Abstract
The whole world is currently facing a pandemic of an infectious disease known as novel coronavirus disease-2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) . This outbreak emerged unexpectedly and imposed a potential threat to humans, associated with the social and economic burden on the individual and federal governments. COVID-19, which initially started in Wuhan City of China and then spread to the whole world, has been declared a Public Health Emergency of International Concern. The continuous increase in the number of confirmed cases leads to high mortality across the world. Growing evidence indicates that the mortality rate is very predominant in elderly people and those with preexisting health conditions. However, the potential pathogenesis of SARS-CoV-2 infection in humans is still unknown. The dysregulated/exuberant immune response may have substantially contributed to the SARS-CoV-2-mediated pathology. Nevertheless, there is no clinically approved drug/vaccine currently available that can restrict its pathogenesis. However, several drugs are currently shown to provide some therapeutic benefits for COVID-19 patients, including antiviral drugs that might have a significant role in restricting the current pandemic of COVID-19. In this article, we highlighted the pharmacological treatment strategies for COVID-19 and purposed the therapeutic targets for the development of vaccines or anti-viral drug molecules against SARS-CoV-2 infection in humans.
Keywords: Novel coronavirus disease, COVID-19, SARS-CoV-2, Anti-viral drugs, Vaccine, Clinical trials
Introduction
In the past 2 decades, three coronavirus (CoV) outbreaks occurred in the world [1]: severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002 [2, 3], Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012 [4], and SARS CoV-2 in 2019 [5]. The SARS-CoV epidemic emerged from an animal market in the Guangdong province of China and spread to 32 countries through air travel routes, infecting 8422 individuals and 916 (10.87%) casualties from November 2002 to August 2003 [6, 7]. MERS-CoV was first reported to cause human infection in Saudi Arabia in 2012, where it remains a major public health concern, and spread over 27 countries, infecting a total of 2,494 individuals and claiming 868 (34.77%) fatalities during the period April 2012 to December 2019 [4, 7, 8].
The recent outbreak of coronavirus disease 2019 (COVID-19) started in December 2019 in Wuhan City of China and spread through human-to-human transmission across the world [9–11]. It continues to cause severe infections in humans, posing significant threats to global public health. China initially reported to the World Health Organization (WHO) on December 31, 2019. Further, on March 11, 2020, the WHO declared COVID-19 a pandemic and imposed Public Health Emergency of International Concern [12]. The fatality rate of coronavirus MERS-CoV was (34.77%) higher than that of SARS-CoV (10.87%); however, SARS-CoV-2 transmitted rapidly in comparison to SARS-CoV and MERS-CoV [13] and accounts for 3.4% deaths all over the world [14]. The mortality rate of COVID-19 varies from one country to another. However, these fatality rates also vary with the age range of the infected persons. COVID-19 primarily spread through respiratory droplets. The manifestation of SARS-CoV-2 infections ranges from fever, cough, shortness of breath, fatigue, and, in a small population of patients, gastrointestinal infection symptoms to acute respiratory distress and pneumonia [15–17]. The loss of taste (ageusia) and loss of smell (anosmia) as one of the major symptoms of COVID-19 was initially ignored. However, further studies demonstrated a significant presence of ageusia and anosmia in the patients with COVID-19 infection [18–20]. The reproductive number of SARS-CoV-2 infection is estimated to be 2–3 [11], and the elderly people with underlying complications such as diabetes, heart disease, lung disease, cancer, etc. are more susceptible to severe infection and fatality [21–24]. Currently, there is no clinically approved drug/vaccine for the treatment of this disease. All the health organizations across the globe are on high alert and treating COVID-19 patients with the available drugs used in other respiratory infections. However, several potent candidates of antivirals and repurposed drugs are under urgent investigation [9]. This article highlighted the recent updates on the epidemiology, antiviral drugs used, and possible therapeutic strategies for the development of a vaccine against SARS-CoV-2 pathology.
Epidemics of COVID-19
According to recent WHO updates, globally, there are about 18 million confirmed cases and rising in American, European, and Southeast Asian countries [14]. To date, 216 countries and territories have been affected by the COVID-19 pandemic with a 5.9% mortality rate estimated by the WHO. Although the disease is now better contained in the suspected origin, the USA has witnessed the biggest surge in coronavirus cases over the past few months which accounts for around one quarter of the total number of infections worldwide. The USA, Brazil, and India are the most affected countries, contributing about half of the COVID-19-infected people across the world [14]. European countries witnessed the maximum number of coronavirus-related deaths than any part of the world followed by the USA. However, the actual total death toll may be higher than the number of confirmed deaths, due to limited testing and problems in the attribution of the cause of death and the difference between reported confirmed deaths and total deaths that varies by country to country. Surprisingly, men are at a significantly higher risk of having severe symptoms and fatalities, compared to women [14]. A maximum number of infected individuals are in the age group of 21–45 years; however, the fatalities are more in elderly patients, i.e., above 60 years. If the current situation prevails for a longer duration and no therapeutic drug/vaccine is available, it is expected that almost one third of the world population may get infected and millions of deaths occur, worldwide.
Etiology of COVID-19
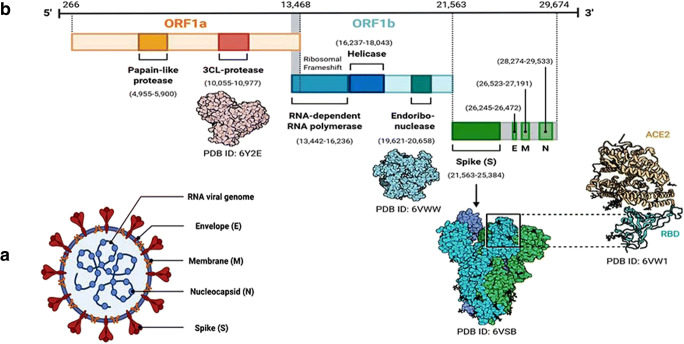
The coronaviruses belong to a large subfamily, Orthocoronavirinae, of the Coronaviridae family within the Nidovirales order and are classified into 4 genera: Alpha (α), Beta (β), Gamma (γ), and Delta (δ) coronavirus. They are single-stranded RNA viruses (+ssRNA) genomes with the size ranging from 26 to 32 kilobases, with a crown-like appearance due to the presence of spike glycoproteins on the envelope. They are known to infect both humans and animals. SARS-CoVs, MERS-CoVs, and SARS-CoV-2 are classified as β-coronavirus family members [25]. There are structural similarities between the MERS-CoV and SARS-CoV; however, they target the host cells through different receptors, dipeptidyl peptidase 4 (DDP4) and angiotensin-converting enzyme 2 (ACE2) respectively [26, 27]. The coronavirus particle, virion, consists of a nucleocapsid, containing single-stranded genomic RNA and phosphorylated nucleocapsid (N) protein, which is covered inside the phospholipid bilayers having two different types of spike proteins (Fig. 1), the spike glycoprotein trimmer (S) that is present in almost all the CoVs and the hemagglutinin-esterase (HE) that is found in few CoVs. The membrane (M) and envelope (E) proteins are located among the S proteins in the virus envelope [28]. SARS-CoV-2 is a positive-sense, single-stranded RNA virus containing 29,891 nucleotides, encoding for 9860 amino acids [29]. Although its origin is not entirely understood, the genomic analyses suggest that SARS-CoV-2 probably evolved from a strain found in bats [30].
Fig. 1.
(A) Structure of SARS-CoV-2 particle, virion, and (B) genomic organization of SARS-CoV-2 (adapted from Chan et al. 2020). A SARS-CoV-2 particle is approximately 70–90 nm in size, 30 kb, an ssRNA virus, similar in structure to the SARS-CoV virion, and belongs to the family Coronaviridae. It has a crown shape with spikes on the membrane that are used to embed in the host membrane-derived lipid bilayer. (B) There are 6–11 open reading frames (ORFs) with 5′ and 3′ flanking untranslated regions (UTRs). The nsps constitutes dependent RNA polymerase (nsp12), main protease (nsp5), helicase (nsp13), and papain-like protease (nsp3)
Recently, bioinformatic analysis of a virus genome from a patient with COVID-19 shows that the genome of SARS-CoV-2 shows 89% nucleotide identity with batSARS-like-CoVZXC21 and 82% with that of human SARS-CoV [29]. The phylogenetic trees of their orf1a/b, spike, envelope, membrane, and nucleoprotein also clustered closely with those of the bat, civet, and human SARS coronaviruses. Further, additional genomic studies suggest that SARS-CoV-2 shared 79% nucleotide identity to SARS-CoV and 51.8% identity to MERS-CoV [31, 32]. Figure 2 shows the comparative analysis of the genomic organization of various coronaviruses in different species. All these studies indicated a high genetic homology among SARS-CoV-2, MERS-CoV, and SARS-CoV. SARS-CoV-2 uses the same receptor, angiotensin-converting enzyme 2 (ACE2), as that for SARS-CoV to enter the target cell, and mainly spreads through the respiratory tract. ACE2 enzyme is involved in the regulation of blood pressure and is expressed by cells of the heart, lungs, kidneys, and intestines. SARS-CoV-2 binds to ACE2 through spike proteins. However, the external subdomain of the spike’s receptor-binding domain of SARS-CoV-2 shares only 40% amino acid identity with other SARS-related coronaviruses [29]. So, the drugs targeting SARS-CoV and MERS-CoV might not be fit for the prevention of COVID-19.
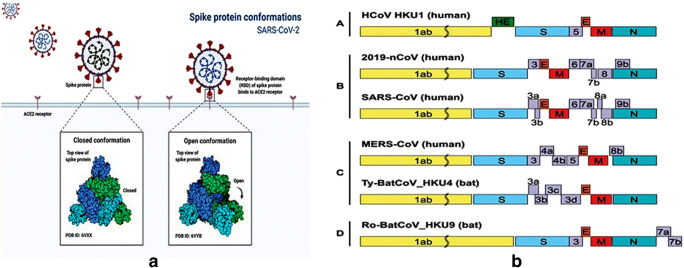
Fig. 2.
a Spike protein conformation of SARS-CoV-2 and b schematic comparison of the genome organization of SARS-CoV and MERS-CoV
Current Scenario of Possible Therapeutic Drugs Against SARS-CoV-2 Infection
To date, we do not have any clinically approved antiviral drug or vaccine that can cure this disease. However, the battle to find a specific therapy for the recent pandemic of COVID-19 is still going on. Currently, various preventive measures including social distancing, hand sanitization, avoiding non-essential international/national travels, use of facial masks, etc. are used to restrict the further transmission of SARS-CoV-2 infection across the world. Several pharmacological treatment possibilities are being explored to treat this infection. In the current situation of COVID-19 pandemic, possible repositioning of various antiviral and antiparasitic drugs including hydroxychloroquine and chloroquine, remdesivir, protease inhibitors—lopinavir and ritonavir, etc. previously used for SARS and MERS or other viral infections is now being followed to treat SARS-CoV-2 infection and might have gained significant improvement in pneumonia-associated symptoms of some of the COVID-19 patients [9, 33–38].
Table 1 shows the clinical trials of repurposing various drugs as a possible therapeutic strategy against SARS-CoV-2 infection. Remdesivir, a nucleotide analog, is a broad-spectrum antiviral drug that inhibits RNA replication [30] and may be a promising anti-viral drug therapy for COVID-19. Several studies demonstrated the effectiveness of remdesivir in animal models of SARS-CoV-2-related coronaviruses [30, 39–41]. However, it was less effective against ebolavirus infections in humans [42]. Recently, clinical trials of remdesivir against COVID-19 are ongoing primarily in the USA and China [43].
Table 1.
Current status of clinical trial studies on the interventions against COVID-19 across the world as of July 31, 2020 (Source: National Institute of Health, https://clinicaltrials.gov/) Accessed on Aug 1, 2020
| No. | Title | Status | Conditions | Interventions | Locations |
|---|---|---|---|---|---|
| 1 | Maternal-Foetal Transmission of SARS-Cov-2 | Recruiting |
Maternal fetal infection transmission COVID-19 SARS-CoV 2 |
Diagnostic test: diagnosis of SARS-Cov2 by RT-PCR and IgG, Ig M serologies in the amniotic fluid, the blood cord, and the placenta | • CHR Orléans, Orléans, France |
| 2 | COVID-19 Breastfeeding Guideline for African-Americans | Not yet recruiting |
COVID-19 Exclusive breastfeeding |
Behavioral: COVID-19 breastfeeding support | • Meharry Medical College, Nashville, TN, USA |
| 3 | Comparison of the Efficacy of Rapid Tests to Identify COVID-19 Infection (CATCh COVID-19) | Not yet recruiting | COVID-19 | Diagnostic test: diagnostic tests for COVID-19 infection | |
| 4 | The Role of a Private Hospital in Hong Kong Amid COVID-19 Pandemic | Active, not recruiting | COVID | Diagnostic test: COVID 19 diagnostic test | • Hong Kong Sanatorium & Hospital, Hong Kong, Hong Kong |
| 5 | Lung CT Scan Analysis of SARS-CoV2 Induced Lung Injury | Recruiting | COVID-19 | Other: lung CT scan analysis in COVID-19 patients |
• Ospedale Papa Giovanni XXIII, Bergamo, Italy • Policlinico San Marco-San Donato group, Bergamo, Italy • Azienda Ospedaliero-Universitaria di Ferrara, Ferrara, Italy • ASST di Lecco Ospedale Alessandro Manzoni, Lecco, Italy • ASST Melegnano-Martesana, Ospedale Santa Maria delle Stelle, Melzo, Italy • ASST Monza, Monza, Italy • AUSL Romagna-Ospedale Infermi di Rimini, Rimini, Italy • Istituto per la Sicurezza Sociale-Ospedale della Repubblica di San Marino, San Marino, San Marino |
| 6 | Study Evaluating the Safety and Efficacy of Autologous Non-hematopoietic Peripheral Blood Stem Cells in Covid-19 | Completed | Coronavirus disease 2019 (COVID-19) |
Biological: autologous non-hematopoietic peripheral blood stem cells (NHPBSC) Drug: COVID-19 standard care |
• Abu Dhabi Stem Cells Center, Abu Dhabi, United Arab Emirates |
| 7 | Performance Evaluation of RealDetect™ COVID-19 RT-PCR Kit for the Detection of SARS-CoV-2 Virus | Recruiting | High sensitivity and specificity (with 95% confidence interval) of RealDetect™ COVID-19 RT-PCR Kit | Device: performance evaluation study of RealDetect RT-PCR Kit for COVID-19 detection | • Institute of Epidemiology, Disease Control and Research (IEDCR), Mohakhali, Dhaka (for sample collection and sample storage), Institute for Developing Science & Health Initiatives (ideSHi); Mohakhali, Dhaka (for sample analysis, data collection, data analysis), Dhaka, Bangladesh |
| 8 | Diagnostic Value of Patient-Reported and Clinically Verified Olfactory Disorders (COVID-19) | Completed | COVID-19 | Biological: reporting of anosmia, ageusia, and other clinical symptoms | • Uhmontpellier, Montpellier, France |
| 9 | Smell and Taste Disorders in COVID-19 Patients | Recruiting | COVID-19 | Other: investigation of smell and taste disorders | • ASST Monza-Ospedale San Gerardo, Monza, Italy |
| 10 | Neonatal Complications of Coronavirus Disease (COVID-19) | Recruiting | Neonatal COVID-19 disease | Other: no intervention—exposure is to COVID-19 | • Imperial College, London, UK |
| 11 | COVID-19 Infection in Patients with Hepatocellular Carcinoma | Recruiting |
Hepatocellular carcinoma COVID-19 |
Diagnostic test: nasopharyngeal COVID-19 RT-PCR | • CHU Amiens, Amiens, France |
| 12 | COVID-19 Prevalence and Cognitive Deficits in Neurological Patients | Not yet recruiting |
Neurological diseases or conditions Stroke, acute Seizure disorder |
Diagnostic test: COVID-19 swap test PCR |
• Aalborg University Hospital, Aalborg, DK, Denmark • Aarhus University Hospital, Aarhus, DK, Denmark • Regional Hospital West Jutland, Hostebro, Holstebro, DK, Denmark • Regional Hospital Central Jutland, Viborg, Viborg, DK, Denmark |
| 13 | Frailty in Elderly Patients with COVID-19 | Recruiting | COVID-19 | Other: relation between frailty and clinical outcomes in elderly patients with COVID-19 | • ASST Monza-Ospedale San Gerardo, Monza, Italy |
| 14 | COVID-19 Convalescent Plasma (CCP) Transfusion | Recruiting | COVID-19 | Biological: COVID convalescent plasma | • University of Mississippi Medical Center, Jackson, MS, USA |
| 15 | COVID-19 in the Swedish ICU-Cohort: Risk Factors of Critical Care Admission and Intensive Care Mortality | Not yet recruiting |
COVID-19 Critical illness |
Other: COVID-19 and intensive care | |
| 16 | MURDOCK Cabarrus County COVID-19 Prevalence and Immunity (C3PI) Study | Enrolling by invitation | COVID-19 | Other: COVID-19 PCR and serology testing | • Duke CTSI Translational Population Health Office, Kannapolis, NC, USA |
| 17 | Rapid Diagnostic Test for COVID-19 Based on Antibody Detection (YCOVID) | Active, not recruiting | COVID-19 | Diagnostic test: ELISA and Rapid test to detect antibodies against COVID-19 | • Parc Tauli University Hospital, Sabadell, Barcelona, Spain |
| 18 | The Assessment of the Prevalence, Clinical Course and Treatment of COVID-19 Complications | Enrolling by invitation |
COVID-19 Coronavirus infection SARS-CoV-2 Complications |
Other: complex diagnostic panel | • Silesian Centre for Heart Disease, Zabrze, Silesia, Poland |
| 19 | COVID19-Convalescent Plasma for Treating Patients with Active Symptomatic COVID 19 Infection (FALP-COVID) | Recruiting |
COVID-19 infection Cancer patients General population |
Biological: convalescent plasma from COVID-19 donors | • Fundacion Arturo Lopez Perez, Providencia, Santiago, Chile |
| 20 | COVID-19 Endoscopy Survey | Completed | COVID-19 | Other: practice details |
• Kings County Hospital Center, Brooklyn, NY, USA; Albertson, NY, USA • Faculty of Medicine, Zagazig University, Zagazig, Sharkia, Egypt • Al-Azhar Univerisity, Cairo, Egypt • Ahvaz Imam hospital, Ahvaz, Iran, Islamic Republic of |
| 21 | Survey: COVID-19 Patients Managed in the Operating Theatre of Belgian Hospitals | Recruiting | COVID-19 | • Cliniques Universitaires Saint Luc, Brussels, Belgium | |
| 22 | COVID-19 Surveillance of Patients and Healthcare Workers in a Hospital Department | Enrolling by invitation | COVID-19 | Diagnostic test: COVID-19 test, polymerase chain reaction for SARS-CoV-2 | • Rigshospitalet University Hospital of Copenhagen, Copenhagen, Denmark |
| 23 | Viral Infection and Respiratory Illness Universal Study [VIRUS]: COVID-19 Registry | Recruiting | Coronavirus | Other: observational |
• Mayo Clinic in Arizona, Scottsdale, AZ, USA • Mayo Clinic in Florida, Jacksonville, FL, USA • Society of Critical Care Medicine (150+ sites), Chicago, IL, USA • Rahul Kashyap, Rochester, MN, USA |
| 24 | Predictive Factors COVID-19 Patients | Recruiting | COVID-19 | Other: predictive factors for clinical response in patients with COVID-19 | • ASST Monza-Ospedale San Gerardo, Monza, Italy |
| 25 | Characteristics and Outcome of Coronavirus Disease 2019 (COVID-19) in Egypt | Not yet recruiting |
Characteristic diseases Outcome, fatal |
Other: follow-up | |
| 26 | Detection of Anti-COVID-19 Antibody Levels in an Hospital Population | Recruiting | COVID-19 | Diagnostic test: detection of anti-COVID-19 antibody level | • Humanitas Rozzano/San Pio X, Rozzano, Lombardia, Italy |
| 27 | Isotretinoin in Treatment of COVID-19 | Not yet recruiting | COVID-19 | Drug: isotretinoin only product in oral dose form | |
| 28 | Convalescent Plasma for Patients with COVID-19: a Pilot Study | Not yet recruiting |
Coronavirus Coronavirus infection |
Drug: plasma | • Universidad del Rosario, Bogota, Cundinamarca, Colombia |
| 29 | Slovenian National COVID-19 Prevalence Study | Recruiting |
COVID-19 SARS-CoV-2 |
Diagnostic test: no intervention planned due to the observational study design—only a diagnostic testing | • University of Ljubljana, Ljubljana, Slovenia |
| 30 | Incidence of COVID-19 Test Conversion in Post-surgical Patients | Recruiting | SARS-CoV-2 | Diagnostic test: COVID-19 PCR and serology |
• North Shore University Hospital, Manhasset, NY, USA • Long Island Jewish Medical Center, New Hyde Park, NY, USA |
| 31 | Novel COVID-19, a National Analysis | Recruiting | COVID-19 | Other: prevalence of COVID-19 | • Assiut University Hospitals, Assiut, Egypt |
| 32 | Convalescent Plasma for Patients with COVID-19: a Randomized, Open Label, Parallel, Controlled Clinical Study | Not yet recruiting |
Coronavirus Coronavirus infection |
Drug: plasma Drug: hydroxychloroquine |
• Universidad del Rosario, Bogota, Cundinamarca, Colombia |
| 33 | Methodist Health System COVID-19 Patient Registry | Not yet recruiting | COVID-19 | Other: treatment for COVID-19 | • Clinical Research Institute Methodist Health System, Dallas, TX, USA |
| 34 | Safety and Efficacy of Melatonin in Outpatients Infected with COVID-19 | Not yet recruiting | COVID-19 |
Drug: melatonin Other: placebo (methylcellulose) capsule |
• University at Buffalo, Buffalo, NY, USA |
| 35 | Discovery Stage (Proof-of-Concept) COVID-19 Antigen Presentation Therapeutic Vaccine | Active, not recruiting | COVID-19 |
Biological: COVID-19 therapeutic vaccine - Nucleocapsid-GM-CSF protein lactated Ringer’s injection |
• Medicine Invention Design Incorporation (MIDI) - IORG0007849, North Bethesda, MD, USA |
| 36 | Dynamic Evaluation of COVID-19 Diagnostic Tests | Not yet recruiting | COVID-19 | Diagnostic test: COVID-19 diagnostic test | |
| 37 | NGS Diagnostic in COVID-19 Hosts - Genetic Cause Relating to the Course of Disease Progression | Not yet recruiting | COVID-19 |
Genetic: whole genome analysis Genetic: T cell receptor (TCR) repertoire Genetic: SARS-CoV-2 viral composition |
|
| 38 | Beaumont Health Large-Scale Automated Serologic Testing for COVID-19 | Recruiting |
COVID-19 Coronavirus infection Severe acute respiratory syndrome coronavirus 2 |
Diagnostic test: EUROIMMUN assay |
• Beaumont Health System, Royal Oak, MI, USA • Beaumont Health, Royal Oak, MI, USA |
| 39 | Assessment of Obstetric, Fetal and Neonatal Risks and Vertical SARS-CoV-2 Transmission During COVID-19 Pandemic | Recruiting | Pregnancy | Diagnostic test: COVID 19 diagnostic test by PCR | • University Hospital of Toulouse, Toulouse, France |
| 40 | Minimal Invasive Autopsies to Investigate Changes in Deceased COVID-19 Patients | Recruiting | COVID-19 | Procedure: CT-scan with minimal invasive autopsy | • Jessa Hospital, Hasselt, Belgium |
| 41 | Exchange Transfusion Versus Plasma from Convalescent Patients with Methylene Blue in Patients with COVID-19 | Recruiting | COVID-19 |
Biological: exchange blood transfusion from normal donor Biological: plasma from convalescent patients with COVID-19 Drug: Methylene Blue 5 MG/ML |
• Ain Shams University, Cairo, Egypt |
| 42 | Clinical Characteristics and Outcomes of Pediatric COVID-19 | Recruiting |
COVID-19 SARS-CoV-2 infection Pediatric ALL Pneumonia, viral Pandemic response |
Other: exposure (not intervention)—SARS-CoV-2 infection | • University of Calgary/Alberta Children’s Hospital, Calgary, Alberta, Canada |
| 43 | Relation Between Lab Finding and COVID-19 Severity | Not yet recruiting | COVID-19 |
Diagnostic test: D-dimer, CBC. ESR, CRP Diagnostic test: liver function tests, serum ferritin, and PCR for COVID-19 |
|
| 44 | Setting Up a COVID-19 Care Facility at a Prison in Pakistan | Completed | COVID-19 | Other: COVID-19 facility | • Camp Jail/COVID-19 care facility/SIMS, Lahore, Pakistan |
| 45 | Impact of COVID-19 on Marshallese Communities in the U.S. | Not yet recruiting | COVID-19 | Other: assessing impact of COVID-19 | |
| 46 | Thorax Computed Tomography Severity Score and Outcome in COVID-19 Patients | Recruiting | COVID-19 | Diagnostic test: CT scan | • Tepecik Training and Research Hospital, Izmir, Izmir, Konak, Turkey |
| 47 | Antibody Based Tests for SARSCOV-2 COVID-19-Evaluation of Patients and Healthcare Providers in the Confines of Healthcare Settings | Recruiting | COVID-19 | Diagnostic test: CoronaCideTM COVID-19 IgM/IgG Rapid Test and Premier Biotech COVID-19 IgM/IgG Rapid Test | • St. David’s Medical Center, Austin, TX, USA |
| 48 | Spartan COVID-19 System: Evaluation of Clinical Sample Collection | Recruiting | COVID-19 | Device: Spartan COVID-19 System |
• Humber River Hospital, North York, Ontario, Canada • The University of Ottawa Heart Institute, Ottawa, Ontario, Canada |
| 49 | Classification of COVID-19 Infection in Posteroanterior Chest X-rays | Completed | COVID-19 | Device: CovX | • Dascena, Oakland, CA, USA |
| 50 | Rapid, Onsite COVID-19 Detection | Enrolling by invitation |
COVID-19 SARS-CoV-2 |
Device: Rapid Onsite COVID-19 detection | • University of Wisconsin, Madison, WI, USA |
| 51 | Safety and Efficacy Of Hydroxychloroquine for At Risk Population (SHARP) Against COVID-19 | Not yet recruiting |
Coronavirus infection Hydroxychloroquine adverse reaction |
Drug: hydroxychloroquine sulfate 200 mg (mg) tab | |
| 52 | Validation of COVID-19 Tests | Active, not recruiting | COVID-19 |
Diagnostic test: Covid-19 Rapid Test Kit (RAPG-COV-019) Diagnostic test: quantitative IgG test |
• Richmond Pharmacology Ltd. 1a Newcomen St, London Bridge, London, UK |
| 53 | Pulmonary Optical Coherence Tomography in COVID-19 Patients | Recruiting |
COVID-19 Pulmonary embolism |
Diagnostic test: optical coherence tomography (OCT) |
• Incor - Heart Institute - University of Sao Paulo, São Paulo, Brazil • IRCCS San Raffaele, Milano, Italy |
| 54 | COVID-19 Surveillance Based on Smart Wearable Device | Not yet recruiting | COVID-19 | • Peking University First Hospital, Beijing, Beijing, China | |
| 55 | Descriptive and Retrospective Analysis of Acute Myocarditis Associated with Pandemic COVID-19 in Children | Not yet recruiting | COVID-19 |
• Hôpital Bicêtre, Le Kremlin-Bicêtre, France • Hôpital Armand Trousseau, Paris, France • Hôpital Necker-Enfants Malades, Paris, France • Hôpital Robert Debré, Paris, France |
|
| 56 | Diagnostic Value of New COVID-19 Antibodies Testing Among Laboratory Healthcare Workers | Not yet recruiting | COVID-19 | Diagnostic test: COVID-19 antibodies testing | |
| 57 | A Clinical Trial of Mycobacterium w in Critically Ill COVID 19 Patients | Recruiting | COVID-19 |
Drug: suspension of heat killed (autoclaved) Mycobacterium w Drug: standard therapy of COVID-19 |
• All India Institute of Medical Science, Raipur, Raipur, Chhattisgarh, India • All India Institute of Medical Sciences, Bhopal, Bhopal, Madhya Pradesh, India • Postgraduate Institute of Medical Education and Research, Chandigarh, India • All lndia Institute of Medical Science, Delhi, Delhi, India |
| 58 | Convalescent Plasma In ICU Patients With COVID-19-Induced Respiratory Failure | Recruiting |
COVID-19 SARS-CoV-2 |
Biological: multiple doses of anti-SARS-CoV-2 convalescent plasma |
• 8700 Beverly Blvd., Los Angeles, CA, USA • Johns Hopkins University, Baltimore, MD, USA |
| 59 | Immunoglobulin G Antibody Immune Response Profile Following Infection with SARS-CoV-2 in COVID-19 Egyptian Patients | Recruiting | COVID-19 | • Sohag University Hospital, Sohag, Egypt | |
| 60 | Efficacy and Safety of Early COVID-19 Convalescent Plasma in Patients Admitted for COVID-19 Infection | Recruiting | Severe acute respiratory syndrome coronavirus 2 | Biological: COVID-19 convalescent plasma | • Hospital Clínico Universidad Católica, Santiago, Chile |
| 61 | Will Hydroxychloroquine Impede or Prevent COVID-19 | Recruiting |
COVID-19 Coronavirus Coronavirus infections SARS-CoV-2 |
Drug: Hydroxychloroquine—daily dosing Drug: hydroxychloroquine—weekly dosing Other: placebo oral tablet Diagnostic test: monitoring visit—baseline Diagnostic test: monitoring visit—week 4 Diagnostic test: monitoring visit—week 8 Other: weekly assessment |
• Henry Ford Hospital, Detroit, MI, USA • Detroit Department of Transportation (DDOT), Detroit, MI, USA • Detroit Fire Department & Detroit EMS, Detroit, MI, USA • Detroit Police Department, Detroit, MI, USA |
| 62 | Various Combination of Protease Inhibitors, Oseltamivir, Favipiravir, and Hydroxychloroquine for Treatment of COVID-19: a Randomized Control Trial | Not yet recruiting |
SARS-CoV-2 infections COVID-19 |
Drug: oral | • Assistant Professor Subsai Kongsaengdao, Bangkok, Thailand |
| 63 | Predicting the Need for Intubation in Hospitalised COVID-19 Patients (PRED ICU COVID19) | Recruiting | COVID-19 | Other: data monitoring for 48 h within the first 12 h of admission for COVID-19 |
• Louis Mourier hospital (AP-HP), Colombes, France • Brabois Hospital (CHRU de Nancy), Vandœuvre-lès-Nancy, France |
| 64 | Effects on the Qt Interval of COVID-19 Coronavirus Infection | Recruiting |
Coronavirus infection Intensive care patients |
• Servicio de Anestesia, Hospital General Universitario Gregorio Marañon, Madrid, Spain | |
| 65 | COVID-19 Convalescent Plasma for the Treatment of Hospitalized Patients with Pneumonia Caused by SARS- CoV-2 | Recruiting | COVID-19 | Biological: COVID-19 convalescent plasma | • Hospital of the University of Pennsylvania, Philadelphia, PA, USA |
| 66 | COVID-19 Pandemic and Worldwide Organ Procurement | Recruiting | COVID-19 | • Paris Transplant Group, Paris, France | |
| 67 | Testing the Accuracy of a Digital Test to Diagnose Covid-19 | Recruiting | COVID-19 | Diagnostic test: COVID-19 swab PCR test | • King’s College London, London, United Kingdom |
| 68 | Clinical Trial of Allogeneic Mesenchymal Cells from Umbilical Cord Tissue in Patients with COVID-19 | Recruiting | COVID |
Biological: mesenchymal cells Drug: standard of care |
• Hospital Universitario de Getafe, Getafe, Madrid, Spain • Hospital Universitario de Cruces, Barakaldo, Spain • Hospital Universitario de La Princesa, Madrid, Spain • Hospital Infantil Universitario Niño Jesus, Madrid, Spain • Hospital Ramón Y Cajal, Madrid, Spain • Complejo Universitario La Paz, Madrid, Spain |
| 69 | The Heart Hive COVID-19 Study | Not yet recruiting |
COVID-19 Cardiomyopathies |
Other: COVID-19 experience surveys | • Imperial College London, London, UK |
| 70 | National Survey of Symptoms of People Aged 70 and Overs Diagnosed with COVID-19 | Completed |
SARS-CoV-2 COVID-19 |
Other: observation | • Angers University Hospital, Angers, France |
| 71 | Seroconversion Among Staff at a Large Acute Care Hospital in Denmark During the COVID-19 Pandemic | Recruiting |
COVID-19 Health personnel Personnel, hospital |
Other: serial seroconversion measurements in hospital employees during the COVID-19 pandemic |
• Nordsjællands Hospital, Hillerød, Capital Region, Denmark • Nykøbing Falster County Hospital, Nykøbing Falster, Southern Region, Denmark |
| 72 | Clinical Trial of Efficacy and Safety of Sinovac's Adsorbed COVID-19 (Inactivated) Vaccine in Healthcare Professionals | Not yet recruiting | COVID-19 |
Biological: adsorbed COVID-19 (inactivated) vaccine Biological: placebo |
• Universidade de Brasília, Brasilia, DF, Brazil • Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil • Hospital das Clínicas da Universidade Federal do Paraná, Curitiba, PR, Brazil • Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul, Porto Alegre, RS, Brazil • Hospital das Clínicas da UNICAMP, Campinas, SP, Brazil • Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo, Ribeirao Preto, SP, Brazil • Instituto de Infectologia Emílio Ribas, Sao Paulo, SP, Brazil • Centro de Pesquisas Clínicas do Instituto Central do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, Sao Paulo, SP, Brazil • Instituto Israelita de Ensino e Pesquisa Albert Einstein, Sao Paulo, SP, Brazil • Universidade Municipal de São Caetano do Sul, São Caetano do Sul, SP, Brazil • Faculdade de Medicina de São José do Rio Preto - FAMERP, São José Do Rio Preto, São Paulo, Brazil • Instituto de Infectologia Evandro Chagas - Fiocruz, Rio De Janeiro, Brazil |
| 73 | Gut Microbiota, “Spark and Flame” of COVID-19 Disease | Recruiting | COVID-19 | Other: exposure |
• Hospital CUF Infante Santo, S.A., Lisbon, Portugal • Hospital de São Francisco Xavier, Lisbon, Portugal • Centro Hospitalar Universitário São João, Oporto, Portugal |
| 74 | Antioxidant Therapy for COVID-19 Study | Not yet recruiting | COVID-19 |
Dietary supplement: antioxidation therapy Other: standard of care |
• Abia State Isolation Centre, Amachara, Umuahia, Abia State, Nigeria • Benue State University Teaching Hospital, Makurdi, Benue State, Nigeria • Brigadier Abba Kyari Memorial Hospital, Borno, Borno State, Nigeria • University of Maiduguri Teaching Hospital, Maiduguri, Borno State, Nigeria • University of Calabar Teaching Hospital, Calabar, Cross River, Nigeria • Federal Medical Centre Idi-Aba, Abeokuta, Ogun State, Nigeria • Olabisi Onabanjo University Teaching Hospital, Sagamu, Ogun State, Nigeria • Infectious Disease Hospital, Amanawa, Sokoto State, Nigeria • Murtala Muhammad Speciaist Hospital, Sokoto, Sokoto State, Nigeria • Occupational Therapy Center, Sokoto, Sokoto State, Nigeria • Usmanu Danfodiyo University Teaching Hospital, Sokoto, Sokoto State, Nigeria |
| 75 | COST (COvid STudio) ACTION: Study for the Evaluation of Specific Antibodies Anti Covid-19 Linked to Covid-19 Infection, Symptoms and Genetic Expression of ACE2 Polymorphisms in Patients (COST ACTION) | Recruiting | COVID-19 | Other: BioMedomics COVID-19 IgM-IgG Rapid Test | • AO San Paolo, Milan, IT, Italy |
| 76 | Experience of an Emergency Intensive Care Unit During COVID-19 Pandemic | Completed | COVID-19 | Diagnostic test: COVID-19 diagnostic PCR | • Seda Yilmaz Semerci, Istanbul, Turkey |
| 77 | Inhaled Nitric Oxide for Preventing Progression in COVID-19 | Recruiting | COVID-19 | Drug: nitric oxide | • Tufts Medical Center, Boston, MA, USA |
| 78 | Cohort of Patients Infected with SARS-CoV2 (COVID-19) or Suspected of Being | Recruiting | COVID-19 | Other: blood samples | • GH Pitié-Salpêtrière / Service d’Accueil des Urgences, Paris, Ile- de-France, France |
| 79 | Hydrogen-Oxygen Generator with Nebulizer in the Improvement of Symptoms in Patients Infected with COVID-19 | Recruiting | COVID-19 |
Device: oxyhydrogen Device: oxygen |
• First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China |
| 80 | Endovascular Thrombectomy in COVID-19 Infected Patients | Completed |
Ischemic stroke COVID-19 |
Other: mechanical trombectomy | • Uhmontpellier, Montpellier, France |
| 81 | Pre-exposure Prophylaxis with Hydroxychloroquine for High- Risk Healthcare Workers During the COVID-19 Pandemic | Suspended | COVID-19 |
Drug: hydroxychloroquine Drug: placebos |
• ISGlobal, Barcelona, Spain |
| 82 | Evaluating Clinical Parameters of COVID-19 in Pregnancy | Not yet recruiting |
COVID-19 2019 novel coronavirus infection COVID-19 infection |
• Chelsea and Westminster Hospital NHS Foundation Trust, London, UK | |
| 83 | Characteristics of COVID-19 Infection Among PREGnant Women | Recruiting |
Coronavirus infection Pregnancy related |
Other: COVID-19 positive via testing | • INOVA Health System, Falls Church, VA, USA |
| 84 | Combination of Chest Scanography and Nasal Viral Detection Test to Detect COVID-19 Positive Patients Before Surgical Intervention in a University Hospital During Coronavirus Pandemia | Active, not recruiting |
COVID-19 SARS-CoV-2 |
• Uh Montpellier, Montpellier, France | |
| 85 | Implementation of Physiotherapy on COVID-19 Patients in ICU | Recruiting |
SARS-CoV-2 COVID-19 |
Other: physiotherapy | • Uhmontpellier, Montpellier, France |
| 86 | Tableted COVID-19 Therapeutic Vaccine | Active, not recruiting | COVID-19 | Biological: V-SARS |
• Immunitor Inc., Vancouver, BC - British Columbia, Canada • Aldar Bourinbayar, Ulaanbaatar, BZD, 3-khoroo, Mongolia |
| 87 | Effects of COVID-19 Convalescent Plasma (CCP) on Coronavirus-Associated Complications in Hospitalized Patients | Recruiting |
COVID-19 SARS-CoV-2 |
Biological: COVID-19 convalescent plasma (CCP) Biological: placebo |
• San Francisco General Hospital, San Francisco, CA, USA • UCSF Medical Center at Mount Zion, San Francisco, CA, USA • University of California, San Francisco Medical Center (Parnassus Campus), San Francisco, CA, USA |
| 88 | Kerecis Oral and Nasal Spray for Treating the Symptoms of COVID-19 | Recruiting | COVID-19 |
Device: Kerecis oral and nasal spray Other: placebo |
• National Hospital of Iceland (Landspítali), Reykjavík, Iceland |
| 89 | Anti COVID-19 Convalescent Plasma Therapy | Not yet recruiting | COVID-19 | Biological: anti-SARS-CoV-2 convalescent plasma | |
| 90 | Influence of COVID-19 Infection in Thromboembolic Venous Disease: National Cohort Study | Not yet recruiting | COVID-19 | Other: deep venous disease diagnostic | • Red de Investigacion Vascular (SEACV), Madrid, Spain |
| 91 | Elmo Respiratory Support Project - COVID-19 | Not yet recruiting |
COVID-19 Respiratory failure with hypoxia |
Other: Elmo Project at COVID-19: study in humans Device: Elmo Project at COVID-19: proof of concept and usability |
|
| 92 | Convalescent Plasma vs Human Immunoglobulin to Treat COVID-19 Pneumonia | Recruiting | COVID-19 pneumonia |
Drug: plasma from COVID-19 convalescent patient Drug: human immunoglobulin |
• Centenario Hospital Miguel Hidalgo, Aguascalientes, Mexico |
| 93 | Observational Cohort of COVID-19 Patients at Raymond- Poincare | Recruiting | COVID-19 | • Department of Infectiology, Raymond Poincaré Hospital, APHP, Garches, France | |
| 94 | Longitudinal Energy Expenditure and Metabolic Effects in Patients with COVID-19 (LEEP-COVID) | Recruiting | COVID-19 |
Device: Q-NRG Metobolic Cart Device Device: MuscleSound Ultrasound Device: multifrequency bioimpedance spectroscopy |
• Duke University Medial Center, Durham, NC, USA |
| 95 | Part Two of Novel Adoptive Cellular Therapy with SARS- CoV-2 Specific T Cells in Patients with Severe COVID-19 | Recruiting | COVID-19 | Biological: SARS-CoV-2 specific T cells |
• Changi General Hospital, Singapore, Singapore • KK Women’s and Children’s Hospital, Singapore, Singapore • National University Hospital, Singapore, Singapore • Sengkang General Hospital, Singapore, Singapore • Singapore General Hospital, Singapore, Singapore |
| 96 | A Study Evaluating the Efficacy and Safety of High-Titer Anti-SARS-CoV-2 Plasma in Hospitalized Patients with COVID-19 Infection | Recruiting | COVID-19 | Biological: anti-SARS-CoV-2 convalescent plasma | • Froedtert Hospital, Milwaukee, WI, USA |
| 97 | Identification of Predictors for the Evolution of COVID-19 Related Pneumonia by Transcriptomic and Seroproteomic | Recruiting |
COVID-19 interstitial pneumonia |
• IRCCS Policlinico San Donato, San Donato Milanese, MI, Italy | |
| 98 | CORonavirus (COVID-19) Diagnostic Lung UltraSound Study | Not yet recruiting | COVID-19 | Diagnostic test: lung ultrasound | |
| 99 | Convalescent Plasma (PC) and Human Intravenous Anti-COVID-19 Immunoglobulin (IV Anti COVID-19 IgG) in Patients Hospitalized for COVID-19 | Not yet recruiting | Coronavirus disease 2019 (COVID-19) |
Biological: COVID-19 convalescent plasma Biological: anti-COVID-19 human immunoglobulin Drug: standard (specific) therapy for COVID-19 |
• LifeFactors Zona Franca SAS, Medellín, Antioquia, Colombia |
| 100 | Safety and Efficacy of Tramadol in COVID-19 Egyptian Patients | Not yet recruiting | COVID-19 |
Drug: tramadol Other: standard care delivered in the isolation hospitals |
Hydroxychloroquine is FDA-approved to prevent and treat malaria, as well as to treat the autoimmune diseases rheumatoid arthritis and lupus [44–48]. Some preliminary reports have suggested that hydroxychloroquine, alone or in combination with the FDA-approved antibiotic azithromycin, may benefit people with COVID-19. Numerous clinical trials are planned or underway, including a recently launched study supported by NIH’s National Heart, Lung and Blood Institute, evaluating the safety and effectiveness of hydroxychloroquine for treatment of adults hospitalized with COVID-19.
The preliminary studies indicate that chloroquine and hydroxychloroquine have the potential to improve disease outcomes and possibly slow COVID-19’s progression [49]. Other in vitro and clinical studies demonstrated that the antiviral action of hydroxychloroquine might be effective in limiting SARS-CoV-2 infection [49, 50]. Further, a combination of hydroxychloroquine and azithromycin has also been used against the pathophysiology of COVID-19 [51] and is under clinical trial against SARS-CoV-2.
The chloroquine drug appears to interfere with terminal glycosylation of ACE2 and exerts direct antiviral effects by inhibiting pH-dependent steps of the replication in several viruses including coronaviruses showing a strong impact on SARS-CoV infection [52–55]. Moreover, chloroquine and hydroxychloroquine are known to modulate the immune response in some infectious diseases via inhibition of autophagy, controlling Toll-like receptor (TLR) signaling, and diminishing the cytokine storm by suppressing the production of TNF-α and IL-6 [52, 56] and worked at both entry and post-entry stages of the COVID-19 infection [35]. Currently, we are looking for therapeutic strategies to counter the severe effects of the SARS-CoV-2 infection. A preliminary clinical trial of chloroquine repurposing against SARS-CoV-2 infection has shown some positive results, which led to the start of several clinical trial studies across the world [35, 37]. Recently, the National Institutes of Health (NIH), USA, stopped the clinical trial of hydroxychloroquine as a potential therapy for COVID-19 because some studies show controversial results of this drug against SARS-CoV-2 [57]. Through the fog of alleged misconduct, hope, hype, and politicization that surrounds hydroxychloroquine, the malaria drug touted as a COVID-19 treatment, a scientific picture is now emerging. Recent systematic studies demonstrated no significant benefit of hydroxychloroquine drug in COVID-19 treatment [57, 58].
Besides, the clinical trials of a combination of two anti-HIV drugs—lopinavir and ritonavir—have also been started for these drugs against the SARS-CoV-2 pathology [59]. These drugs are used in a fixed-dose combination for the treatment and prevention of HIV/AIDS [60]. A recent study reported that the β-coronavirus viral loads of a COVID-19 patient in Korea were significantly reduced after lopinavir/ritonavir treatment [61]. In contrast, another clinical trial on hospitalized adult patients with severe COVID-19 shows no benefit with the lopinavir-ritonavir treatment [62].
In the past, vaccines were developed against SARS-CoV and MERS-CoV, but none of them has been clinically approved for use in humans [63]. Currently, several vaccination strategies have been undergoing against SARS-CoV using vaccine candidates including inactivated virus, a live-attenuated virus, viral vectors, subunit vaccines, recombinant proteins, DNA vaccines, etc. across the world. Additionally, many other options are being explored for the treatment of COVID-19 patients, including plasma therapy. Recently, convalescence plasma therapy in which the immunoglobulin of cured COVID-19 patients is injected into a severe patient as a treatment option is explored in India, China, and the USA. Table 1 shows the status of recent clinical trials of vaccines against SARS-CoV-2 infection. Table 2 shows the current status of the clinical trials of vaccines and candidate therapy for cytokine release syndrome (CRS) against SARS-CoV-2 infection.
Table 2.
Current status of the clinical trials of vaccines and candidate therapy for cytokine release syndrome (CRS) against SARS-CoV-2 infection (Source: National Institute of Health, https://clinicaltrials.gov/) Accessed on Aug 1, 2020
| Company | Approach | Stage | Strategy |
|---|---|---|---|
| Arcturus Therapeutics | Vaccine | Phase 1/2 | Engineering RNA with nanoparticle |
| BioNTech | Vaccine | Phase 3 | mRNA |
| Zydus Cadila | ZyCoV-D vaccine | Phase 1/2 | RNA recombinant measles virus |
| Mudoch Childre’s Research Institute | BCG vaccine | Phase 2/3 | Live attenuated virus |
| Medicago, GSK, Dynavax | Plant-based vaccine | Phase 1 | Virus-like particle |
| CureVac | Vaccine | Phase 1 | Man-made mRNA |
| Eli Lilly | Treatment | Phase 3 | Antibody treatment |
| GlaxoSmithkline+ Clover Biopharmaceuticals | Vaccine | Phase 1 | Engineering adjuvants with proteins |
| Inovio Pharmaceuticals | Vaccine | Phase 1 | DNA vaccine |
| Johnson & Johnson | Vaccine and treatment | Phase 1/2 | Deactivated virus |
| Pfizer-BioNTech | Vaccine and treatment | Phase 2/3 |
Has not yet revealed strategy, five-point plan released RNA vaccine |
| Regeneron Pharmaceuticals | Treatment | Phase 3 | Cocktail of antibodies |
| Sanofi | Vaccine and treatment | Phase 3 | Chimera of RNA viruses, Kevzara drug |
| Takeda | Treatment | Treatment phase | Plasma of treated patients |
| Vir Biotechnology | Treatment | Phase 1 | Viral replication inhibitor |
| Ascletis Pharma | Treatment | Phase 1b | Cocktail of danoprevir and titonavir |
| Gamaleya Institute of Epidemiology and Microbiology | Vaccine | Phase 2 | Isolated strain |
| Siberian Vector Institute | Vaccine | Phase 1 | Using a platform first developed for Ebola |
| Moderna Therapeutics | Vaccine | Phase 3 | RNA vaccine (mRNA −1273) |
| CanSino Biologics | Vaccine | To start phase 3 |
SARS-CoV-2 genetic code entwined in harmless virus Non-replicating virus |
| Gilead Sciences | Treatment | Phase 3 | Remdesivir |
| Oxford University | Vaccine | Phase 2/3 trial in UK, phase 3 trials in SA and Brazil |
AZD 1222 Non-replicating virus |
| Bharat Biotech | Vaccine | Phase 1/2 | COVAXIN inactivated virus |
Immune Catastrophe in the Panic of Self-Defense
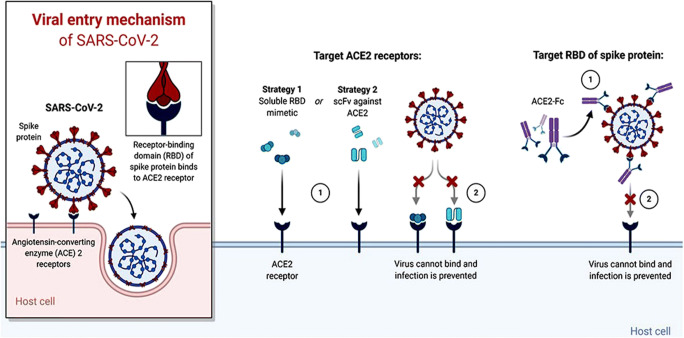
The immune response is a strong defense against invasive pathogens. Recent studies demonstrated an acute inflammation in the severely affected COVID-19 patients, leading to cytokine storm, acute respiratory distress, and then multiple organ failure. Nutritional interventions including vitamin C and D and antioxidants are also used to boost the immune response against this disease. After the recent demonstration of the crystal structure of spikes in SARS-CoV-2 [64], several opportunities had emerged for the development of a vaccine for COVID-19 treatment. The S protein in the spike of SARS-CoV-2 is the major target for vaccine development, and several companies are now in the advancing stage of an effective therapeutic strategy against SARS-CoV-2. Figure 3 shows the proposed treatment strategies targeting ACE2 receptors and the receptor-binding domain (RBD) of the spike for designing a promising drug therapy against COVID-19.
Fig. 3.
Demonstration of viral entry mechanisms and proposed therapeutic strategies against SARS-CoV-2. It uses human ACE2 receptor for entering the host cell by binding its surface spike protein mediated through proteolytic enzyme activity and receptor-binding domain (RBD). The RBD shows strong hACE2 binding affinity allowing SARS-CoV-2 to befool the host defense mechanism
Proposed Hypothesis
We propose a “Pitchfork self-defense hypothesis” where a varied and wide range of effects are observed among the infected population from mild symptoms to multiorgan failure leading to death. This all is owing to the flawed genome and incompetent immune system which acts as a rod for its own back in the rage of self-defense. The peculiar aspect is the reckless and speedy multiplication of virus resulting in the accumulation of monstrous viral copies which in turn calls for a “save the ship” signal activation. The defense system, sensing this as a hulking danger, apparently must be reacting in an exaggerated manner, deploying and marching its armed forces to the site of infection in the form of the cytokine storm syndrome. This storm would do more harm than good, engulfing the healthy RBCs and WBCs, and further inviting more of the type to the site of infection. Thus, this likely stimulates the blood vessels to leak out the immune cells in adjoining tissues, pouring fluid into the lungs. This pneumonia-like condition undoubtedly must be activating cell suicide in lung tissue and hence tearing off the alveoli. Air sacs failing to exchange gases would lead to chronic oxygen starvation which finally culminates in respiratory distress. Thus, in almost 100% of cases, the first critical clinical sign is respiratory unrest which fits in the said hypothesis perfectly. Once the blood vessels lose their fluid, a sudden drop in blood pressure is observed. This may probably be causing clogging of blood vessels and clotting of blood in several tissues leading to a shortage of blood supply and oxygen to the vital organs. This expectedly would have a shocking climax of multiple organ failure. But, interestingly, not all individuals meet with the same fate. Moreover, there are different categories into which individuals can be divided into, as follows: (1) extremely vulnerable category which includes people with an impaired immune system, a chronic respiratory condition, cancer, etc. who cannot afford a competent immune response and are sure to succumb to the disease; (2) high-risk category which includes old people, pregnant women, obese people, and diabetic patients, who are highly susceptible owing to low immunity; and (3) low-risk category which includes healthy and younger individuals who are well equipped to give a tough fight. Frontline workers (mainly doctors, workers, and police personnel) remain in the high-exposure zone and so have a greater chance of contacting COVID-19. Unreasonable and hectic service schedules make the fall prey to the virus since a tired and restless body temporarily loses on its immunity. But there are also reports where young, healthy, and shielded individuals were victimized to death due to COVID-19. The abnormal immune system rests on a faulty genome; thus, the strongest are selected and the defective perish. This can be explained with the present hypothesis in the sense that some immune defects remain latent and are expressed only when a virus triggers them. In the first few days of catching any infection majorly, natural killer cells and macrophages take charge in containing the pathogen and preventing against any severe damage in the body. In case these fail to fight out the infection, then B and T lymphocytes come in the picture and rage a stronger defense response [65]. People with impaired immune systems are more vulnerable to severe infections. Many evidences support this view. (1) One of the most buttressing exemplar is that patients having impaired perforins (perforinopathies) tend to trigger a very severe cytokine storm. These perforins are stocked in natural killer cells and cytotoxic T lymphocytes. Since perforins are glycoproteins that are critically responsible for pore formation in the host cell (infected with virus) causing immune cell-mediated death of the target cell, thus, reduced attenuated expression of perforins invites a cytokine storm. Thus, if such patients are provided with sufficient amounts of perforin, then it may help generate a healthy immune response against SARS-CoV-2 infection. (2) Also IL-6 blocks the expression of granzyme B and perforins and prevents the killing of the viral-infected cell (experimentation studies on mice). Therefore, it is of concern to note that in COVID-19 patients succumbing to heart attack, IL-6 prevented the activity of STAT-5 signal transducer, thereby decreasing perforin levels. Thus, a treatment involving anti IL-6R would be quite effective against COVID-19 infection. In a nutshell, patients with weakened immune systems tend to show severe symptoms because of elevated levels of IL-6 mediated by poor perforin functionality [66]. It has been discovered by the Indo-US research team that the Indian population is better equipped with these killer cell immunoglobulin-like receptor genes (KIR genes) and therefore can contain the infection in the initial stage itself. Thus, Indian populations are less susceptible to these infections and also confer Indians with stronger immunity against other viruses, autoimmune diseases, and even tumors [67]. However, recently, this claim was opposed and declared baseless by a few science researchers and science magazine editors [68]. Thus, the answer lies in developing artificial adaptive immunity through an effective medicine.
Genome Analysis/Genomics Could Help Resolve Some Unanswered Questions
A comprehensive genome analysis of the virus as well as the host population to predict genetic determinants is the need of the hour. Such a large-scale collaborative task of genome sequencing may unmask hidden mysteries like severity and susceptibility of COVID-19. Many such initiatives using high-throughput sequencing technologies are in the pipeline (namely Genomics England, UK Biobank). Since we know that genome is unique to an individual and it is the sole dower of phenotypic or functional expression of a trait, so it is imperative to first analyze the defect at the molecular level. This would open a gamut of doors for therapeutic and preventive strategies and may help answer all the puzzles concerning COVID-19. Once we know the gene, we may easily correlate any defect in its protein expression and biochemical pathway concerning the disease. This gene structure would also give an insight into new and ameliorating drugs and therapies.
Current Therapeutic Strategies for the Development of an Effective Vaccine Against COVID-19
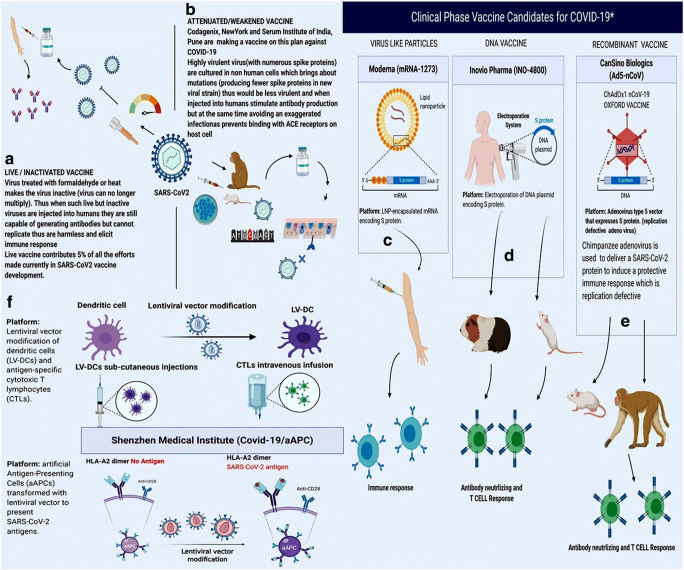
In this hour of COVID-19 pandemic, a population of 7.8 billion can be safeguarded only by active immunity. Natural active immunity cannot be expected from the diseased chunk of the population supporting a fragile immune system. Also, isolation alone cannot help, since it is not an indelible solution. Thus, developing an effective vaccine is utmost required to develop adaptive immunity to fight upcoming viral infections. Thus, today, countries vie with each other for developing an effective vaccine against SARS-CoV-2. To date, approximately 100 designs of different vaccines have been proposed worldwide and more than six groups of these vaccines have already undergone trial (Fig. 4).
Fig. 4.
A–F Possible therapeutic strategies for the development of an effective vaccine against SARS-CoV-2
Angiotensin-Converting Enzyme-2 (ACE-2) Receptor-Viral Doorknob-Based Therapy
Dozens of pharmaceutical companies are targeting this key to unlock a SARS-CoV-2 vaccine. Different strategies are being worked upon. It was recently discovered that ACE receptors are present not only on lung, heart, gut, and kidney cells but also on the nose. Using ACE receptors in therapeutics holds a contrasting version. Some research teams undermine its potentials citing that non-steroidal inflammatory drugs may spoil the show by increasing the expression of ACE receptors, while others emphasize that using a floating enzyme strategy may prove effective. These ACE receptor mimics may befool viruses and make them latch on (Apeiron Biologics, an Austrian pharmaceutical company). We propose that a gene variation study of the ACE-2 gene specific to lung tissue can be instrumental in discovering effective drugs against viral entry since this gene deals with the production of surfactant for the lungs. Any defect or overexpression of this gene may either prevent viral entry into the host cell or may make it detrimental for the host. One such research to support our proposal is the CCR5 gene on blood cells. Genetic analysis revealed that people with defective CCR5 gene prevented HIV from entering the host cell [69]. Thus, in this case, a defective gene was at an advantage since it made some people immune to HIV infection by preventing viral entry. One of the observed trends in COVID-19 is the cytokine storm (similar to the one observed in H1N1 flu). This also results from some impaired gene which causes a hyperexpressive immune response. Thus, a genetic variation study among populations would help figure out who is less vulnerable and who is more prone to such deadly viruses. The viral genomics and proteomics studies would help to discover some relevant transcription factors that induce overexpression of the host immune system. In a recent proteomics study of SARS-CoV-2-infected host cells, a fresh understanding of some gene therapies effective against COVID-19 was hinted. It includes the modulations in the host cell pathway infected with SARS-CoV-2 in human cell culture studies and suggests that effective therapy can be blocking these pathways by some inhibitor molecules that would halt the process of viral multiplication.
MicroRNA, a Direct Attack on the Viral Genome
The miRNAs are small, non-coding, single-stranded RNA molecules, which are known to modulate a variety of vital physiological processes including RNA silencing, post-transcriptional regulators of gene expression involved in cardiovascular development and health [70–75]. Several thousand human genes, amounting to about one third of the whole genome, are potential targets for regulation by the several hundred miRNAs encoded in the genome [76]. It is evident from the previous studies that novel miRNAs can be used as promising tools as diagnostic biomarkers and therapeutic drug targets for several diseases [77–79]. In the present context, microRNAs specifically mitochondrial microRNA can prove as a panacea. Silico analysis studies have been used to interpret which host microRNAs have complete complementarity with viral RNA so that viral RNA expression can be silenced.
Recently, microRNA-5197-3p has been identified as the most valuable site for interaction with SARS-CoV-2, and complete complementary miRNA regions in the viral genome have been predicted. This proposed site lacks any side effect or competition and a synthetic microRNA constructed based on this template may act as a remarkable candidate for the treatment of COVID-19. These may then be packed into exosomes and either injected into the blood for multiple organ treatment or simply inhaled into the lungs through nebulizers. Antiviral miRNAs present in the host target specific genes of the virus and interfere with viral molecular processes vital for reproduction, namely replication, protein synthesis, and phenotypic expression. Thus, microRNAs are vital tools for tailoring and silencing viral genome as these complementarily bind to the viral genome and prevent their expression. It has been found that hsa-miR-27b is uniquely specific to only SARS-CoV-2 found in India, and so, Indian populations are naturally endowed with a miraculous antidote to this virus. Alnylam and Vir, two big companies, are working on temporarily silencing the ACE2 receptors using interference RNA technology. They believe that when there will be no receptors, then viruses would not be able to enter. But another big challenge is delivering these RNA molecules to the target site. It has been proposed that these microRNA would probably be packed in fat vesicles and inhaled in the form of a dry powder. But most researchers are skeptical about this as knocking down these vital blood pressure-regulating receptors may prove fatal. To speed up the emergency medical need of vaccines and therapies against COVID-19, the Food and Drug Administration (FDA) of the USA has provided fast-track designation to Mrna Vaccine mRNA-1273 created by Moderna. It is in phase II of the clinical trial. Moderna is highly trusted as earlier it could fast-track the Zika vaccine.
Recombinant Vaccine
It is a recombinant adenovirus vaccine developed by the University of Oxford. ChAdOx1 nCoV-19 has been genetically engineered using weakened common cold adenovirus (ChAdOx1) infective only to chimpanzees. Genes coding for spike glycoprotein (S) of SARS-CoV-2 virus have been inserted into these viruses as these spike proteins are vital to bind on to ACE2 receptors on human cells. Its peculiar feature is that MenACWY has been used as an active control instead of saline solution. It gave very encouraging positive results on monkeys, generating antibodies within 28 days of its administration. It also drastically reduced respiratory distress in the patients and also prevented viral replication. Its human trials are in the process, and the university has already signed an agreement for its global manufacture and sale with Astra Zeneca. It is under trial on humans and is estimated that the initial study would include more than 1100 healthy subjects between 18 and 55 years of age. Exclusion criteria include pregnant, breastfeeding mothers and COVID-19 patients.
Epitope-Based Vaccines
The coronavirus has spike proteins on its shell which are essential to latch on to the host cellular receptors. Viruses have been smart enough to evade the host’s defense system by hiding their receptor binding motif (RBM). Thus, one of the strategies could be to design a vaccine targeting the RBM of SARS-CoV-2 virus. Tel Aviv University (TAU) of Israel is working on the same plan in association with Neovii, a pharmaceutical company. They aim to come up with a COVID-19 epitope vaccine based on in silico or computational prediction. It will be a cost-effective solution with great therapeutic efficacy.
Drug Against Cytokine Storm
The National Institutes of Health (NIH), USA, has sponsored a biotechnology company, CytoAgents, to fast-track the production of GP1681. This would be effective in controlling the cytokine storm resulting from the SARS-CoV-2 infection. These are small molecules known to show a host-directed methodology to spot the fundamental reason of cytokine storm, altering the host’s natural immune system. This molecule would be effective against other viral diseases like influenza. It is currently under phase I and II of the human trial. There are enough case studies that have reported an exaggerated and abnormal production of IL-6 that led to “cytokine storm” and which finally ended up in heart failure due to stimulation of the coagulation process. Also, tissue necrosis and infiltration of monocytes and macrophages are commonly observed in both COVID-19 patients showing severe symptoms and postmortem pathological analysis. The authors propose that there is a good possibility that anti-interleukin-6 (IL-6) therapy may manage COVID-19-induced cytokine storm (hemophagocytic lymphohistiocytosis, macrophage activation syndrome, and sepsis) [80]. Cytokines IL-6, IL-2, IL-1β, IL-8, IL-17, IL-10, and IL-4 all have known to increase excessively during COVID-19. Tocilizumab is a human monoclonal anti-IL-6 receptor antibody, extracted from patients with cytokine release syndrome. This is in phase 4 for SARS-CoV-2 trial. Tocilizumab can be associated with soluble IL-6R and mIL-6R, and inhibit signal transduction. However, it is a very costly treatment and its safety risks need to be tested in clinical trials [80–82].
Adjuvant Vaccine for COVID-19
Adjuvant is generally a chemical/drug or immunological agent added to a vaccine in small amounts to increase the production of antibodies. Thus, an adjuvant vaccine would probably provide instant and long-lasting immunity. GlaxoSmithKline and Sanofi Pasteur (French Pharma-Company) together are working on the development of an adjuvant vaccine against SARS-CoV-2.
Synthetic Chimeric COVID-19 Vaccine
Reconstruction of live synthetic less virulent SARS-CoV-2 virus which may provide vaccine protection against COVID-19 is in the pipeline. This commendable project is being taken up by Tonix Pharmaceuticals which had earlier developed the horsepox virus vaccine on a similar plan. University of Alberta, Canada, has signed a licensing agreement with Tonix Pharmaceuticals. Their basic plan is to synthesize some antigen genes of SARS-CoV-2 to innervate T cell immune response. They have a license to create 3 vaccines for COVID-19, namely TNX-1810, TNX-1820, and TNX-1830. Currently, it is at the pre-investigation of new drug stage. This kind of vaccine no doubt can prove miraculous but at the same time may instigate certain criminal minds to construct deadly bioweapons.
Emetine Injections
National Center for Advancing Translational Sciences (NCATS) and Acer Therapeutics (pharmaceutical company) have agreed on placebo-controlled and random tests of this antiviral drug on COVID-19 patients.
NVX-CoV2373 Vaccine
The Coalition for Epidemic Preparedness Innovations (CEPI) has agreed to fund $384m to Novavax for manufacturing Matrix-M adjuvant and NVX-CoV2373 vaccine antigen of COVID-19 vaccine which would boost rapid production of antibodies.
Antibody Cocktail COVI-SHIELD
Some researchers at Mount Sinai Health System have come up with the idea of developing a triple-antibody prophylactic and therapy. This antibody cocktail specifically would safeguard frontline workers and doctors who are frequently exposed to this virus They aim at screening approximately 1500 COVID-19 recovered patients for identifying and isolating at least 3 vital antibodies against spike proteins of SARS-CoV-2 virus from their blood plasma. Then, monoclonal antibodies would be synthesized in collaboration with Sorrento Therapeutics. This would specifically provide immunity to the high-risk population for at least 2 months. This therapy may also provide resistance to future virus mutations and is currently filing an Investigational New Drug (IND) application.
Novel Decoy Cellular Vaccine
Transgenic antigen-expressing cells: In this innovative strategy, nonreplicating, irradiated cells (I-cells) would be deployed as presenting carriers of antigens of SARS-Cov-2 so that these can be recognized by the host immune cells. These are irradiated to prevent in vivo replication and will act as a vaccine to protect against COVID-19 disease. It is thus a transgenic antigen-expressing cell.
ACCORD program
Official records depict that the UK was worst affected by the COVID-19 pandemic; thus, the UK government has constituted an Accelerating COVID-19 Research & Development (ACCORD) Program to step up large-scale research and development strategies to fight COVID-19. The first drug has been fast-tracked is bemcentinib. This drug is a selective inhibitor of the AXL kinase protein and was originally developed to prevent cancer cell metastasis. It has been proposed to use this drug against SARS-CoV-2 since this virus uses AXL kinase protein for its entry into the host cell. Thus, by inhibiting this protein, the virus can be prevented from infecting cells. This drug is a panacea and has also proven effective against the Ebola and Zika viruses. Another important characteristic of this drug is that it is safe in case of adults as its target is a protein that is least essential in adulthood. This drug can prevent the virus from both entering the host cell in the first stage and controlling the progression of disease in patients already suffering from COVID-19. Thus, it is a broad-spectrum drug. Apart from this drug, the program includes tyrosine kinase inhibitor interleukin 33 and Calquence drug. Under this program, approximately $8billion funding would be provided to several international agencies, research organizations, and industries by UK Research and Innovation (UKRI) and Department of Health and Social Care (DHSC).
BCG Vaccine for COVID-19
The BCG vaccine is known to improve frontline immunity. Every year, approximately 130 million children are vaccinated with BCG for tuberculosis. In Australia, Murdoch Children’s Research Institute is conducting a clinical trial that shows that BCG vaccine reduces viral infections. It is estimated to be tried on more than 4000 frontline workers.
Killed Virus Vaccine
Viruses are border organisms that are categorized neither among living nor among dead as these become activated only when they get into a host body. Therefore, a dead virus simply means the one in which infective capacity is removed off so that they cannot enter our body. This can be done either by chemical alteration or by extreme temperature treatment. Thus, using such dead virus vaccines may serve our purpose, but this is not always the case as dead viruses possess different altered protein than one produced by the live virus and so they do not prove very effective. Examples are flu, hepatitis A, etc.
Attenuated Virus Vaccine
Such viruses are more effective than dead viruses as are live and draw out a strong immune response. But these vaccines provide only short-term immunity and their booster doses are required. Such weakened viruses are actual viruses with some mutations that make them more attracted to other animals than humans. Such viruses are created by culturing them in tissues of some other animal. Thus, a mutant form of the virus is chosen that would prefer other non-human animal hosts and would hardly infect humans. Vaccines made from such weakened viruses when injected into humans may provide immunity against viruses and at the same time avoid exaggerated infection. Thus, such vaccines usually show mild side effects post-treatment. Some of the examples are rotavirus, measles, mumps, rubella (MMR combined vaccine), smallpox, etc. Thus, both live and attenuated vaccines train our body to fight against infection as they are more natural. Some of the limitations of such vaccines include refrigeration to keep them alive; costly instrumentation to protect them from any new virulent mutation (or reversion) during transportation to distant places; reliability deficit in the effectiveness of the vaccine and safety after administration.
Conjugate Vaccines
Sometimes viruses have some modulating or disguising outer envelope proteins that have the capacity to befool the host’s immune system by concealing their antigens. Thus, such viruses go unnoticed by the host defense system and invade different organs. Live or weakened vaccines generally do not work in such cases, and thus, conjugate or subunit vaccines are made by attaching a characteristic antigen from some other familiar pathogen on to the virulent virus’s coat. It works because the body’s immune system learns to recognize this masked virus as a potential threat in the light of a known pathogen, e.g., Haemophilus influenzae type B (Hib).
Nucleic Acid Vaccines
In this approach, DNA from the virus is genetically engineered (with specific spike protein genes) and introduced into the host body as a plasmid, thus eliciting an immune response against those antigens. This probably remains harmless but, in turn, teaches the body to identify harmful viruses and produce antibodies against them. But this has never been tested on humans to date and is not yet licensed (20% of current vaccine research focuses on this approach).
Recombinant Vaccines
Specific spike protein-producing gene segments are inserted into vectors (adenovirus) for vaccine delivery. Adenoviruses are good vectors for vaccine development because they can infect a wide range of hosts; cause effective expression of transgene; can be cultured in laboratories at low cost; do not allow lysogeny of viral genes into host genes; and can trigger an immune response by infecting dendritic cells and target both systemic and mucosal immune response. Johnson and Johnson, a US-based company, is working on this project (26% of current vaccine research focuses on this approach).
Virus-Like Particles (VLP)
Specialized empty (without genetic material) lipid vesicles are prepared with spike proteins on the surface that mimic a true virus, and the body’s immune system reacts in response to this. These could serve as excellent vaccine candidates (33% of current vaccine research focuses on this approach).
Conclusions and Future Challenges
Worldwide, millions of people are infected, and thousands of deaths are occurring due to the pandemic situation of COVID-19. Unfortunately, no therapeutics have yet been proven effective for the treatment of severe illness caused by SARS-CoV-2. The identification of effective strategies against SARS-CoV-2 is a major challenge. Currently, we are fighting a twenty-first century disease with twentieth century weapons. The clinical trials of repurposing the existing antiviral agents against this potentially fatal disease are going on across the world. Recently, several possibilities including targeting viral binding receptors (ACE2) and spike proteins, stimulating an immune response, monoclonal antibodies, peptides, small-molecule drugs, etc. are being explored against emerging SARS-CoV-2 infection. However, the whole world is facing a challenge in dealing with a new coronavirus infection that has just emerged in humans and we are not having the existing vaccine or a drug against this potentially fatal disease. We hope that through these accelerated and determined plans, very soon, an effective vaccine that targets SARS-CoV-2 will be developed to settle this global COVID-19 issue. In closing, we express our concern about fading away from these big programs and funds offered to sort this public health crisis (generally offered around such pandemic). We would be witnessing many new and far deadly viral pandemics in the ensuing time, and thus, we really need to be prepared to tackle this unseen danger. Thus, we need to continue this research with the same enthusiasm and zeal to discover reliable and affordable therapeutic strategies to curb such pandemics.
Acknowledgments
The research presented in this article was supported by the National Institutes of Health (NIH) grants AG042178, AG047812, NS105473, AG060767, and AG069333 (to PHR). This research was also supported by the Alzheimer’s Association New Investigator Research Grant 2016-NIRG-39787, the Center of Excellence for Translational Neuroscience and Therapeutics (PN-CTNT20115-AR), Alzheimer’s Association through a SAGA grant, and NIH grant AG063162 (to AR). We acknowledge support from the Central University of Punjab, Bathinda, India. We thank all the doctors, nurses, health workers, and research scientists who are working day and night to fight against the COVID-19 pandemic. The figures were assembled using dynamic BioRender assets.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jasvinder Singh Bhatti and Gurjit Kaur Bhatti contributed equally to this work.
Contributor Information
Jasvinder Singh Bhatti, Email: jasvinder.bhatti@cup.edu.in.
Gurjit Kaur Bhatti, Email: bhattigk@yahoo.com.
Naina Khullar, Email: naina306@gmail.com.
Arubala P. Reddy, Email: arubala.reddy@ttuhsc.edu
P. Hemachandra Reddy, Email: hemachandra.reddy@ttuhsc.edu.
References
- 1.Guarner J. Three emerging coronaviruses in two decades. Am J Clin Pathol. 2020;153(4):420–421. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drosten C, Günther S, Preiser W, Van Der Werf S, Brodt H-R, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 3.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 4.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO guidelines for the global surveillance of severe acute respiratory syndrome (SARS): updated recommendations, October 2004. Geneva: World Health Organization; 2004. [Google Scholar]
- 7.Meo SA, Alhowikan AM, Al-Khlaiwi T, Meo IM, Halepoto DM, Iqbal M, Usmani AM, Hajjar W, Ahmed N. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci. 2020;24(4):2012–2019. doi: 10.26355/eurrev_202002_20379. [DOI] [PubMed] [Google Scholar]
- 8.Cui J, Li F, Shi Z-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Military Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan JF-W, Yuan S, Kok K-H, To KK-W. Chu H, Yang J, Xing F, Liu J, Yip CC-Y, Poon RW-S. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KS, Lau EH, Wong JY. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization (2020) Coronavirus disease (COVID-19) outbreak. URL https://www.whoint/emergencies/diseases/novel-coronavirus-2019 Accessed March
- 13.Meo S, Alhowikan A, Al-Khlaiwi T, Meo I, Halepoto D, Iqbal M, Usmani A, Hajjar W, Ahmed N. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci. 2020;24(4):2012–2019. doi: 10.26355/eurrev_202002_20379. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (2020) Coronavirus disease 2019 (COVID-19): situation report, 194 (1st Aug 2020).
- 15.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zavascki AP, Falci DR. Clinical characteristics of Covid-19 in China. N Engl J Med. 2020;382:1859–1862. doi: 10.1056/NEJMc2005203. [DOI] [PubMed] [Google Scholar]
- 17.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for C (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 10.1056/NEJMoa2002032, 382, 1708, 1720 [DOI] [PMC free article] [PubMed]
- 18.Passarelli PC, Lopez MA, Mastandrea Bonaviri GN, Garcia-Godoy F, D'Addona A. Taste and smell as chemosensory dysfunctions in COVID-19 infection. Am J Dent. 2020;33(3):135–137. [PubMed] [Google Scholar]
- 19.Dawson P, Rabold EM, Laws RL, Conners EE, Gharpure R, Yin S, Buono SA, Dasu T et al (2020) Loss of taste and smell as distinguishing symptoms of COVID-19. Clin Infect Dis. 10.1093/cid/ciaa799 [DOI] [PMC free article] [PubMed]
- 20.Gautier JF, Ravussin Y. A new symptom of COVID-19: loss of taste and smell. Obesity. 2020;28:848. doi: 10.1002/oby.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX, China Medical Treatment Expert Group for C (2020) Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J 10.1183/13993003.00547-2020, 55, 2000547 [DOI] [PMC free article] [PubMed]
- 22.Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. COVID-19 and cancer: a comprehensive review. Curr Oncol Rep. 2020;22(5):53. doi: 10.1007/s11912-020-00934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang W, Li H, Zhang P, Song X, Chen X, Xiang M, Zhang C, Bai L, Xiang D, Chen MM, Liu Y, Yan Y, Liu M, Mao W, Zou J, Liu L, Chen G, Luo P, Xiao B, Zhang C, Zhang Z, Lu Z, Wang J, Lu H, Xia X, Wang D, Liao X, Peng G, Ye P, Yang J, Yuan Y, Huang X, Guo J, Zhang BH, Li H. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22(2):69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, Zhang Q, Wu J. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan JF, Kok KH, Zhu Z, Chu H, To KK. Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9(2):e00221-00218. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L, Xu T, Jiang YZ, Xiong Y, Li YJ, Li XW, Li H, Fan GH, Gu XY, Xiao Y, Gao H, Xu JY, Yang F, Wang XM, Wu C, Chen L, Liu YW, Liu B, Yang J, Wang XR, Dong J, Li L, Huang CL, Zhao JP, Hu Y, Cheng ZS, Liu LL, Qian ZH, Qin C, Jin Q, Cao B, Wang JW. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J. 2020;133:1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/s0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK, Washington State - nCo VCIT (2020) First case of 2019 novel coronavirus in the United States. N Engl J Med 382 (10):929–936. 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed]
- 34.Aguiar ACC, Murce E, Cortopassi WA, Pimentel AS, Almeida M, Barros DCS, Guedes JS, Meneghetti MR, Krettli AU. Chloroquine analogs as antimalarial candidates with potent in vitro and in vivo activity. Int J Parasitol Drugs Drug Resist. 2018;8(3):459–464. doi: 10.1016/j.ijpddr.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019- nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yazdany J, Kim AHJ. Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know. Ann Intern Med. 2020;172:754–755. doi: 10.7326/M20-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D (2020) Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents:105932. 10.1016/j.ijantimicag.2020.105932 [DOI] [PMC free article] [PubMed]
- 38.Guastalegname M, Vallone A. Could chloroquine /hydroxychloroquine be harmful in coronavirus disease 2019 (COVID-19) treatment? Clin Infect Dis. 2020;71:888–889. doi: 10.1093/cid/ciaa321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown AJ, Won JJ, Graham RL, Dinnon KH, III, Sims AC, Feng JY, Cihlar T, Denison MR, Baric RS, Sheahan TP. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antivir Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulangu S, Dodd LE, Davey RT, Jr, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 44.Rainsford K, Parke AL, Clifford-Rashotte M, Kean W. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231–269. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- 45.JCD S, Mariz HA, LFd RJ, PSSd O, Dantas AT, ALBP D, IdR P, Galdino SL, MGdR P. Hydroxychloroquine decreases Th17-related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients. Clinics. 2013;68(6):766–771. doi: 10.6061/clinics/2013(06)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42(2):145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gladman DD, Urowitz MB, Senecal JL, Fortin PJ, Petty RE, Esdaile JM, Carrette S, Edworthy SM, Smith CD, Thorne JC. Aspects of use of antimalarials in systemic lupus erythematosus. J Rheumatol. 1998;25(5):983–985. [PubMed] [Google Scholar]
- 48.Taylor JK, McMurray RV. Medical therapy for systemic lupus erythematosus. J Miss State Med Assoc. 2011;52(2):39–43. [PubMed] [Google Scholar]
- 49.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honore S, Colson P, Chabriere E, La Scola B, Rolain JM, Brouqui P, Raoult D (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents:105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Retracted]
- 52.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rolain JM, Colson P, Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30(4):297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devaux CA, Rolain JM, Colson P, Raoult D (2020) New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents:105938. 10.1016/j.ijantimicag.2020.105938 [DOI] [PMC free article] [PubMed]
- 55.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3(11):722–727. doi: 10.1016/s1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golden EB, Cho HY, Hofman FM, Louie SG, Schonthal AH, Chen TC. Quinoline- based antimalarial drugs: a novel class of autophagy inhibitors. Neurosurg Focus. 2015;38(3):E12. doi: 10.3171/2014.12.FOCUS14748. [DOI] [PubMed] [Google Scholar]
- 57.Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM (2020) Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. 10.7326/M20-2496 [DOI] [PubMed]
- 58.Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi M, Engen NW, Cheng MP, LaBar D, Lother SA, MacKenzie LJ, Drobot G, Marten N, Zarychanski R, Kelly LE, Schwartz IS, McDonald EG, Rajasingham R, Lee TC, Hullsiek KH. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.National Institutes of Health (2020) Coronavirus (COVID-19). https://www.nih.gov/health- information/coronavirus. Accessed April 11, 2020
- 60.Hull MW, Montaner JS. Ritonavir-boosted protease inhibitors in HIV therapy. Ann Med. 2011;43(5):375–388. doi: 10.3109/07853890.2011.572905. [DOI] [PubMed] [Google Scholar]
- 61.Lim J, Jeon S, Shin H-Y, Kim MJ, Seong YM, Lee WJ, Choe K-W, Kang YM et al (2020) Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci 35(6) [DOI] [PMC free article] [PubMed]
- 62.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang N, Shang J, Jiang S, Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol. 2020;11:298. doi: 10.3389/fmicb.2020.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L (2020) Crystal structure of the 2019-nCoV spike receptor-binding domain bound with the ACE2 receptor. bioRxiv [DOI] [PubMed]
- 65.Estefanía E, Flores R, Gómez-Lozano N, Aguilar H, López-Botet M, Vilches C. Human KIR2DL5 is an inhibitory receptor expressed on the surface of NK and T lymphocyte subsets. J Immunol (Baltimore, Md : 1950) 2007;178(7):4402–4410. doi: 10.4049/jimmunol.178.7.4402. [DOI] [PubMed] [Google Scholar]
- 66.Cunningham L, Kimber I, Basketter DA, McFadden JP (2020) Why judiciously timed anti-IL 6 therapy may be of benefit in severe COVID-19 infection. Autoimmun Rev:102563 10.1016/j.autrev.2020.102563 [DOI] [PMC free article] [PubMed]
- 67.Du Z, Sharma SK, Spellman S, Reed EF, Rajalingam R. KIR2DL5 alleles mark certain combination of activating KIR genes. Genes Immun. 2008;9(5):470–480. doi: 10.1038/gene.2008.39. [DOI] [PubMed] [Google Scholar]
- 68.Shaikh S, Rao A, Prasad P (2020) Indians do not have genetic protection against coronavirus, published research incorrectly interpreted.
- 69.Lopalco L. CCR5: from natural resistance to a new anti-HIV strategy. Viruses. 2010;2(2):574–600. doi: 10.3390/v2020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 71.Kato M, Natarajan R. Diabetic nephropathy--emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10(9):517–530. doi: 10.1038/nrneph.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kato M, Natarajan R. MicroRNAs in diabetic nephropathy: functions, biomarkers, and therapeutic targets. Ann N Y Acad Sci. 2015;1353(1):72–88. doi: 10.1111/nyas.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, Sun X, Icli B, Feinberg MW. Emerging roles for microRNAs in diabetic microvascular disease: novel targets for therapy. Endocr Rev. 2017;38(2):145–168. doi: 10.1210/er.2016-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song R, Hu XQ, Zhang L (2019) Mitochondrial MiRNA in cardiovascular function and disease. Cells 8(12). 10.3390/cells8121475 [DOI] [PMC free article] [PubMed]
- 75.Cao Q, Chen XM, Huang C, Pollock CA. MicroRNA as novel biomarkers and therapeutic targets in diabetic kidney disease: an update. FASEB Bioadv. 2019;1(6):375–388. doi: 10.1096/fba.2018-00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackson RJ, Standart N. How do microRNAs regulate gene expression? Science's STKE: Signal Transduction Knowledge Environment. 2007;2007(367):re1. doi: 10.1126/stke.3672007re1. [DOI] [PubMed] [Google Scholar]
- 77.Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, van Rooij E. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124(14):1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dickinson BA, Semus HM, Montgomery RL, Stack C, Latimer PA, Lewton SM, Lynch JM, Hullinger TG, Seto AG, van Rooij E. Plasma microRNAs serve as biomarkers of therapeutic efficacy and disease progression in hypertension-induced heart failure. Eur J Heart Fail. 2013;15(6):650–659. doi: 10.1093/eurjhf/hft018. [DOI] [PubMed] [Google Scholar]
- 79.Pinti MV, Hathaway QA, Hollander JM. Role of microRNA in metabolic shift during heart failure. Am J Phys Heart Circ Phys. 2017;312(1):H33–H45. doi: 10.1152/ajpheart.00341.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 81.immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents:105982 10.1016/j.ijantimicag.2020.105982 [DOI] [PMC free article] [PubMed]
- 82.Michot JM, Albiges L, Chaput N, Saada V, Pommeret F, Griscelli F, Balleyguier C, Besse B, Marabelle A, Netzer F, Merad M, Robert C, Barlesi F, Gachot B, Stoclin A. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Ann Oncol. 2020;31:961–964. doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]