develop recommendations and core sets of outcomes as promoted by the Core Outcome Measures in Effectiveness Trials (COMET) initiative [10] in the field of knee prosthetic replacement.
Small Improvement in HKA Alignment
We found that about 10% more patients could achieve an HKA of within 3° of neutral when navigation was used compared with when standard guides or patient-specific instruments were used. We caution readers against making too much of even this incremental difference. The evidence is scant and contradictory that such small alignment differences are likely to improve implant survivorship or function [9, 20, 31] and even larger differences—such as ± 5°—may not matter as much as once was thought [36]. We would have preferred to set the cutoff to 5° or even analyze it as a continuous variable, but the data were not available in the studies we evaluated. Reducing the likelihood of HKA outliers in a small minority of patients undergoing TKA by widespread adoption of navigation would result in substantial increases in costs and operative time [2], at least initially.
No Improvements in Outcomes Scores
We found no evidence that these technologies improved KSS or WOMAC scores at either 6 months, 1 year, or 2 years after TKA. For clinical outcomes, similar results were found in other studies regarding KSS comparing navigation, patient-specific instrumentation, and standard cutting guides [28, 37]. Petursson et al. [37] found that navigation had a better WOMAC after 2 years. We did not find similar results at short or medium terms.
No Increase in Complications but Longer Surgical Time
Navigation is often criticized because of increased operative time and the risk of iatrogenic fracture due to the pin insertions. In this network meta-analysis, navigation increased the mean operative time by 20 minutes compared with standard cutting guides and patient-specific instrumentation. However, postoperative complications were similar among all cutting guides. We were probably underpowered to detected rare but serious complications. Thus, in our view, the benefit/risk balance between a more precise tool, such as navigation, allowing more reproducible cuts but without evidence of an impact on function and an increase of surgical time does not favor the navigation approach.
Conclusions
No new approach in this study demonstrated superiority over standard cutting guides on functional outcome, and the alignment differences affected few patients, were small in magnitude, and were of questionable clinical importance. Thus, unless these new approaches either convincingly demonstrate superior survivorship or convincingly demonstrate superior long-term functional outcomes, there is no reason to use these approaches from either the patient’s point of view or the hospital’s point of view since they add cost, have a learning curve (during which some patients may be harmed), and have the risks associated with novelty.
Acknowledgments
We thank Professor Sylvie Chevret MD, PhD, director of the biostatistics department in the Saint Louis Hospital, for her advice. Furthermore, we thank every corresponding author who made the effort to provide us with the requested supplementary data.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution waived approval for the reporting of this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Hôpital Saint-Louis, INSERM, Paris, France.
References
- 1.Banerjee S, Cherian JJ, Elmallah RK, Jauregui JJ, Pierce TP, Mont MA. Robotic-assisted knee arthroplasty. Expert Rev Med Devices. 2015;12:727–735. [DOI] [PubMed] [Google Scholar]
- 2.Bauwens K Matthes G Wich M Gebhard F,Hanson B Ekkernkamp A,Stengel D, Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89:261-269. [DOI] [PubMed] [Google Scholar]
- 3.Berger RA, Crossett LS, Jacobs JJ, Rubash HE. Malrotation causing patellofemoral complications after total knee arthroplasty. Clin Orthop Relat Res. 1998; 356:144–153. [DOI] [PubMed] [Google Scholar]
- 4.Brooks SP, Gelman A. General Methods for Monitoring Convergence of Iterative Simulations. J Comput Graph Stat. 1998;7:434-355. [Google Scholar]
- 5.Clark GW, Wood DJ. Robotics in arthroplasty: Where are we today? Bone & Joint 360 2015;4:2-7. [Google Scholar]
- 6.Deep K, Shankar S, Mahendra A. Computer assisted navigation in total knee and hip arthroplasty. SICOT-J. 2015;3:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–944. [DOI] [PubMed] [Google Scholar]
- 8.Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Mak. 2013;33:641–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Y, Wang M, Liu Y, Fu Q. Alignment outcomes in navigated total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20:075–1082. [DOI] [PubMed] [Google Scholar]
- 10.Gargon E, Williamson PR, Altman DG, Blazeby JM, Tunis S, Clarke M. The COMET Initiative database: progress and activities update (2015). Trials. 2017; 18:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gharaibeh MA, Solayar GN, Harris IA, Chen DB, MacDessi SJ. Accelerometer-based, portable navigation (KneeAlign) vs conventional instrumentation for total knee arthroplasty: a prospective randomized comparative trial. J Arthroplasty. 2017;32: 777–782. [DOI] [PubMed] [Google Scholar]
- 12.Haglin JM, Eltorai AEM, Gil JA, Marcaccio SE, Botero-Hincapie J, Daniels AH. Patient-specific orthopaedic implants. Orthop Surg.2016;8:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC, Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ . 2011;18:343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2015;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty.2015;30:1906–1910. [DOI] [PubMed] [Google Scholar]
- 16.Huijbregts HJTAM, Khan RJK, Sorensen E, Fick DP, Haebich S. Patient-specific instrumentation does not improve radiographic alignment or clinical outcomes after total knee arthroplasty. Acta Orthop. 2016;87:386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikawa T, Takemura S, Kim M, Takaoka K, Minoda Y, Kadoya Y. Usefulness of an accelerometer-based portable navigation system in total knee arthroplasty. Bone Joint J. 2017;99:1047–1052. [DOI] [PubMed] [Google Scholar]
- 18.Jacofsky DJ, Allen M. Robotics in arthroplasty: A Comprehensive Review. J Arthroplasty. 2016;31:2353–2363. [DOI] [PubMed] [Google Scholar]
- 19.Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73:709–714. [DOI] [PubMed] [Google Scholar]
- 20.Kim YH, Kim JS, Yoon SH. Alignment and orientation of the components in total knee replacement with and without navigation support: a prospective, randomised study. J Bone Joint Surg. 2007;89:471–476. [DOI] [PubMed] [Google Scholar]
- 21.Kinney MC, Cidambi KR, Severns DL, Gonzales FB. Comparison of the iAssist handheld guidance system to conventional instruments for mechanical axis restoration in total knee arthroplasty. J Arthroplasty. 2018;33:61–66. [DOI] [PubMed] [Google Scholar]
- 22.Kuzyk PRT, Higgins GA, Tunggal JAW, Sellan ME, Waddell JP, Schemitsch EH. Computer navigation vs extramedullary guide for sagittal alignment of tibial components: radiographic study and meta-analysis. J Arthroplasty.2012;27:630–637. [DOI] [PubMed] [Google Scholar]
- 23.Laurent P. Patient-specific instruments in orthopaedics. In: Ritacco LE, Milano FE, Chao E, eds. Computer-assisted Musculoskeletal Surgery. Philadelphia, PA: Springer; 2016:163–179. [Google Scholar]
- 24.Leopold SS. Pencil and paper research? Network meta-analysis and other study designs that do not enroll patients. Clin Orthop Relat Res. 2015;473:2163-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liow MHL, Goh GSH, Wong MK, Chin PL, Tay DKJ, Yeo SJ. Robotic-assisted total knee arthroplasty may lead to improvement in quality-of-life measures: a 2-year follow-up of a prospective randomized trial. Knee Surg Sports Traumatol Arthrosc Off; 2017;2942–2951. [DOI] [PubMed] [Google Scholar]
- 26.Liow MHL, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis: A prospective randomised study. J Arthroplasty. 2014;29:2373–2377. [DOI] [PubMed] [Google Scholar]
- 27.Lovelock TM, Broughton NS, Williams CM. The popularity of outcome measures for hip and knee arthroplasties. J Arthroplasty. 2018;33:273–276. [DOI] [PubMed] [Google Scholar]
- 28.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. [DOI] [PubMed] [Google Scholar]
- 29.Mannan A, Akinyooye D, Hossain F. A Meta-analysis of functional outcomes in patient-specific instrumented knee arthroplasty. J Knee Surg. 2017;30:668–674. [DOI] [PubMed] [Google Scholar]
- 30.Mannan A, Smith TO. Favourable rotational alignment outcomes in PSI knee arthroplasty: A Level 1 systematic review and meta-analysis. The Knee. 2016;23:186–190. [DOI] [PubMed] [Google Scholar]
- 31.Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22:1097–1106. [DOI] [PubMed] [Google Scholar]
- 32.Mattei L, Pellegrino P, Calò M, Bistolfi A, Castoldi F. Patient specific instrumentation in total knee arthroplasty: a state of the art. Ann Transl Med. 2016;4:126-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moskal J, Capps S, Mann J, Scanelli J. Navigated versus conventional total knee arthroplasty. J Knee Surg. 2013;27:235–248. [DOI] [PubMed] [Google Scholar]
- 34.Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: A randomized, controlled trial: Winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29:288–294. [DOI] [PubMed] [Google Scholar]
- 35.Parratte S, Blanc G, Boussemart T, Ollivier M, Corroller TL, Argenson JN. Rotation in total knee arthroplasty: no difference between patient-specific and conventional instrumentation. Knee Surg Sports Traumatol Arthrosc. 2013;21:2213–2219. [DOI] [PubMed] [Google Scholar]
- 36.Parratte S, Pagnano MW, Trousdale RT, Berry DJ. Effect of postoperative mechanical axis alignment on the fifteen-year survival of modern, cemented total knee replacements. J Bone Joint Surg Am. 2010;92:2143-2149. [DOI] [PubMed] [Google Scholar]
- 37.Petursson G, Fenstad AM, Gothesen O, Dyrhovden GS, Hallan G, Rohrl SM, Aamodt A, Furnes O. Computer-assisted compared with conventional total knee replacement: a multicenter parallel-group randomized controlled trial. J Bone Joint Surg Am. 2018;100:1265–1274. [DOI] [PubMed] [Google Scholar]
- 38.Rebal BA, Babatunde OM, Lee JH, Geller JA, Patrick DAJ, Macaulay W. Imageless computer navigation in total knee arthroplasty provides superior short term functional outcomes: a meta-analysis. J Arthroplasty. 2014;29:938–944. [DOI] [PubMed] [Google Scholar]
- 39.Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153–156. [PubMed] [Google Scholar]
- 40.Salanti G, Giovane CD, Chaimani A, Caldwell DM, Higgins JPT. Evaluating the Quality of Evidence from a Network Meta-Analysis. PLoS One. 2014;9:e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sikorski JM. Alignment in total knee replacement. J Bone Joint Surg Br. 2009;90:1121–1127. [DOI] [PubMed] [Google Scholar]
- 42.Stulberg SD, Loan P, Sarin V. Computer-assisted navigation in total knee replacement: results of an initial experience in thirty-five patients. J Bone Joint Surg Am. 2002;84(A Supple 2):90–98. [PubMed] [Google Scholar]
- 43.Thiengwittayaporn S, Fusaku Y, Kangkano N, Jarupongprapa C, Charoenphandhu N. Hand-held navigation may improve accuracy in minimally invasive total knee arthroplasty: a prospective randomized controlled trial. Int Orthop. 2016;40:51–57. [DOI] [PubMed] [Google Scholar]
- 44.Thienpont E, Schwab PE, Fennema P. Efficacy of patient-specific instruments in total knee arthroplasty: a systematic review and meta-analysis. J Bone Joint Surg Am. 2017;99:521-530. [DOI] [PubMed] [Google Scholar]
- 45.Trinquart L, Attiche N, Bafeta A, Porcher R, Ravaud P. Uncertainty in treatment rankings: reanalysis of network meta-analyses of randomized trials. Ann Intern Med. 2016;164:666-673. [DOI] [PubMed] [Google Scholar]
- 46.Van Valkenhoef and Joel Kuiper. gemtc: Network Meta-Analysis Using Bayesian Methods. R package version 0.8-2. https://CRAN.R-project.org/package=gemtc. Accessed March 20, 2020. [Google Scholar]
- 47.Vanlommel L, Vanlommel J, Claes S, Bellemans J. Slight undercorrection following total knee arthroplasty results in superior clinical outcomes in varus knees. Knee Surg Sports Traumatol Arthrosc . 2013;21:2325-2330. [DOI] [PubMed] [Google Scholar]
- 48.Weir CJ, Butcher I, Assi V, Lewis SC, Murray GD, Langhorne P, Brady MC. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: a systematic review. BMC Med Res Methodol . 2018;18:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Victor J, Dujardin J, Vandenneucker H, Arnout N, Bellemans J. Patient-specific guides do not improve accuracy in total knee arthroplasty: A prospective randomized controlled trial knee. Clin Orthop Relat Res . 2014;472:263-271. [DOI] [PMC free article] [PubMed] [Google Scholar]