Abstract
A novel coronavirus termed as COVID-19 by WHO has been the causative agent of an unprecedented pandemic in the history of humanity. The global burden of mortality and morbidity associated with this pandemic continues to increase with each passing day as it is progressively leading to multiorgan dysfunction. In most cases, the cause of death has been attributed to respiratory failure, sepsis, cardiac failure, kidney injury, or coagulopathy. As more knowledge is being unfolded, an in-depth understanding of various systemic manifestations and complications of SARS-CoV2 is vital for optimum management of these patients. This novel virus is known to spread faster than its two ancestors, the SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), demonstrating a case fatality ranging from 5 to 8% [1]. Hematological abnormalities such as lymphopenia, thrombocytopenia, elevated D-Dimer, elevated fibrinogen, elevated fibrinogen degradation products as well as cytokines such as IL-6 are emerging as important prognostic marker for worse outcome of COVID-19. Among various systemic manifestations, hematological complications such as venous thrombosis causing pulmonary embolism or deep vein thrombosis, and arterial thrombosis causing myocardial infarction, strokes or limb ischemia are being noted to be directly linked to high mortality from COVID-19. An attempt to understand the pathophysiology of various hematological abnormalities including cytokine storm, hypercoagulable state and some rare presentations of this disease hence becomes imperative. Through this review, we aim to provide an up-to-date summary of current evidence-based literature of hematological manifestations, their consequences and management including role of anticoagulation and drugs targeting cytokine storm in patients with SARS-CoV-2.
Keywords: COVID-19, Thrombosis, Cytokine release syndrome, Thrombocytopenia, Coagulation, Anticoagulation
1. Background
A novel coronavirus termed as COVID-19 (named by WHO on Feb 11, 2020)has been the causative agent of an unprecedented pandemic in the history of humanity that originated in Wuhan China in December 2019 [2]. COVID-19 is caused by a virus called SARS-CoV2 belonging to a group of beta-coronaviruses, a large family of single-stranded RNA viruses [3]. As of June 28, 2020, there are more than 10 million cases across the world with 501,281 deaths as per the Johns Hopkins Coronavirus resource center in the United States of America [4].
Bubonic Plague (1347–1351) and Smallpox (1520) have been the worst outbreaks known to humans each leading to deaths of approximately 200 million and 56 million people respectively. The Spanish flu pandemic of 1918, the deadliest among them, infected an estimated 500 million people worldwide and killed an estimated 50 million victims. While the deadly Spanish flu had a case fatality rate (CFR, the ratio between confirmed deaths and confirmed deaths) of about 2%, the global case fatality rate of COVID-19 is estimated to be 7.13% (as of April 24, 2020) [1].
The COVID-19 outbreak has posed serious challenges to the public and healthcare community and an adequate understanding of the systemic effects of this novel coronavirus is key to its prevention and cure. Early hematological abnormalities in COVID-19 have been described in the literature which is directly linked to mortality in these patients. This review provides an overview of the hematological presentations, pathophysiology, and complications that are being manifested in this outbreak. We also aim to highlight the existing and under investigation treatments that are being used for various abnormalities and complications.
2. Transmission and pathophysiology
COVID-19 had 88% similarity to two bat-derived severe acute respiratory syndromes (SARS)-like coronaviruses on genomic sequence analysis, providing plausible evidence of transmission of the virus from mammals to humans [5]. Person-to-person transmission of SARS-CoV-2 is mainly via respiratory droplets or direct contact from an infected patient. The virus was found to be viable in aerosol for at least 3 h, on copper for 4 h, cardboard for 24 h, and remained stable on plastic and stainless steel up to 72 h after application to these surfaces [6,7]. Though the fecal-oral route of transmission has been reported, it does not appear to be significant according to the WHO-China Joint report [8]. Further, studies have shown that the transmission potential and viral load are similar in asymptomatic as well as symptomatic carriers accounting for the rapid spread of this pandemic [9].
It has been well described that ACE-2 (angiotensin-converting enzyme −2) receptor acts as a site for cellular binding for SARS-CoV-2 [10]. However, its affinity for the ACE-2 receptor is 10–20 folds higher than other coronaviruses, amounting to its higher transmissibility [11]. ACE-2 receptor expression is found in pulmonary and extrapulmonary tissues including type 2 alveolar epithelial cells in the lungs, bronchus, nasal mucosa, heart, esophagus, kidney, stomach, bladder, and ileum making them susceptible entry sites [12]. With the help of TMPRSS2 (transmembrane serine protease 2), the virus is endocytosed by proteolytic cleavage of ACE-2, which is followed by its cytosol replication and cell-to-cell transmission [12].
3. Hematological LAB abnormalities (Fig. 1)
Fig. 1.
Hematological Parameters and Prognostic correlation with COVID-19. Abbreviations: ICU- Intensive Care Unit, ARDS- Acute Respiratory Distress Syndrome, VTE-Venous Thromboembolism.
3.1. Complete blood count overview
3.1.1. White blood cells (WBC)
3.1.1.1. Presentation
During the early phase of COVID-19, most patients present with normal leukocyte count. In later stages, either leukopenia or leukocytosis can occur though leukopenia has been more frequently reported [13]. Rodriguez et al. performed a metanalysis of 8 studies involving 511 patients and concluded that lymphopenia is present in 43.1% of the patients [14]. In another study by Guan et al. lymphopenia was observed in 82.1%, leukopenia in 33.7% vs leukocytosis in 5.9% of 1099 patients from 552 hospitals in China [15]. In contrast, a correspondence by Fan BE et al., in their small cohort of 69 patient observed that ICU patients were more likely to develop neutrophilia during the hospitalization with a median peak Absolute Neutrophil Count of 11.6 × 109/L, compared to 3.5 × 109/L in the non-ICU group (P-value < 0.001) [16].
3.1.1.2. Etiology
The frequency of lymphopenia found suggests that COVID-19 might act on lymphocytes, especially T lymphocytes causing depletion of CD4 and CD8 cells. Potential mechanisms include: a)lysis of lymphocytes through direct target of viruses through ACE2 receptor, b)Cytokine-induced atrophy of lymphatic organs may decrease the lymphocyte turnover (although direct damage of thymus and spleen has not been proven), c) pro-inflammatory mediators induced direct lymphocyte apoptosis or d) coexisting metabolic disorders in certain patients like lactic acidosis can lead to inhibition of lymphocyte production and decrease their cytotoxic activity [[17], [18], [19]].
3.1.1.3. Prognostic correlation with COVID-19
Patients with severe COVID-19 were noted to have more prominent laboratory abnormalities than those with a non-severe disease [15]. Lymphopenia is a reliable indicator of the severity and hospitalization in COVID-19 patients [17]. Wang et al. followed changes in six clinical laboratory parameters from day 1 to day 19 at 2-day intervals and found that non-survivors had higher WBC with higher neutrophil and lower lymphocyte counts than those in survivors [13]. A meta-analysis revealed increased Neutrophil-to-lymphocyte ratio and decreased lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease as compared to non-severe patients [20].
3.1.2. Platelets
3.1.2.1. Presentation
Thrombocytopenia in most patients with COVID-19 is mild (platelet counts 100–150/mm3) [15]. Qu et al. found that in severe COVID-19 patients, platelet counts had a trend of initial increase followed by decrease with worsening clinical status, eventually leading to a lengthier hospital stay [21].
3.1.2.2. Etiology
It is known that thrombocytopenia was reported in up to 55% of patients in the outbreak of SARS. The possible mechanisms for thrombocytopenia in SARS include a) direct endothelial damage by virus leading to platelet activation, b) aggregation and thrombosis in lungs resulting in platelet consumption, c) infection of bone marrow resulting in abnormal hematopoiesis or d) triggering of an auto-immune response against blood cells. We can extrapolate that these mechanisms might not be specific to SARS and thrombocytopenia in COVID-19 likely have similar etiology.
3.1.2.3. Prognostic correlation with COVID-19
Many studies comparing the platelet counts in ICU vs non-ICU COVID-19 patients showed that ICU patients and non-survivors were more likely to have lower counts [[22], [23], [24]]. A meta-analysis of nine studies of 1779 COVID-19 patients showed that thrombocytopenia is associated with a fivefold increased risk of severity and mortality, therefore serving as a clinical indicator of worsening illness during hospitalization [25]. Another interesting finding, albeit from a small study of 30 patients, showed that larger difference in Platelet- Lymphocyte ratio predicted worse outcomes and increased hospital stay of COVID-19 patients [21].
3.1.3. Red blood cell
In patients with COVID-19 anemia has not been reported frequently [15].
Overall, patients with severe COVID-19 release markers of hyper-inflammation and identification of this subgroup might be beneficial, as early immunosuppression in such patients might decrease mortality. H-score [26], generally used as a score for the diagnosis of reactive hemophagocytic syndrome, has been proposed in patients with severe COVID-19 to screen for hyper-inflammation markers to assess the need for treatment [27].
3.2. Coagulation profile overview
3.2.1. Presentation
Hematological profile in COVID-19 has been consistently associated with a hypercoagulable picture with elevated D- dimer, elevated fibrinogen degradation products (FDP), elevated fibrinogen, prolonged prothrombin time (PT) and activated partial thromboplastin time (APTT) [28].
3.2.2. Etiology
There is evolving evidence that SARS-COV-2 causes activation of inflammatory cells such as neutrophils, monocytes, and endothelial cells that results in cytokine release and excess production of pro-coagulants such as tissue factor and von Willebrand factor [29]. Circulation of free thrombin leads to the formation of a fibrin clot that stimulates fibrinolysis along with platelet activation which ultimately results in widespread microvascular thrombosis [29]. Another consideration that liver-related dysfunction from COVID-19 can affect the hemostatic changes remains to be investigated [30].
3.2.3. Prognostic correlation with COVID-19
D-dimer has been reported to be an important predictor of development of complications such as acute respiratory distress syndrome (ARDS) requiring admission to ICU, mechanical ventilation, and death in COVID-19. As early as December 2019, a small study of 41 patients in Wuhan, China have detected that D- Dimer and PT had significant differences in ICU vs non-ICU patients (median D-dimer level 2.4 mg/L vs 0.5 mg/L, p = 0.0042; median PT 12.2 s vs 10.7 s,p = 0.012) [31]. In another retrospective analysis of 183 patients by Tang N et al. non-survivors with pneumonia had significantly higher D-dimer and FDP levels and longer PT and APTT compared to survivors [29]. Fibrinogen and anti-thrombin activity were found to be significantly lower in non-survivors. D-dimer was found to be increased early in the disease course (day 1–2) of non- survivors, FDP was elevated by day 4 and both remained markedly elevated till day 14. Fibrinogen, being an acute phase reactant was found to be elevated initially, followed by a decrease to less than 200 mg/dL by day 10–14 onwards among non-survivors. Disseminated intravascular coagulation (DIC) was associated with most of the deaths and is a predictor of poor prognosis [29]. However, this was a single-center study and the patients' characteristics may not be representative of the general population.
Similar findings were reported by Wu C et al. (23)where patients with severe infection and ARDS were found to have elevated D-dimers compared to patients without ARDS (D-Dimer difference 0.52 microgram/ml). Among the patients with ARDS who died, the D-dimer difference was 2.1 microgram/ml compared to the survivors with ARDS (p < 0.01). Elevated PT was also significantly associated with higher risks of the development of ARDS [23].
In the analysis by Guan et al., D-dimer of >0.5 mg/L was observed in 46.4% of patients with 59.6% of them having a severe disease and 69.4% of the patients with elevated D-dimers required mechanical ventilation [15]. Mounting evidence suggests that patients who have markedly raised D- dimers (approximately three to four-fold increase) should be considered for hospital admission even in the absence of severe symptoms [32].
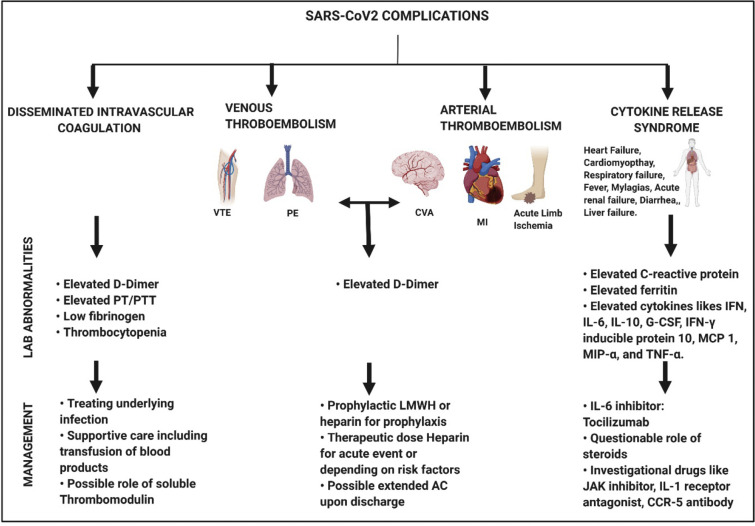
4. Hematological complications (Fig. 2)
Fig. 2.
Figure illustrating the hematological complications, Lab abnormalities and possible treatments due to SARS- CoV2. Abbreviations: VTE, venous thromboembolism; PE, pulmonary embolism; CVA, cerebrovascular accident; MI, myocardial infarction; sHLH, secondary hemophagocytic lymphohistiocytosis; G-CSF, granulocyte-colony stimulating factor (g-CSF); IFN- γ, interferon-γ; MCP 1, monocyte chemoattractant protein 1; MIP- α, macrophage inflammatory protein 1-α; and TNF- α, tumor necrosis factor-α. Created with BioRender.com
4.1. Thrombosis
4.1.1. Presentation
The initial reports on clinical characteristics of novel COVID-19 Pneumonia (NCP) mentioned DIC as a presenting feature however, the incidence of thrombosis was not described. But in recent times, more studies have emerged that highlight cases with both arterial and venous thrombosis associated with this infection.
4.1.2. Venous thrombosis
Most patients of COVID-19 had venous thrombosis in the lower extremity, with few catheter-related thrombosis. A few case reports also described NCP presenting with co-existing pulmonary embolism without any risk factor for VTE [33].
A multi-institutional Dutch study of ICU-admitted patients showed an incidence of 27% VTE despite prophylactic standard dose anticoagulation [34]. A similar single-institution analysis of 81 ICU-admitted patients in Wuhan reported VTE in 25% of the patients with severe NCP. This study, however, did not report whether the patients received prophylactic anticoagulation [35]. A recent study of COVID-19 patients with a high risk of thrombosis found that despite 40% of patients in the study who were predicted to have a high risk of VTE based on Padua score, only 7% of patients received prophylactic anticoagulation [24]. This suggested at least some patients were not anticoagulated appropriately. Another single-institution study of 198 patients from the Netherlands reported VTE incidence of 11%,15%, and 34% at 5,7 and 14 days respectively despite prophylactic use of anticoagulants [36]. It must be assumed that these figures of VTE events are conservative because of the practical difficulty of getting a documented pulmonary embolism (PE)/VTE in patients with strict isolation policies and comorbidities like renal failure.
The incidence of pulmonary embolism in the setting of NCP has been reported in multiple studies so far. Among 150 patients admitted for NCP in a French study, 25 patients had pulmonary embolism (37.5%), 9 troncular, 8 lobar, 5 segmental and 3 subsegmental pulmonary embolisms. The incidence of pulmonary embolism was much higher in COVID-19 ARDS, as assessed through matching with non-COVID-19 patients (11.7 vs. 2.1%). The median time to diagnose PE was around 5.7 days [37]. In an updated analysis by Klok et al. of 184 critically ill ICU patients with nCOVID-19, 68 patients developed VTE out of which 65 were pulmonary embolism (46 in proximal or segmental pulmonary arteries and 19 subsegmental arteries). This finding was observed despite the patients being on prophylactic anticoagulation based on hospital protocol for ICU patients [34]. Lodigiani et al. reported the incidence of Pulmonary embolism among hospitalized patients with NCP as 2.8% (4.2% of ICU patients and 2.5% general ward patients). Albeit, the study also quotes that this might be an underestimate due to low screening among the patients [38]. Pamelo Rodriguez et al., reported that the incidence of asymptomatic deep vein thrombosis (DVT) in patients admitted with COVID-19 pneumonia and elevated D dimer (14.7%) was not greater than that described in other series of non COVID affected patients admitted to general medicine or orthopedic wards [39].
All these data suggest that while the incidence of DVT may not be increased in COVID-19, incidence of PE is clearly much higher. Local thrombi may form in the lung vessels because of strong activation of inflammatory processes within the lung, with associated cytokine storm and resultant pulmonary endothelial dysfunction or damage. This has been confirmed in at least prospective autopsy series in which thrombosis of pulmonary arteries were seen in all the patients in small and medium sized arteries [40].
4.1.3. Arterial thrombosis
Arterial thrombotic events have also been described in COVID-19, but the incidence is much less reported. These events can manifest in the form of arterial occlusions causing strokes, myocardial infarction (MI) or rarely acute limb ischemia. A case series of COVID-19 patients which included 8 (44%)patients who received a diagnosis of MI had elevated D-Dimer levels in contrast to the previous study in which 64% of patients with ST-elevation MI (STEMI) had normal D- Dimer levels [41,42]. In another observational case series in France involving 58 patients with COVID-19 and ARDS who had neurological sequelae, workup revealed that only 3 patients had radiological evidence of ischemic stroke [43]. However, adequate data whether this was specific to SARs-CoV-2 or if the patients had baseline risk factors that could precipitate cerebrovascular accident (CVA) was not provided.
An interesting study in Wuhan described 7 patients, who all had significant limb ischemia and acrocyanosis together with elevated myocardial injury markers, suggesting that they had substantial microcirculation disorders. Of note, none of the patients were in a state of shock when the limb ischemia occurred and no high-dose vasoactive drugs were used [44]. Possibility of COVID-19 causing extensive micro thrombosis by activating the coagulation system and aggravating organ ischemia should thus be considered.
A French prospective observational study comparing thrombosis in COVID and non-COVID ARDS showed a significantly increased thrombosis incidence in COVID ARDS (11.7 vs 2.1 percentage p < 0.008). In the same study 64/150 COVID-19 patients developed clinically significant thrombotic complications (25 patients developed PE, 28/29 developed clotting of dialysis circuit on renal replacement therapy and 3/12 patients on ECMO support had occlusion of ECMO circuit) [45].
4.1.3.1. Pathophysiology
The pathophysiology of thrombus formation is probably multifactorial in COVID-19. Several reports mention cytokine storm as an important factor affecting the severity of illness [46]. These cytokines result in severe endothelial damage which can lead to ARDS, hypercoagulation, and DIC. DIC could also be part of the primary pathophysiology of SARS-CoV-2 infection, rather than a final common pathway. “Endothelitis” has been demonstrated in COVID-19 infection which might explain the unusually high incidence of thrombosis in these patients [47]. Hyaline thrombi of micro-vessels were found postmortem in a large proportion of severe cases, although this could represent ARDS picture rather than a primary thrombotic event [23]. The presence of antiphospholipid antibodies has also been described in a few critically ill patients, specifically anti-cardiolipin IgA and anti-beta2 glycoprotein antibodies. It is unclear if they are associated with thrombosis or coincidental findings since antibody titers are not available [48].
D-Dimer is a marker of lysis of fibrin clots and can be nonspecifically elevated in inflammatory conditions. In COVID-19, D-dimer could potentially be a sign of diffuse microvascular thrombosis. Severe lung injury can cause microthrombi in the lung resulting in elevated D-Dimer levels. Severe cases of SARS-CoV-2 have been characterized by extreme levels of D dimer elevation which is prognostic in mortality assessment [22,29]. Elevated D dimer has also been reported to be a sensitive marker with good positive predictive value in patients with VTE in COVID-19 infection. If 1.5 microgram/ml is used as the cut-off, the reported sensitivity for VTE is 85%, specificity is 88.5% and negative predictive value is 94.7% [35].
4.1.3.2. Management
Empirical full dose anticoagulation in patients with ARDS with H1N1 has been known to have decreased the incidence of VTE without increasing the risk of bleeding. Similarly, LMWH appears to be associated with better prognosis in severe COVID-19 patients meeting sepsis-induced coagulopathy (SIC) criteria or with markedly elevated D-dimer [49]. Heparin use (LMWH dose of 40-60 mg/day) in patients with higher SIC scores and higher D-dimer levels >6-fold of upper limit of normal were found to have lower 28-day mortality. (40.0% vs 64.2%, P = 0.029) versus (32.8% vs 52.4%, P = 0.017) in a single-center study in China. Interestingly the study reported a slightly increased mortality in patients with lower D-dimer levels treated with systemic anticoagulation although there was no significant difference in overall mortality. Of note, the study used only prophylactic LMWH and the number of patients with actual VTE is not reported. Also, the fact that the general Asian population has a lower incidence of VTE compared to other populations should be taken into account during the interpretation of the study [49].
Multiple studies have shown increased risk of VTE /pulmonary thrombosis in nCOVID-19 however there are no prospective data on how to manage patients with nCOVID-19 who require hospitalization. There is indication that despite prophylactic anticoagulation some patients develop thrombotic complication. In the absence of clear data regarding the appropriate intensity of anticoagulation it is prudent to base management based on indirect evidence.
Since hospitalized COVID-19 patients have additional risk for DVT including diminished mobility, inflammation and infection, it has been generally accepted that all patients should receive prophylactic dose of anticoagulation regardless of VTE risk assessment score based on IMPROVE, Padua or Caprini unless a contraindication such as active bleed exists. Intensification of anticoagulation in critically ill patients or patients with high D dimer is controversial although it is well known that these patients have poorer prognosis. The fact that pathogenesis of thrombosis might be related to thrombo-inflammation and the lack of prospective data for high intensity anticoagulation makes it difficult to recommend such doses in critically ill patients. Until such data is available the care of such patients should be decided by the clinical judgement of the treatment team, however it makes eminent sense to treat patients with low risk of bleeding and high risk of thrombosis with higher intensity anticoagulation.
Patient with nCOVID-19 and DVT/PE should be treated with Heparin or LMWH or direct oral anticoagulants, each having its on advantages and disadvantages. UFH and LMWH are known to have anti-inflammatory property [50,51] potential antiviral action and indirect evidence of improving surrogate markers of inflammation/thrombosis like D-dimer with their use. In a retrospective analysis of 2773 hospitalized COVID-19 patients in New York city, a longer duration of systemic anticoagulation with heparin was associated with a reduced risk of mortality (adjusted hazard ratio of 0.86 per day, p < 0.001). [52]. Bleeding events were more common among patients who received systemic anticoagulation (3%) as compared to those who did not (1.9%), but the difference was not statistically significant. There has been at least one case series describing acquired antithrombin 3 deficiency due to COVID-19 who were resistant to heparinization but treated with argatroban successfully [53].
Direct oral anticoagulants (DOACS) are attractive based on their ease of administration and lack of monitoring however lack of anti-inflammatory properties, potential drug interactions and lack of effective reversal agents in all healthcare settings are issues that need to be considered.
As per the American Society of Hematology recommendations, all patients with COVID-19 should receive thromboprophylaxis with LMWH, in the absence of contra-indications (abnormal PT or APTT is not a contraindication). For patients who are already on therapeutic anticoagulation for other indications, heparin may be continued and withheld only if the platelet count is lower than 50,000 or if fibrinogen is <1 g/L. The effects of LMWH in anticoagulation could be due to its reported anti-inflammatory effect [54]. This is supported by a small retrospective study of 42 patients out of which 21 received LMWH with a reduction in inflammatory markers like IL-6, d-dimer, and FDP [55]. Also, the interaction between heparin and SARS-CoV-2 spike S1 protein receptor-binding domain has been studied. Heparan sulfate proteoglycans (HSPGs) are the basic cell surface molecule required for SARS-CoV-2 entry. Since LMWH is composed of HSPGs, it can reduce the ability of SARS CoV-2 to bind to the cell surface and reduce viral replication [56].
A caveat with using unfractionated heparin is the frequent need to monitor APTT. This could be a challenge in DIC associated with infection because heparin can bind non-specifically to various proteins resulting in higher dose requirements to reach the therapeutic APTT levels. Hence, it is advisable to measure anti-Xa levels with heparin use as it bypasses the issue with nonspecific binding. Heparin resistance is also a concern due to acute phase reactants in infection binding heparin and causing progressive thrombosis.
Studies in the past have established that extended anticoagulation with either prophylactic LMWH or NOACs like Rivaroxaban (10 mg) after an acute medical illness has reduced the risk of VTE although there is a slightly more bleeding risk with NOAC [57]. The common risk factors for increased risk of thrombosis include older age, prolonged immobilization, congestive heart failure, and obesity [58,59] which are also commonly seen in patients with COVID-19. Although there are no established guidelines on discharged COVID-19 patients, the decision of extended VTE prophylaxis should be made by weighing the risk factors and bleeding risk. Similarly, the role of VTE prophylaxis in COVID-19 patients who are quarantined at home is not established but is generally recommended to have an active lifestyle to prevent thrombosis [60].
In the study by Yan Z et al., COVID-19 infected patients with pneumonia who developed limb ischemia received low molecular weight heparin with a resulting decline in d-dimer and FDP levels with no significant improvement in clinical symptoms noted. 5 out of the 7 patients died, with the median time from limb ischemia to death being 12 days. This indicates that earlier initiation of anticoagulation in patients with positive findings on lab parameters may have better outcomes than starting it at the onset of ischemic events. [44]
4.2. Cytokine release syndrome and storm
4.2.1. Presentation
Cytokine release syndrome (CRS) is a systemic inflammatory response due to the release of pro-inflammatory soluble mediators called cytokines triggered by factors such as infections and drugs. The term “cytokine storm” is used to describe a condition when there is an unusually high production of cytokines triggering immunopathological reactions [61]. Based on literature there is substantial evidence of CRS in patients diagnosed with COVID-19. CRS can present with a variety of symptoms ranging from fever or myalgias in mild cases to life-threatening cardiovascular, pulmonary, and renal manifestations in severe cases [62].
4.2.2. Pathophysiology
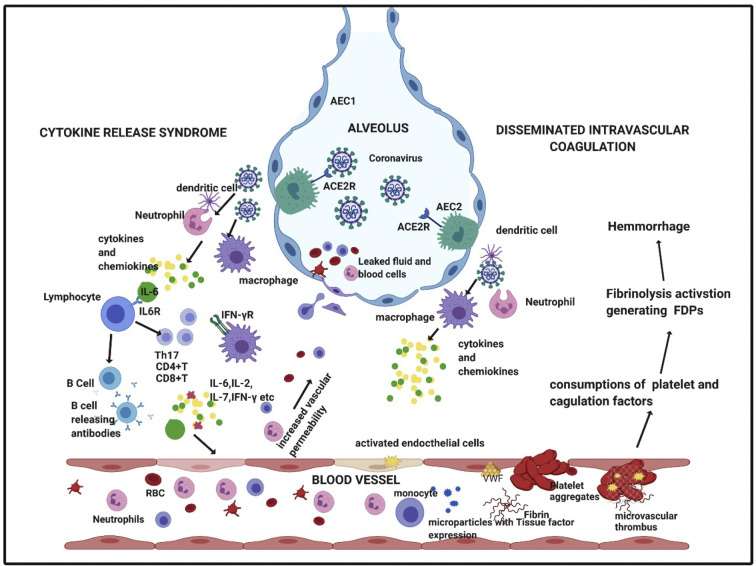
Evidence suggests poorer clinical outcomes in respiratory viral infections in humans and animal models associated with elevated levels of cytokine and chemokine production [[63], [64], [65]]. The possible pathophysiology of action of SARS-CoV-2 involves the binding of the virus to the alveolar epithelial cells and activating the innate and adaptive immune system and consequentially causing release of cytokines. These pro-inflammatory markers increase vascular permeability and cause exudate of a large number of fluid and blood cells into the alveoli, resulting in dyspnea and eventually respiratory failure (Fig. 3 ) [[65], [66], [67]].
Fig. 3.
Possible mechanism of Cytokine Release Syndrome and Disseminated Intravascular Coagulation in severe COVID-19 patients. Abbreviations: RBC- red blood cell; IL, interleukin; IFN-γ, interferon gamma; IFN- γR, interferon gamma receptor; AEC2, alveolar epithelial cell type 2; AEC1, alveolar epithelial cell type 2; ACE2R- angiotensin converting enzyme receptor 2; FDPs, Fibrin degrading products. Created with BioRender.com (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In severe cases, CRS can have a presentation that resembles secondary hemophagocytic lymphohistiocytosis (sHLH). Secondary HLH is a hyperinflammatory syndrome characterized by multiorgan failure due to fatal hyper-cytokinaemia. The typical clinical and laboratory findings include high fevers, elevated ferritin and triglyceride levels in patients with CRS associated HLH [62]. A cytokine profile characterized by increased IL-2, IL-7, granulocyte-colony stimulating factor (g-CSF), interferon-γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and tumor necrosis factor-α has been noted in severe COVID-19 disease markedly resembling sHLH [68]. Gao et al. investigated 43 adult patients with COVID-19 and found that IL-6 and D-Dimer together were closely related to severity of the disease with a specificity of 93.3% and sensitivity of 96.4% for early prediction [69]. A recent study of 150 COVID-19 patients from Wuhan showed that elevated ferritin (mean 1297·6 ng/ml in non-survivors vs 614·0 ng/ml in survivors; p < 0·001) and IL-6 (mean 11.4 ng/ml vs 6.8 ng/ml; p < 0.001) were strong predictors of mortality suggesting that hyper-inflammation from the infection plays a major role [70].
4.2.3. Management
With emerging data, it is more evident that cytokine storm is an important cause of death, and hence the reversal of cytokine action can be a key step in the management of these patients. Among the cytokines, interleukin-6 (IL-6) plays a prominent role in the inflammatory cascade and hence its potential blockade might be useful in mitigating the cytokine storm. Tocilizumab is a recombinant humanized monoclonal antibody against the IL-6 receptor of the immunoglobulin IgG1 subtype. It binds to soluble and membrane-bound IL-6 receptors (sIL-6R and mIL-6R) and inhibits signal transduction mediated by these receptors. A retrospective study in Anhui, China was designed to study the effectiveness of tocilizumab in the treatment of 21 patients with severe COVID-19. It demonstrated that 75.0% of treated patients had reduced oxygen requirements and the lowered lymphocyte count returned to normal in 52.6% of patients on the fifth day after treatment. Further, the radiological improvement was also shown in the CT scan manifestation of lung opacities which improved in 90.5% patients [71]. A case report by Michot et al. in France describes a patient with severe respiratory failure from COVID-19 who had a rapid favorable outcome after two infusions of tocilizumab [72]. Another case of successful treatment with tocilizumab in a patient with multiple myeloma showed that administration of a one-time dose of 8 mg/kg IV of tocilizumab led to a gradual decrease in IL-6 level over 10 days (from 121.59 to 20.81 pg/ml), followed by a rapid increase to the peak (317.38 pg/ml, likely indicating immune recovery), and then final decrease to a low level (117.10 pg/ml) and accompanied improvement in ground-glass opacities on CT scan [73]. More recently, results from a prospective trail at single center in Brescia, Italy show that at 10 days, the respiratory condition was improved or stabilized in 77/100 (77%) patients who received tocilizumab, of whom 61% showed a significant improvement in chest x-ray as well [74]. There are currently more than 15 active trials in various parts of the world that have been approved for studying the efficacy of Tocilizumab in the treatment of NCP.
Safety concerns associated with tocilizumab include risk of GI perforation, hepatotoxicity, infusion-related reactions, increased risk of certain cancers and infections as it alters the immune system and caution is advised in patients with thrombocytopenia and neutropenia [75]. Sarilumab and Siltuximab (drugs of the same class as Tocilizumab) are currently under investigation for their clinical efficacy for COVID-19 (Table 1 ). We anticipate a clearer picture of the safety profile and potential drug interactions through these clinical trials.
Table 1.
List of active trials in phase 2 or 3 throughout the world that are currently evaluating the efficacy of investigational drugs targeting cytokine release in COVID-19.
| Drug | Study title | Clinical trial identifier | Country | Phase | Status |
|---|---|---|---|---|---|
| Anakinra Emapalumab |
Efficacy and safety of Emapalumab and Anakinra in reducing hyperinflammation and respiratory distress in patients with COVID-19 | NCT04324021 | Italy | Phase 2/3 | Recruiting |
| Anakinra Tocilizumab |
Personalized Immunotherapy for SARS-CoV-2 associated with organ dysfunction (ESCAPE) | NCT04339712 | Greece | Phase 2 | Recruiting |
| Anakinra Siltuximab Tocilizumab |
Treatment of COVID-19 patients with Anti-interleukin drugs (COV-AID) | NCT04330638 | Belgium | Phase 3 | Recruiting |
| Anakinra | Trial evaluating efficacy of Anakinra in patients with COVID-19 Infection | NCT04341584 | France | Phase 2 | Not yet recruiting |
| Sarilumab Azithromycin Hydrooxychloro-quine |
Study of immune modulatory drugs and other treatments in COVID-19 patients: Sarilumab, azithromycin, hydroxychloroquine trial (CORIMUNO-VIRO) | NCT04341870 | France | Phase 2/3 | Recruiting |
| Sarilumab | Evaluation of efficacy and safety of Sarilumab in patients hospitalized with COVID-19 | NCT04315298 | United States | Phase 2/3 | Recruiting |
| Sarilumab | Sarilumab COVID-19 | NCT04327388 | Canada | Phase 2/3 | Recruiting |
| Sarilumab Tocilizumab |
Anti-IL6 treatment of serious COVID-19 disease with threatening respiratory failure (TOCIVID) | NCT04322773 | Denmark | Phase 2 | Recruiting |
| Siltuximab | An observational case-control study of the use of Siltuximab in ARDS patients diagnosed with COVID-19 infection. | NCT04322188 | Italy | – | Recruiting |
| Siltuximab Methylprednisolone |
Efficacy and safety of Siltuximab vs corticosteroids in hospitalized patients with COVID-19 pneumonia | NCT04329650 | Spain | Phase 2 | Recruiting |
| Baricitinib Sarilumab Hydroxychloroquine |
Efficacy and safety of novel treatment options for adults with COVID-19 pneumonia (CCAP) | NCT04345289 | Denmark | Phase 3 | Not yet recruiting |
| Baricitinib | Baricitinib in symptomatic patients infected by COVID-19: an open-label, pilot study (BARI-COVID). | NCT04320277 | Italy | Phase 3 | Recruiting |
| Baricitinib Sarilumab |
Treatment of moderate to severe coronavirus disease (COVID-19) in hospitalized patients. | NCT04321993 | Canada | Phase 2 | Not yet recruiting |
| Baricitinib | Safety and efficacy of Baricitinib for COVID-19 | NCT04340232 | USA | Phase 2/3 | Not yet recruiting |
| Leronlimab | Study to evaluate the efficacy and safety of Leronlimab for mild to moderate COVID-19 | NCT04343651 | United States | Phase 2 | Recruiting |
| Leronlimab | Study to evaluate the efficacy and safety of Leronlimab with severe to critical coronavirus disease 2019 (COVID-19). | NCT04347239 | United States | Phase 2 | Recruiting |
Other drugs that target the cytokine release phenomenon and are presently undergoing clinical trials (Table 1) include 1) Janus Kinase (JAK) inhibitor- Baricitinib 2) Recombinant Human Interleukin-1(IL-1) receptor antagonist- Anakinra: IL-1 3) Investigational humanized monoclonal antibody to the chemokine receptor CCR5-Leronlimab [76].
Based on the anti-inflammatory properties of corticosteroid a trial using methylprednisolone to study its efficacy in the treatment of severe NCP was started and is currently in its phase 4 (ClinicalTrials.gov Identifier: NCT04263402). Despite the proven efficacy of corticosteroids in ARDS, it did not improve mortality in SARS and MERS pneumonia [77] and the consensus from WHO is not in favor of using steroids in current COVID-19 pandemic. However, prelim data from RECOVERY trial showed that dexamethasone given 6 mg once daily reduced 28-day mortality among those receiving invasive mechanical ventilation (29.0% vs. 40.7%, RR 0.65 [95% CI 0.51 to 0.82]; p < 0.001) or oxygen at randomization (21.5% vs. 25.0%, RR 0.80 [95% CI 0.70 to 0.92]; p = 0.002), but not among patients not receiving respiratory support [78].
5. Rare Presentations
5.1. Sickle cell disease and COVID-19
Prior studies with the H1N1 influenza pandemic have demonstrated that sickle cell patients have worse complications if infected. In one study, comparing the severity of H1N1 influenza with seasonal influenza in Sickle cell disease (SCD) patients, those with H1N1 influenza more often developed acute chest syndrome (ACS), severe pain, and illness requiring intensive care [79]. Thus, there is a dire need to vaccinate this immunocompromised population yearly with the influenza vaccine.
There have been few reported cases of SCD patients presenting as vaso-occlusive crisis (VOC) with COVID-19 as a precipitating factor. Biemond et al. reported a case of a 20-year-old female with SCD presenting with VOC but no respiratory symptoms and normal chest CT initially. A decline in oxygen levels led them to suspect SARS-CoV-2 which later was found to be positive [80]. They also reported another case of SCD and SARS-CoV-2 who presented with ACS and was treated conservatively. Similarly, a 21-year-old man with a history of SCD (HbS/β0-thalassemia) who initially presented with VOC and later on developed fever, desaturation and new infiltrates on chest X-ray was diagnosed with ACS due to COVID-19 [81]. The patient was initially given simple transfusion but subsequently required exchange transfusion due to worsening respiratory status.
These cases illustrate that in the current times, any SCD patient presenting with VOC or ACS should be tested for SARS-CoV-2 and treating physicians should have a low threshold for exchange transfusion.
5.2. ITP and COVID-19
A single case report in literature has raised the possibility of occurrence of immune thrombocytopenia (ITP) in patients infected with COVID-19. In this case, presented by Zulfiqar et al., a 65-year female with a known history of autoimmune hypothyroidism was admitted for COVID-19 and was found to have isolated thrombocytopenia of 66,000/mm3 and lower extremity purpura on day 4 of hospitalization [82]. On blood work, she had normal coagulation profile and elevated thyroid peroxidase antibody (TPO). A bone marrow biopsy revealed normal cellularity with an increase in pleomorphic megakaryocytes only. The patient did not respond appropriately to IVIG treatment and by day 9, the platelet count had dropped to 2000/mm3 and had developed subarachnoid micro-hemorrhage. The patient was then started on prednisone and eltrombopag with improvement in platelet count to 139,000/mm3 by day 13. Although there are other factors that could contribute to thrombocytopenia in this patient including the possibility of secondary ITP in the setting of elevated TPO antibodies, the temporal sequence, suggests that COVID-19 could be the reason for ITP in this patient.
6. Conclusion and future direction
It has been well established that COVID-19 is a systemic infection with a significant impact on the hematopoietic system and hemostasis. The key is to recognize these laboratory abnormalities and complications sooner and act upon them appropriately. As the pandemic continues to expand and the plausible treatments are still in the nascent stage, it is expected that healthcare workers will be battling this disease for a long time. Hematological parameters may be an early indicator for hospitalization, disease severity, prognosis and may help physicians take timely clinical decisions. Also, several proposed therapeutic agents that target receptors involved in cytokine release syndrome are now being tested in clinical trial. As the pathophysiology and complications associated with COVID-19 continue to unfold, further basic sciences research in areas of pathophysiology and randomized control trials for successful management of this pandemic are needed.
7. Practice points
-
•
Lymphopenia is noted to be a reliable indicator of disease severity and hospitalization related to COVID-19.
-
•
Elevated D-Dimer is an important predictor for development of ARDS and requirement for mechanical ventilation.
-
•
The incidence of venous thromboembolism is higher than arterial thrombosis.
-
•
Use of low molecular weight heparin (LMWH) may be associated with improved prognosis in severe disease.
-
•
Severe cases of COVID-19 with cytokine release syndrome (CRS) can have similar presentation to secondary HLH.
-
•
Antibody against IL-6 receptor may have a therapeutic effect in cytokine release syndrome associated with COVID-19
8. Research agenda
-
•
Does a larger difference in platelet-lymphocyte ratio predicts worse outcomes?
-
•
Role of hepatic dysfunction and its effect on homeostasis in COVID-19
-
•
Is COVID-19 an independent risk factor for development of CVA?
-
•
Randomized controlled trial evaluating role of therapeutic vs. prophylactic doses of anticoagulation and VTE incidence.
-
•
Post discharge VTE prophylaxis- who to give?
Declaration of Competing Interest
None.
References
- 1.Max Roser H.R., Ortiz-Ospina Esteban, Hasellx Joe. Coronavirus disease (COVID-19) published online at. OurWorldInData.org
- 2.Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) 2020. https://coronavirus.jhu.edu/map.html Available from.
- 5.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organization WH Coronavirus disease (COVID-2019) situation reports. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports Available from.
- 8.Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) 2020. 16–24 February 2020. [Google Scholar]
- 9.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020:1–8. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J.P., et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.W-j Guan, Ni Z.-y., Hu Y., Liang W.-H., Ou C.Q., He J.-x., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan B.E., Chong V.C.L., Chan S.S.W., Lim G.H., Lim K.G.E., Tan G.B., et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020 Mar 4 doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 17.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.-Q., et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:1–3. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M., et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 20.Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qu R, Ling Y, Zhang YH, Wei Ly, Chen X, Li X, et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with Corona Virus Disease-19. J. Med. Virol. 2020. 2020; 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed]
- 22.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C, Chen X, Cai Y, Zhou X, Xu S, Huang H, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;e200994. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed]
- 24.Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol 2020. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed]
- 25.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fardet L., Galicier L., Lambotte O., Marzac C., Aumont C., Chahwan D., et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613–2620. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- 27.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.COVID-19 and coagulopathy American Society of Hematology. 2020. https://www.hematology.org/covid-19/covid-19-and-coagulopathy Available from.
- 29.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Hemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Hemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41:1858. doi: 10.1093/eurheartj/ehaa254. 10.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klok F., Kruip M., Van der Meer N., Arbous M., Gommers D., Kant K., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Muller MC, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. 2020. J Thromb Haemost. 2020. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed]
- 37.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demelo-Rodríguez P., Cervilla-Muñoz E., Ordieres-Ortega L., Parra-Virto A., Toledano-Macías M., Toledo-Samaniego N., et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020 doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bangalore S., Sharma A., Slotwiner A., Yatskar L., Harari R., Shah B., et al. ST-segment elevation in patients with Covid-19 — a case series. N Eng JMed. 2020 doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi S., Jang W.J., Song Y.B., Lima J.A., Guallar E., Choe Y.H., et al. D-dimer levels predict myocardial injury in ST-segment elevation myocardial infarction: a cardiac magnetic resonance imaging study. PloS One. 2016;11(8) doi: 10.1371/journal.pone.0160955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., et al. Neurologic features in severe SARS-CoV-2 infection. N Eng J Med. 2020 doi: 10.1056/NEJMc2008597. NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y., Cao W., Xiao M., Li Y., Yang Y., Zhao J., et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua xue ye xue za zhi= Zhonghua xueyexue zazhi. 2020;41:E006. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PubMed] [Google Scholar]
- 45.Helms J, Charles Tacquard, et al. High risk of thrombosis in patients in severe SARS-CoV-2 infection: a Multicenter prospective cohort study. Intensive Care Med DOI: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed]
- 46.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poterucha T.J., Libby P., Goldhaber S.Z. More than an anticoagulant: do heparins have direct anti-inflammatory effects? Thromb Haemost. 2017;117:437–444. doi: 10.1160/TH16-08-0620. [DOI] [PubMed] [Google Scholar]
- 51.Mousavi S., Moradi M., Khorshidahmad T., Motamedi M. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci. 2015;2015 doi: 10.1155/2015/507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paranjpe I., Fuster V., Lala A., Russak A., Glicksberg B.S., Levin M.A., et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arachchillage D.J., Remmington C., Rosenberg A., Xu T., Passariello M., Hall D., et al. Anticoagulation with Argatroban in patients with acute antithrombin deficiency in severe COVID-19. Br J Haematol. 2020 doi: 10.1111/bjh.16927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008;122(6):743–752. doi: 10.1016/j.thromres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 55.Shi C., Wang C., Wang H., Yang C., CAI F., ZENG F., et al. Clinical observations of low molecular weight heparin in relieving inflammation in COVID-19 patients: a retrospective cohort study. medRxiv. 2020 doi: 10.1101/2020.03.28.20046144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mycroft-West C, Su D, Elli S, Guimond S, Miller G, Turnbull J, et al. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 Receptor Binding Domain undergoes conformational change upon heparin binding. bioRxiv. 2020. doi: 10.1101/2020.03.28.20046144. [DOI]
- 57.Cohen A.T., Spiro T.E., Büller H.R., Haskell L., Hu D., Hull R., et al. Rivaroxaban for Thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368(6):513–523. doi: 10.1056/NEJMoa1111096. [DOI] [PubMed] [Google Scholar]
- 58.Samama M.-M., Group ftSS An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med. 2000;160(22):3415–3420. doi: 10.1001/archinte.160.22.3415. [DOI] [PubMed] [Google Scholar]
- 59.Alikhan R., Cohen A.T., Combe S., Samama M.M., Desjardins L., Eldor A., et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX study. Arch Intern Med. 2004;164(9):963–968. doi: 10.1001/archinte.164.9.963. [DOI] [PubMed] [Google Scholar]
- 60.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teijaro J.R. Cytokine storms in infectious diseases. Semin Immunopathol. 2017;39(5):501–503. doi: 10.1007/s00281-017-0640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M., et al. Cytokine release syndrome. J Immunother Cancer. 2018;6 doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y., Li J., Zhan Y., Wu L., Yu X., Zhang W., et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun. 2004;72(8):4410–4415. doi: 10.1128/IAI.72.8.4410-4415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Jong M.D., Simmons C.P., Thanh T.T., Hien V.M., Smith G.J., Chau T.N.B., et al. Fatal outcome of human influenza a (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12(10):1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cillóniz C., Shinya K., Peng X., Korth M.J., Proll S.C., Aicher L.D., et al. Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes. PLoS Pathog. 2009;5(10) doi: 10.1371/journal.ppat.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leiva-Juárez M.M., Kolls J.K., Evans S.E. Lung epithelial cells: therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol. 2018;11(1):21–34. doi: 10.1038/mi.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brune K., Frank J., Schwingshackl A., Finigan J., Sidhaye V.K. Pulmonary epithelial barrier function: some new players and mechanisms. Am J Physiol Lung Cell Mol Physiol. 2015;308(8):L731–L745. doi: 10.1152/ajplung.00309.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao Y, Li T, Han M, Li X, Wu D, Xu Y, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed]
- 70.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv. 2020;202003(00026):v1. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Michot J.-M., Albiges L., Chaput N., Saada V., Pommeret F., Griscelli F., et al. Tocilizumab, an anti-IL6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X., Song K., Tong F., Fei M., Guo H., Lu Z., et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4(7):1307–1310. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7) doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Actemra (tocilizumab) injection package insert. 2020. https://www.actemra.com/?c=act-170f429dc8b&gclid=Cj0KCQjws_r0BRCwARIsAMxfDRh8syRDjmJOC57MuWUab7TloGs5mCt_eLIpkC50kyvtSDM5OmCIFvoaAhUKEALw_wcB&gclsrc=aw.ds Available from.
- 76.Miao M., De Clercq E., Li G. Clinical significance of chemokine receptor antagonists. Expert Opin Drug Metab Toxicol. 2020;16(1):11–30. doi: 10.1080/17425255.2020.1711884. [DOI] [PubMed] [Google Scholar]
- 77.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horby P., Shen Lim W., Emberson J., et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. 2020 doi: 10.1101/2020.06.22.20137273. [DOI] [Google Scholar]
- 79.Strouse J.J., Reller M.E., Bundy D.G., Amoako M., Cancio M., Han R.N., et al. Severe pandemic H1N1 and seasonal influenza in children and young adults with sickle cell disease. Blood. 2010;116(18):3431–3434. doi: 10.1182/blood-2010-05-282194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nur E, Gaartman A, van Tuijn C, Tang M, Biemond B. Vaso-occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID-19). Am J Hematol 2020 Apr 8. doi: 10.1002/ajh.25821. [DOI] [PMC free article] [PubMed]
- 81.Beerkens F., John M., Puliafito B., Corbett V., Edwards C., Tremblay D. COVID-19 pneumonia as a cause of acute chest syndrome in an adult sickle cell patient. Am J Hematol. 2020 Apr 3 doi: 10.1002/ajh.25809. [DOI] [PubMed] [Google Scholar]
- 82.Zulfiqar A.-A., Lorenzo-Villalba N., Hassler P., Andrès E. Immune thrombocytopenic Purpura in a patient with Covid-19. N Engl J Med. 2020;382(18):e43. doi: 10.1056/NEJMc2010472. [DOI] [PMC free article] [PubMed] [Google Scholar]