Abstract
During the period of pregnancy, several processes and physiological adaptations occur in the body and metabolism of pregnant woman. These physiological adaptations in pregnant woman end up leading to a suppression in immune system favoring obstetric complications to the mother, fetus and placental tissue. An effective pharmacological therapy for these complications is still a challenge, since some drugs during pregnancy can have deleterious and teratogenic effects. An emerging alternative to pharmacological therapy during pregnancy is drugs encapsulated in nanoparticles (NP), recent area called nano-obstetrics. NP have the advantage of drug targeting and reduction of side effects. Then, maternal, placental or fetal uptake can be expected, depending on the characteristics of NP. Inorganic NP, crossing placental barrier effectively, but have several nanotoxicological effects. While organic NP appear to have a better targeting capacity and have few toxicological effects, but the studies are still scarce. Thus, in this review, were examined questions related to use and impact of physicochemical aspects of inorganic and organic NP during pregnancy.
Keywords: Nanoparticles, Pregnancy, Drug targeting, Placenta, Fetus
Highlights
-
•
Use of organic and inorganic nanoparticles during pregnancy.
-
•
Impact of use of nanotherapy in pregnancy.
-
•
Role of inorganic nanoparticles in nanotoxicological effects during pregnancy.
1. Introduction
During pregnancy in different animal species, adaptive physiological processes occurs, so that another life can be generated. Because these adaptations, pregnant are more susceptible to infectious diseases due to suppression of immune system [1,2]. Currently, the main infectious disease that affects pregnant is Coronavirus 2019 (COVID-19). The etiologic agent of disease is severe acute respiratory distress syndrome-associated coronavirus-2 (SARS-CoV-2) [3]. Some studies have already shown evidence of COVID-19-intrauterine transmission [4,5]. Still, Mulvey et al. [6], demonstrated that the placenta of patients infected with COVID-19 presented thrombotic events. Also, in study of Dashraath et al. [7], they showed that mortality during pregnancy is related to predisposition of superimposed bacterial infections, suppression of immune response and alteration of respiratory microbiome after pneumonia COVID-19-caused.
For the fetus, the most common complications are miscarriage, changes in intrauterine growth and preterm birth [3,7]. Due to these complications, the large number of cesarean sections performed on pregnant infected by COVID-19 is justified [8]. In view of above, an effective and safe pharmacological therapy for pregnant and fetus must develop with emergency. Preferably, with the ability to carry the drug to target/organ tissue, avoiding side effects. In this context, nanotechnology appears as a pioneering alternative to solve the aforementioned problem.
Nanotechnological innovations have helped to overcome several problems in the biomedical and pharmaceutical area. Among the advances in nanotechnology, highlighted the synthesis of nanoparticles (NP) and their biomedical application. Different NP have been documented with a wide range of applications, including cancer therapy, drug administration, tissue engineering, regenerative medicine, biomolecule detection and as antimicrobial agents [9]. Despite the use of NP for treatment of different pathologies and some metabolic disorders, studies with NP in pregnancy and impact on the fetus are still scarce. Therefore, nanoparticles can be exploited to precisely control the administration of drug during pregnancy, providing minimal risk of side effects on the fetus and mother, giving a new approach to treat pathological implications in pregnancy [10].
Given the above, the present review examines questions related to the use and impact of inorganic and organic NP during pregnancy, focusing on technological aspects associated to NP and their biological implications.
2. Pregnancy: A recurring challenge for pharmacological therapy
Medication exposure during pregnancy can affect both mother and fetus. In fact, in clinical routine, treatment of pregnant is a recurring challenge. The opacity of studies, lack of reliable sources of information and the insecurity of pregnant towards systemic drug treatment leads to difficult clinical decision making. A careful evaluation of potential benefits and risks of a therapy and adequate control of clinical symptoms are crucial for a safe pregnancy [11]. The potential effects of drugs on fetal development depend on several factors, including gestational age, dose, dosage, route of administration and clearance of drug [12,13]. According to the Center for Disease Control and Prevention (CDC) [14] 9 out of 10 women take at least one medication during pregnancy and 70% of pregnant take at least one prescription medication. Over the past 30 years, the use of prescription drugs during the first quarter has increased by more than 60% [15].
The pregnancy induces significant changes in some physiological parameters, as the decrease in plasma proteins which increase the unbound fractions of the drug [16]. Besides that, the question of plasma proteins, pregnancy induces an increase of almost 50% in the circulating blood volume and cardiac output, leading to increased blood flow in organs. This leads to a smaller area under the curve and potential therapeutic failure, although it is compensated for to some extent by increased bioavailability during pregnancy due to prolonged intestinal transit time [17]. Other changes include increased glomerular filtration rate (increased renal clearance) and altered activity of drug-metabolizing enzymes in liver (affecting hepatic clearance) [18]. All these changes have an impact on the pharmacological results for this population.
Considering the various physiological alterations in metabolization of drugs described during pregnancy, it is clear that specific guidelines are needed for use of medications in pregnant. Despite the increase in use of medication by pregnant, previously mentioned, the amount of data available to guide decision-making is limited. The caution surrounding drug use in pregnancy became widespread after unexpected adverse events from thalidomide and diethylstilbestrol, which led to the exclusion of pregnant from clinical trials [19,20]. Scaffidi et al. [21] researched 16 clinical trial records comprising 301.538 trials and found that only 0.32% of all active trials were pregnancy drug trials. It is known that fetal drug toxicity can occur at any time during pregnancy, although the most vulnerable period for anomalies is the first trimester [22].
The great challenge for development of safe medications for use in pregnancy is to reduce or eliminate the exposure of unwanted tissues (maternal or fetal) while still providing the necessary therapeutic effect [13]. Placenta is the organ that represents the maternal-fetal interface, responsible for the transfer of oxygen, nutrients, waste and other molecules between the maternal and fetal bloodstream [23]. In humans, the placenta is of the hemochorial type and its composition consists of a maternal portion, called deciduous and a fetal portion, the chorionic plate. Fibroblasts are the origin of deciduous cells, which differentiate and form the deciduous, while the chorionic plate presents a structure of villous trees that form the placental barrier [24].
After implantation of the blastocyst, placental development begins. Initially, placental membrane is composed of four layers, the syncytiotrophoblast facing the mother, a layer of cytotrophoblastic cells, connective tissue of the villi called the stroma of the villi (containing vascular endothelium, fibroblasts and macrophages), the endothelium that lines the fetal capillaries, as well as the basement membranes of these cells [24]. During implantation and the onset of placentation, trophoblastic cells undergo extensive proliferation and differentiation. The cytotrophoblast penetrates the syncytiotrophoblast layer around the initial concept to form columns of extravenous cytotrophoblast cells. As the walls of maternal endometrial vessels are corroded, maternal blood cells reach the gaps. Arterial entrances to the lacunar system and venous exits from the gaps are established, and the branching of trophoblastic cells in the lacunar spaces results in formation of villous trees [23]. Lacunar spaces become the intervillous space where maternal blood flows between villous trees. Fetal capillaries and larger vessels carry oxygenated blood from the villi to the umbilical vein, and deoxygenated fetal blood is returned from the fetus to the placental villi via the umbilical arteries. Maternal blood in the intervillous space and fetal blood in villus capillaries are separated by a continuous layer of syncytiotrophoblast, a discontinuous layer of cytotrophoblastic cells, basal lamina, connective tissue and fetal endothelial cells [12].
The transfer between maternal and fetal circulation occurs through the endothelial-syncytial membrane and depends on several factors, including membrane surface area and thickness, blood flow, hydrostatic pressure in the intervillous chamber and the difference in fetal and maternal osmotic pressure. Maternal blood, therefore, is in direct contact with the fetal chorionic tissue (trophoblast), which is the main barrier that separates the maternal circulation from fetal microvasculature. The fetus is also surrounded by a membranous sac, consisting of amnion, chorion and parietal decidua, which is quite impervious to most xenobiotics from the maternal bloodstream [12,25,26].
The placenta plays a pivotal role in fetal and maternal health, placental pathology is implicated in all common obstetric complications such as preeclampsia and intrauterine growth restriction. As a capture and exchange organ, with access to substances that circulate in maternal bloodstream, the placenta is a potential channel for the administration of fetal drugs, as well, as offering an excellent therapeutic target. However, there are currently few placental-specific therapeutics [27]. The consequences of fetal (and placental) drug exposure can be benign or involve structural or behavioral teratogenicity, or even termination of pregnancy, and are often unknown [26].
3. Nanotherapy
3.1. Nanoparticles: Characteristics, advantages and pharmaceutical considerations
Conceptually, NP are nanometer-sized (1–1000 nm) particles formed by different nanomaterials of organic or inorganic origin. In general, NP are dispersed in a colloidal system, also known as nanoformulations [28]. Among the particles of inorganic origin, metallic NP are the most used [25]. Due to its ultra-small size, they interact with DNA and enzymes, altering the pathophysiology of some diseases [29]. Still, among the particles of organic origin, the polymeric NP are highlighted, due to their biocompatibility, biodegradability and high applicability in biomedical area. Generally, polymeric NP are coated by a polymer of synthetic or natural origin which attributes different physicochemical characteristics to particle [30]. Polymeric NP are excellent drug carriers, targeting the target tissue/organ [31]. Depending on the polymeric coating used in NP, it may generate a greater interaction of drug with cell membrane or increase the time in blood circulation by reducing the recognition of phagocytic system [32,33].
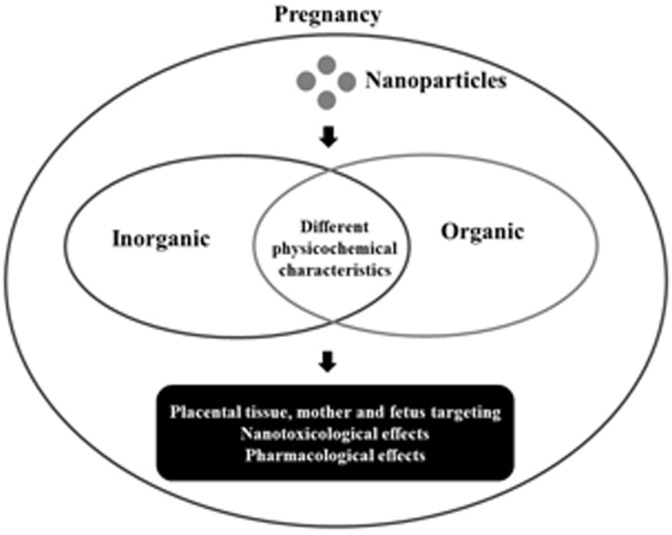
The physicochemical characteristics of NP determined the type of interaction with biological systems (in vitro and in vivo). The physicochemical characteristics most analyzed in NP are size, morphology, pH, morphology and zeta potential. One of those characteristics that is very important is zeta potential. Because it is known that the different charges (negative or positive) present in NP determined the type of interaction with biological system [34]. Besides that, zeta potential is directly related to NP stability in the colloidal system [35] (Fig. 1 ).
Fig. 1.
Overview of review. Interaction of organic and inorganic NP with biological system during pregnancy.
In view of these facts, the development of stable and effective NP requires a knowledge of nanomaterials physicochemical characteristics used and intended applications. In addition, the development of new therapies based on NP in pregnancy requires a thorough understanding of the mechanisms of placental transfer, nanoparticulate systems and their dependence on distinct characteristics and functionality [27]. According to the pharmaceutical purpose, NP can be made from a wide variety of materials and structures, with or without targeting binders and/or ‘stealthy’ portions. The main variables that affect NP biodistribution are chemical composition, size, shape, surface chemistry, porosity and elasticity, which can be adjusted for a specific clinical application [19].
The biodistribution and clearance of NP in the bloodstream (pregnant or non-pregnant) is mainly dependent on the extent of renal elimination and ability to prevent sequestration by endothelial reticulum system (RES) [19,36]. RES removes molecules and particles from the circulation via immune cell phagocytosis and/or liver and spleen sequestration/elimination. Surface opsonization (spontaneous coating by serum proteins) is a fundamental step to facilitate this process [37]. The natural role of opsonins is to promote the approach of bacteria and viruses by phagocytic cells, both systems with the same negative charge that inhibits the interaction between bacteria/viruses and phagocytes due to charge repulsion. After the coating of bacteria and viruses, opsonins undergo conformational rearrangements that induce biorecognition by phagocytes through specific membrane receptors. The opsonization of the xenoparticle by complement system proteins (more than 30 soluble and membrane-bound proteins) finally promotes the phagocyte removal process [36].
Opsonins interact with NP by van der Waals, electrostatic, ionic and hydrophobic/hydrophilic forces. Therefore, the surface characteristics of nanocarriers play a central role in this process. Hydrophobic and charged particles undergo higher opsonization when compared to hydrophilic and uncharged particles. In this way, “stealth” strategies can be employed to reduce opsonization, prevent phagocytic activation and increase tissue uptake [19,36]. It is known that, long circulation nanocarriers can be obtained by coating the surface with hydrophilic polymers that prevent the opsonization process. The consequence of avoiding opsonization is the prolongation of NP permanence in the bloodstream and, with that, tissue uptake [37].
The role of surface loading in the transport of NP for placental barrier has been assessed in a limited number of studies with conflicting results. For example, Aengenheister et al. [38], tested titanium dioxide NP (TiO2 NP) with different surface charges (positive and negative) in BeWo cell cultures (HPEC-A2), and there was no significant transplacetary transfer, but a considerable accumulation of TiO2 NP in placental tissue (showed studies of transplacental passage in Table 1 ).
Table 1.
In vitro/ex vivo studies showing different results regarding transplacental passage.
| Reference | Origin/Type of NP | Model | Results |
|---|---|---|---|
| [42] | Inorganic | BeWo cell | Ability to cross the placental barrier and low toxicity to fetal development |
| Ag | b30 and embryonic stem cells | ||
| [54] | Organic | Ex vivo images | NP did not cause toxicity to fetus |
| PEG-PLA | |||
| [62] | Organic | BeWo cell | Evidence that NP are transcellularly transported |
| Fluoresbrite | |||
| [63] | Organic | BeWo cell | There was no toxicity for cell culture and NP transport was observed by endocytosis and pinocytosis |
| Pullulan acetate | |||
| [64] | Organic | BeWo cell | Nanoencapsulation improved the maternal-fetal transplacental permeation of dexamethasone |
| Poly (lactic-co-glycolic acid) loaded with dexamethasone | |||
| [65] | Organic | BeWo cell | The permeability of the NP was dependent on charge, the negatively charged NP were not detected in basolateral compartment. |
| Polystyrene (cationic and anionic) | |||
| [66] | Inorganic | Placental perfusion | There was no significant decrease in blood flow from the maternal to fetal artery |
| Au | |||
| [67] | Organic | Ex vivo human placenta perfusion and BeWo cell | NP was translocated through the placental barrier without causing toxicity to same. |
| Poly-(HPMA)115-b-poly (DMAEMA)115 |
Studies available in scientific literature from the year 2012–2020. Ag (silver NP). Au (gold NP).
3.2. Drug targeting in pregnancy
Drug targeting aims to modulate and fully direct the distribution of a substance to biophase, associating appropriate system (nanocarrier). In the scientific literature, studies on the uptake or placental transfer of NP acting in drug targeting are scarce. It is known that the use of NP in pregnancy can have three different approaches: maternal, placental or fetal treatment [27]. In this context, nanotechnology can benefit the mother, thus being a useful tool for the treatment of gestational diabetes [39]. For example, in the treatment of preeclampsia, NP are able to release drugs directed to specific tissues without influencing uterine blood flow and fetal safety [24,40]. In postpartum depression and chronic diseases that affect the central nervous system [41].
However, pregnancy represents a major challenge for drug targeting. NP are an alternative to overcome the problem previously exposed. Because that the NP can easily reach the placental barrier. Moreover, depending on the size, NP can cross the placental barrier. Considering that the pores of the placental barrier vary between 15 and 25 nm, a NP of up to 25 nm is able to cross the barrier via passive transport (simple diffusion) [10]. Abdelkhaliq et al. [42], developed silver-NP (Ag NP), with the ability to cross placental barrier (BeWo cell culture model) and presented a low toxicity index in fetal development. Still, the transport was described as dependent on particle size, where NP greater than 50 nm did not cross the placental barrier. In another study (in vivo), Teng et al. [43], proved that ZnO NP with 13 nm of size crossed for placental barrier while ZnO NP with 57 nm did not have this capacity. Thus, the size of NP is directly related to your ability a drug targeting.
It is known that exposure of fetus to NP occurs mainly through the mother circulation to the fetal circulation, but this exposure may also occur through amniotic fluid [24,44]. In view of above, myometrial and placental targeting allows to increase the protection to fetus with regard to the treatment of obstetric diseases [[45], [46], [47]]. For example, in the treatment of malaria, it is necessary to obtain a limited transplacental passage, as the purpose in this case is to prevent Plasmodium from infecting erythrocytes in placental tissue [48]. The use of a nanocarrier aimed at placenta will certainly strengthen the safety and efficiency of therapy [49]. NP are also able to cross the placenta and treat the fetus, as in hypoxia situations [50], and through the transport of macromolecules such as immunoglobulin G to fetal circulation [51]. In the studies (in vitro) of Hua and Vaughan [52] and Hua [53] was demonstrated the potential of NP (liposomes) for drug targeting to myometrial tissue. Moreover, Li et al. [54] (ex vivo), tested NP by targeting of placental tissue for preeclampsia-treatment. Still, Elsharawy et al. [55] (in vivo), analyzed the impact of a biosynthesized NP with a polymeric coating on possible targeting to placenta. But aforementioned studies have stopped on the nanotoxicity question, due to the majority using inorganics NP. Thus, liposomal NP seem to be safer for drug targeting.
In view of above, the use of NP during pregnancy has proved to be an area of recent knowledge, with much to be understood. As mentioned in previous section, zeta potential is directly related to the interaction of NP with the placental barrier or other tissue during pregnancy [35]. When the objective is to drug targeting during pregnancy, this physicochemical characteristic of NP is very important to direct the NP (loaded with drug or not) to target tissue. It is also reiterated that depending on whether the NP is of organic or inorganic origin, they will have a different targeting due to their different physicochemical characteristics. Thus, in current review, we seek to select studies that can exemplify the effects of exposure to NP during pregnancy. Through in vivo studies (showed in Table 2 ) using NP, it was possible to evaluate the impact of organic and inorganic NP on placental tissue, mother and fetus as well as the advantages related to the three (targeting).
Table 2.
In vivo studies and the results obtained from use of NP during pregnancy.
| Reference | Origin/Type of NP | Animal Specie | Route of administration | Results |
|---|---|---|---|---|
| [39] | Inorganic | Mice | Oral | NP abrogated the diabetes-induced embryopathy through your antioxidant effects. |
| Mettalic | ||||
| Cerium | ||||
| [43] | Inorganic | Mice | Oral | NP caused toxicity to fetus. |
| Mettalic | ||||
| ZnO | ||||
| [45] | Organic | Ratss | Intravenous | NP prevented oxidative stress in placenta, but not in the fetus. |
| Polymeric | ||||
| Poly (γ-glutamic acid) and l-phenylalanine ethylester | ||||
| [46] | Organic | Rats | Intravenous | NP caused beneficial effects dependent on sex and age on the cardiovascular function of adult offspring. |
| Polymeric | ||||
| Poly (γ-glutamic acid) and l-phenylalanine ethylester | ||||
| [48] | Inorganic | Mice | Oral | NP caused a decrease in angiogenesis and activation of apoptotic pathways through caspase-3 in placental tissue. |
| Mettalic | ||||
| TiO2 | ||||
| [50] | Organic | Mice | Oral | NP improved the delivery of compound to the maternal and fetal brains and also reduced the accumulation of fatty acids in fetal liver. |
| Protein | ||||
| Zein | ||||
| [55] | Inorganic | Rats | Intraperitoneal | NP had a toxic effect on maternal, placenta and fetus tissues. |
| Mettalic | ||||
| Ag coated with chitosan | ||||
| [57] | Inorganic | Rats | Inhalation | NP caused severe impacts causing fetal resorption, decreased estrogen and increased proinflammatory cytokines levels. |
| Mettalic | ||||
| Ag | ||||
| [59] | Organic | Rats | Intravenous | There was a tissue distribution depending on the particle charge. |
| Polymeric | ||||
| Poly (glycidyl methacrylate) | ||||
| [68] |
Inorganic Mettalic Au |
Mice | Intravenous | There was a tissue distribution depending on the particle size. |
| [69] | Organic | Mice | Intravenous | Np with diameters up to 500 nm were absorbed by placenta and were able to cross the placental barrier. |
| Polymeric | ||||
| Polystyrene | ||||
| [70] | Inorganic | Mice | Intravenous | In clinical and histopathological evaluation, there were no changes in placental and fetal development. |
| Mettalic | ||||
| SiO2 | ||||
| [71] | Inorganic | Mice | Subcutaneous | Maternal exposure to NP influenced the offspring central dopaminergic system. |
| Mettalic | ||||
| TiO2 | ||||
| [72] | Organic | Mice | Intravenous | Prevented fetal exposure and minimized placental exposure. |
| Liposome | ||||
| Phosphatidylcholine + cholesterol | ||||
| [73] | Inorganic | Mice | Intravenous | Remarkable accumulation of silver in maternal liver and spleen, may have affected embryonic growth. |
| Mettalic | ||||
| Ag | ||||
| [74] | Inorganic | Rats | Oral | NP induced oxidative stress and apoptosis in the fetal liver. |
| Mettalic | ||||
| Ag | ||||
| [75] | Inorganic | Chicken | Oral | NP caused abnormal expression of genes and proteins in offspring liver. |
| Mettalic | ||||
| ZnO | ||||
| [76] | Organic Polymeric Poly(ϵ-caprolactone) | Rats | Oral | NP did not cause toxicological effects in pregnant rats and your fetuses. |
Studies available in scientific literature from the year 2010–2020. Ce (Cerium NP). ZnO (zinc oxide NP). TiO2 (titanium dioxide NP) Ag (silver NP). Au (gold NP). SiO2 (silicon dioxide NP).
3.3. Implications in use of NP during pregnancy
Currently, one of the biggest challenges for use of NP during pregnancy is the nanotoxicological question. Nanotoxicological studies have to assess the impact of NP-use on the mother, fetus and placenta. In view of this problem, NP targeting is fundamental for the advancement of these studies Thus, markers of oxidative stress, inflammatory status, DNA damage, epigenetic changes and fetal malformations must be mandatorily evaluated by nanotoxicological studies [49].
Gestational age is an important factor to be taken into account when assessing the impact of NP-using. Fennell et al. [56], evaluated the effect of using Ag NP (administered per oral and intravenously route) on gestational day 18 in female rats. In this study, was reported that Ag NP bioaccumulated in placenta after 48 h and still transfer and deposition of NP occurred from this tissue to fetuses. NP can have a direct impact on the fetus even at a more advanced gestational age. In another study, Campagnolo et al. [57], demonstrated that inhalation of Ag NP during the first gestational day until the fifteenth gestational day in female rats has severe impacts causing fetal resorption, decreased estrogen and increased proinflammatory cytokines levels in mothers. In the studies aforementioned, three different routes of administration are used for Ag NP. Again, reiterating that use of inorganic NP during pregnancy requires several precautions, mainly the Ag NP.
In view of above, Zhang et al. [48] evaluated the effect of maternal exposure (mice) to NP-TiO2 with an emphasis on placental analysis. The researchers found that exposure to TiO2 NP caused a decrease in angiogenesis and activation of apoptotic pathways through caspase-3 in placental tissue. Corroborating with the studies aforementioned. In opposite manner, Lee et al. [58], found no changes in toxicity markers when they administered TiO2 NP to pregnant rats for 13 days during pregnancy, however, only clinical markers were used and no biochemical determinations. Thus, metallic NP despite the advantage of crossing the placental barrier due to their size, require attention in relation to toxicological effect.
On the other hand, Ho et al. [59], evaluated the effect of polymeric (poly (glycidyl methacrylate)) NP (cationic and anionic, organic NP) during the 10th and 20th gestational day in rats, NP had a tendency to accumulate in placenta at two periods tested. Although poly (glycidyl methacrylate) is a synthetic polymer, this study may represent an advance on using NP vs nanotoxicological question during pregnancy, since different polymers have already been tested toxicologically and have had few or no toxicological effects [60,61].
Thus, in our present review, analyzing the grouped studies (Table 2), we found that despite the nanotoxicological challenge, NP are promising for carrying drugs during pregnancy treating obstetric disorders related to the placental tissue, mother and fetus.
4. Conclusion
In current review, we analyzed through the scientific literature, the impact of NP-use during pregnancy. Each different nanomaterial used to form the NP will endow it with different physicochemical characteristics that directly influence the targeting and toxicity, that is, NP ability to reach the target tissue. Moreover, the NP-size is directly related to the ability to cross placental barrier. Despite several promising studies, nanotoxicology is a factor that prevents the application and use of NP in clinical practice. We found that the use of inorganic NP despite the advantage of crossing placental barrier, has a tendency to have deleterious effects on the placental tissue, mother and fetus. On the other hand, studies that used polymeric organic NP, seem to represent an advance for use of NP during pregnancy, due to your effectiveness in targeting and low toxicity, but they are still scarce. It can be a therapeutic alternative for complications or diseases during pregnancy. Therefore, in the future, NP will be an effective and safe alternative for use in pregnancy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare that there are no conflicts of interest.
Acknowledgment
The authors thank a Federal University of Pampa for their financial support. This study was financed in part by the Coordination for the Improvement of Higher Education Personnel-Brazil (CAPES) – Finance Code 001.
References
- 1.Ewing A.C. Predictors of perinatal HIV transmission among women without prior antiretroviral therapy in a resource-limited setting: the breastfeeding, antiretrovirals and nutrition study. Pediatr. Infect. Dis. 2019;38:508–512. doi: 10.1097/INF.0000000000002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kourtis A.P., Read J.S., Jamieson D.J. Pregnancy and infection. N. Engl. J. Med. 2014;23:2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu N. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020;5(2020):559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;10226:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulvey J.J. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients. Ann. Diagn. Pathol. 2020;46:151530. doi: 10.1016/j.anndiagpath.2020.151530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dashraath P. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020;222:521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Della Gatta A.N. Coronavirus disease 2019 during pregnancy: a systematic review of reported cases. Am. J. Obstet. Gynecol. 2020;S0002–9378(2020):30438–30445. doi: 10.1016/j.ajog.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudramurthy G.R., Swamy M.K. Potential applications of engineered nanoparticles in medicine and biology: an update. J. Biol. Inorg. Chem. 2018;23:1185–1204. doi: 10.1007/s00775-018-1600-6. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B. Surface-functionalized nanoparticles as efficient tools in targeted therapy of pregnancy complications. Int. J. Mol. Sci. 2019;15:3642. doi: 10.3390/ijms20153642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riedel M., Kuschel B. Systemic drug treatment during pregnancy. Hautarzt. 2020;71:313–323. doi: 10.1007/s00105-020-04560-z. [DOI] [PubMed] [Google Scholar]
- 12.Al-enazy S. Placental control of drug delivery. Adv. Drug Deliv. Rev. 2017;116:63–72. doi: 10.1016/j.addr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi M.D. Drug delivery during pregnancy: how can nanomedicine be used? Ther. Deliv. 2017;12:1023–1025. doi: 10.4155/tde-2017-0084. [DOI] [PubMed] [Google Scholar]
- 14.Center for Disease Control and Prevention. 2018. https://www.cdc.gov/pregnancy/meds/treatingfortwo/research.html USA.
- 15.Mitchell A.A. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am. J. Obstet. Gynecol. 2011;205:1–8. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pariente G. Pregnancy-associated changes in pharmacokinetics: a systematic review. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tasnif Y., Morado J., Hebert M.F. Pregnancy-related pharmacokinetic changes. Clin. Pharmacol. Ther. 2016;100:53–62. doi: 10.1002/cpt.382. [DOI] [PubMed] [Google Scholar]
- 18.Costantine M. Physiologic and pharmacokinetic changes in pregnancy. Front. Pharmacol. 2014;5:e65. doi: 10.3389/fphar.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keelan J.A. Therapeutic and safety considerations of nanoparticle-mediated drug delivery in pregnancy. Nanomedicine. 2015;10:2229–2247. doi: 10.2217/nnm.15.48. [DOI] [PubMed] [Google Scholar]
- 20.Pinheiro E.A., Stika C.S. Drugs in pregnancy: pharmacologic and physiologic changes that affect clinical care. Semin. Perinatol. 2020;44 doi: 10.1016/j.semperi.2020.151221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scaffidi J., Mol B.W., Keelan J.A. The pregnant women as a drug orphan: a global survey of registered clinical trials of pharmacological interventions in pregnancy. Bjog. 2017;124:132–140. doi: 10.1111/1471-0528.14151. [DOI] [PubMed] [Google Scholar]
- 22.Ito S. Mother and child: medication use in pregnancy and lactation. Clin. Pharmacol. Ther. 2016;100:8–11. doi: 10.1002/cpt.383. [DOI] [PubMed] [Google Scholar]
- 23.Maltepe E., Fisher S.J. Placenta: the forgotten organ. Annu. Rev. Cell Dev. Biol. 2015;31:523–552. doi: 10.1146/annurev-cellbio-100814-125620. [DOI] [PubMed] [Google Scholar]
- 24.De Araujo T.E. Experimental models of maternal-fetal interface and their potential use for nanotechnology applications. Cell Biol. Int. 2019 doi: 10.1002/cbin.11222. [DOI] [PubMed] [Google Scholar]
- 25.Menezes V., Malek A., Keelan J.A. Nanoparticulate drug delivery in pregnancy: placental passage and fetal exposure. Curr. Pharmaceut. Biotechnol. 2011;12:731–742. doi: 10.2174/138920111795471010. [DOI] [PubMed] [Google Scholar]
- 26.Tetro N. The placental barrier: the gate and the fate in drug distribution. Pharm. Res. (N. Y.) 2018;35:71. doi: 10.1007/s11095-017-2286-0. [DOI] [PubMed] [Google Scholar]
- 27.Muoth C. Nanoparticle transport across the placental barrier: pushing the field forward. Nanomedicine. 2016;11:941–957. doi: 10.2217/nnm-2015-0012. [DOI] [PubMed] [Google Scholar]
- 28.Pirtarighat S., Ghannadnia M., Baghshahi S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem. 2018;9:1–9. [Google Scholar]
- 29.Mody V.V. Introduction to metallic nanoparticles. J. Pharm. BioAllied Sci. 2010;4:282–289. doi: 10.4103/0975-7406.72127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiel L. Diverse deformation properties of polymeric nanocapsules and lipid-core nanocapsules. Soft Matter. 2011;7:7240–7248. [Google Scholar]
- 31.Wen C. Molecular structures and mechanisms of waterborne biodegradable polyurethane nanoparticles. Comput. Struct. Biotechnol. J. 2019;17:110–117. doi: 10.1016/j.csbj.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C. Biodegradable cationic polymeric nanocapsules for overcoming multidrug resistance and enabling drug–gene co-delivery to cancer cells. Nanoscale. 2014;6:1567–1572. doi: 10.1039/c3nr04804g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guterres S. Chitosan gel containing polymeric nanocapsules: a new formulation for vaginal drug delivery. Int. J. Nanomed. 2014;9:3151–3161. doi: 10.2147/IJN.S62599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank L.A. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020;147 [Google Scholar]
- 35.Sur S. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Structures & Nano-Objects. 2019;20 [Google Scholar]
- 36.Zhao Z. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019;143:3–21. doi: 10.1016/j.addr.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Salmaso S., Caliceti P. Stealth properties to improve therapeutic efficacy of drug nanocarriers. J. Drug. Deliv. 2013 doi: 10.1155/2013/374252. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aengenheister L. Investigating the accumulation and translocation of titanium dioxide nanoparticles with different surface modifications in static and dynamic human placental transfer models. Eur; Jour. of Pharm. and Biopha. 2019;142:488–497. doi: 10.1016/j.ejpb.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Vafaei-pour Z. Embryo-protective effects of cerium oxide nanoparticles against gestational diabetes in mice. Iran. J. Pharm. Res. (IJPR) 2018;17:964–975. [PMC free article] [PubMed] [Google Scholar]
- 40.Valero L. Nanomedicine as a potential approach to empower the new strategies for the treatment of preeclampsia. Drug Discov. Today. 2018;23:1099–1107. doi: 10.1016/j.drudis.2018.01.048. [DOI] [PubMed] [Google Scholar]
- 41.Sezgin-Bayindir Z. Investigations on clonazepam-loaded polymeric micelle-like nanoparticles for safe drug administration during pregnancy. J. Microencapsul. 2018;35:149–164. doi: 10.1080/02652048.2018.1447615. [DOI] [PubMed] [Google Scholar]
- 42.Abdelkhaliq A. Combination of the BeWo b30 placental transport model and the embryonic stem cell test to assess the potential developmental toxicity of silver nanoparticles. Part. Fibre Toxicol. 2020;17:11. doi: 10.1186/s12989-020-00342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teng C. Size-dependent maternal-fetal transfer and fetal developmental toxicity of ZnO nanoparticles after oral exposures in pregnant mice. Ecotoxicol. Environ. Saf. 2019;182:109439. doi: 10.1016/j.ecoenv.2019.109439. [DOI] [PubMed] [Google Scholar]
- 44.Saunders M. Transplacental transport of nanomaterials. Nanomedicine and Nanobiotechnology. 2009;1:671–684. doi: 10.1002/wnan.53. [DOI] [PubMed] [Google Scholar]
- 45.J Phillips T. Treating the placenta to prevent adverse effects of gestational hypoxia on fetal brain development. Sci. Rep. 2017;7:9079. doi: 10.1038/s41598-017-06300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aljunaidy M.M. Maternal treatment with a placental-targeted antioxidant (MitoQ) impacts offspring cardiovascular function in a rat model of prenatal hypoxia. Pharmacol. Res. 2018;134:332–342. doi: 10.1016/j.phrs.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Ganguly E. Placenta-targeted treatment strategies: an opportunity to impact fetal development and improve offspring health later in life. Pharmacol. Res. 2020;157:104836. doi: 10.1016/j.phrs.2020.104836. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L. Gestational exposure to titanium dioxide nanoparticles impairs the placentation through dysregulation of vascularization, proliferation and apoptosis in mice. Int. J. Nanomed. 2018;13:777–789. doi: 10.2147/IJN.S152400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mortensen N.P. Biological interactions between nanomaterials and placental development and function following oral exposure. Reprod. Toxicol. 2019;90:150–165. doi: 10.1016/j.reprotox.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 50.Zeng J. A nanoencapsulation suspension biomimetic of milk structure for enhanced maternal and fetal absorptions of DHA to improve early brain development. Nanomedicine. 2019;15:119–128. doi: 10.1016/j.nano.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Zhang B. Surface-functionalized nanoparticles as efficient tools in targeted therapy of pregnancy complications. Int. J. Mol. Sci. 2019;20:3642. doi: 10.3390/ijms20153642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hua S., Vaughan B. In vitro comparison of liposomal drug delivery systems targeting the oxytocin receptor: a potential novel treatment for obstetric complications. Int. J. Nanomed. 2019;14:2191–2206. doi: 10.2147/IJN.S198116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hua S. Synthesis and in vitro characterization of oxytocin receptor targeted PEGylated immunoliposomes for drug delivery to the uterus. J. Liposome Res. 2019;29:357–367. doi: 10.1080/08982104.2018.1556293. [DOI] [PubMed] [Google Scholar]
- 54.Li L. Trophoblast-targeted nanomedicine modulates placental sflt 1 for preeclampsia treatment. Front. Bioeng. Biotechnol. 2020;8:64. doi: 10.3389/fbioe.2020.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elsharawy K. Chitosan coating does not prevent the effect of the transfer of green silver nanoparticles biosynthesized by Streptomyces malachitus into fetuses via the placenta. Reprod. Biol. 2020;20:97–105. doi: 10.1016/j.repbio.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Fennell T.R. Disposition of intravenously or orally administered silver nanoparticles in pregnant rats and the effect on the biochemical profile in urine. J. Appl. Toxicol. 2017;37:530–544. doi: 10.1002/jat.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campagnolo L. Silver nanoparticles inhaled during pregnancy reach and affect the placenta and the foetus. Nanotoxicology. 2017;11:687–698. doi: 10.1080/17435390.2017.1343875. [DOI] [PubMed] [Google Scholar]
- 58.Lee J. Titanium dioxide nanoparticles oral exposure to pregnant rats and its distribution. Part. Fibre Toxicol. 2019;16:31. doi: 10.1186/s12989-019-0313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ho D. Maternal-placental-fetal biodistribution of multimodal polymeric nanoparticles in a pregnant rat model in mid and late gestation. Sci. Rep. 2017;7:2866. doi: 10.1038/s41598-017-03128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pereira M.P. Cationic and anionic unloaded polymeric nanocapsules: toxicological evaluation in rats shows low toxicity. Biomed. Pharmacother. 2019;116:109014. doi: 10.1016/j.biopha.2019.109014. [DOI] [PubMed] [Google Scholar]
- 61.G de Gomes M. Assessment of unloaded polymeric nanocapsules with different coatings in female rats: influence on toxicological and behavioral parameters. Biomed. Pharmacother. 2020;121:109575. doi: 10.1016/j.biopha.2019.109575. [DOI] [PubMed] [Google Scholar]
- 62.Cartwirght L. In vitro placental model optimization for nanoparticle transport studies. Int. J. Nanomed. 2012;7:497–510. doi: 10.2147/IJN.S26601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang H. Uptake and transport of pullulan acetate nanoparticles in the BeWo b30 placental barrier cell model. Int. J. Nanomed. 2018;13:4073–4082. doi: 10.2147/IJN.S161319. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ali H. Preparation, characterization, and transport of dexamethasone-loaded polymeric nanoparticles across a human placental in vitro model. Int. J. Pharm. 2013;454:149–157. doi: 10.1016/j.ijpharm.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kloet S.K. Translocation of positively and negatively charged polystyrene nanoparticles in an in vitro placental model. Toxicol. Vitro. 2015;29:1701–1710. doi: 10.1016/j.tiv.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 66.D'Errico J.N. Identification and quantification of gold engineered nanomaterials and impaired fluid transfer across the rat placenta via ex vivo perfusion. Biomed. Pharmacother. 2019;117:109148. doi: 10.1016/j.biopha.2019.109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson R.L. Nanoparticle mediated increased insulin-like growth factor 1 expression enhances human placenta syncytium function. Placenta. 2020;93:1–7. doi: 10.1016/j.placenta.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang H. Effects of nanoparticle size and gestational age on maternal biodistribution and toxicity of gold nanoparticles in pregnant mice. Toxicol. Lett. 2014;230:10–18. doi: 10.1016/j.toxlet.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 69.Huang J.P. Nanoparticles can cross mouse placenta and induce trophoblast apoptosis. Placenta. 2015;36:1433–1441. doi: 10.1016/j.placenta.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 70.Pietroiusti A. Relevance to investigate different stages of pregnancy to highlight toxic effects of nanoparticles: the example of silica. Toxicol. Appl. Pharmacol. 2018;342:60–68. doi: 10.1016/j.taap.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi Y. Prenatal exposure to titanium dioxide nanoparticles increases dopamine levels in the prefrontal cortex and neostriatum of mice. J. Toxicol. Sci. 2010;35:749–756. doi: 10.2131/jts.35.749. [DOI] [PubMed] [Google Scholar]
- 72.Refuerzo J.S. Liposomes: a nanoscale drug carrying system to prevent indomethacin passage to the fetus in a pregnant mouse model. Am. J. Obstet. Gynecol. 2015;212:508.e1–508.e7. doi: 10.1016/j.ajog.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 73.Austin C.A. Distribution and accumulation of 10 nm silver nanoparticles in maternal tissues and visceral yolk sac of pregnant mice, and a potential effect on embryo growth. Nanotoxicology. 2016;10:654–661. doi: 10.3109/17435390.2015.1107143. [DOI] [PubMed] [Google Scholar]
- 74.Fatemi M. Effects of silver nanoparticle on the developing liver of rat pups after maternal exposure. Iran. J. Pharm. Res. (IJPR) 2017;16:685–693. [PMC free article] [PubMed] [Google Scholar]
- 75.Hao Y. Molecular evidence of offspring liver dysfunction after maternal exposure to zinc oxide nanoparticles. Toxicol. Appl. Pharmacol. 2017;329:318–325. doi: 10.1016/j.taap.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 76.Giacomeli R. Curcumin-loaded poly(ϵ-caprolactone) lipid-core nanocapsules: Evaluation of fetal and maternal toxicity. Food Chem. Toxicol. 2020;144:111625. doi: 10.1016/j.fct.2020.111625. [DOI] [PubMed] [Google Scholar]