To the Editor,
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and first described in December 2019 in Wuhan, continues to spread globally. In European countries and UK, the 14-day case notification rate was reported to be 21.5 (country range: 2.2–209.5) per 100,000 population on August 2nd, with an increasing trend with respect to July 19th [1]. These results, demonstrated that, despite the surveillance programs for containing viral spread [2], a stationary transmission of SARS-CoV-2 among individual, both within and between European countries, is still active. There documented raising trend in viral spread, however, calls for an improvement of efficacy of these programs, to isolate infected individuals quickly, especially for high risk groups that should be not only promptly identified but also monitored by enhanced comprehensive or sentinel surveillance. In this scenario, serology testing for SARS-CoV-2 has been recommended for the rapid triage of symptomatic individuals in community settings, for testing all contacts of people with confirmed infection and for testing the viral spread in sentinel sites [3]. Further important roles of SARS-CoV-2 immunoassays are in understanding the virus epidemiology in the general population, and in identifying the disease prevalence in categories at higher risk of infection (e.g. healthcare workers) [4]. To accomplish these purposes, robust, reliable and accurate results are required from commercial immunoassays measuring serum antibody levels. Here we describe the clinical performances of an ELISA (Novalisa NovaTec Immunodiagnostica, Dietzenbach, Germany) for the detection of SARS-CoV-2 IgA, IgM and IgG and the comparison of results with the neutralization activity. According to the manufacturer’s claims, which we have verified, repeatability and intermediate precision of these assays vary between 4.8% and 10.6% for IgA, 2.7% and 11.9% for IgM, and 4.1% and 8.7% for IgG, in results ranging from 0.2 to 20 Novatec units (NTU). A total of 171 leftover serum samples from 41 SARS-CoV-2 negative subjects (20 healthcare workers, 13 autoimmune patients, 8 pregnant women) and 130 COVID-19 patients (9 asymptomatic/mildly symptomatic recovered at home with supportive care and isolation, and 121 hospitalized, classified with moderate or severe disease following WHO interim guidance [5]) were included in the study. All subjects underwent nasopharyngeal swab testing, analyzed by rRT-PCR as described elsewhere [6]. Healthcare workers were considered negative on the basis of at least three negative sequential rRT-PCR results obtained between February 26th and May 29th, 2020. For patients, the mean time interval from symptoms onset and the serological determinations was 24.6 days (SD ± 18.5; range 4–89 days). Among SARS-CoV-2 positive patients, in a subset of 52 samples plaque reduction neutralization test (PRNT) was also performed. In this assay, neutralization titer was defined as reciprocal of the highest dilution resulting in a reduction of the control plaque count > 50% (PRNT50). Stata v16.1 (StataCorp, LakeWay Drive, TX) was used for the statistical analyses. The study protocol (number 23307) was approved by the Ethics Committee of the University-Hospital of Padova (Padova, Italy).
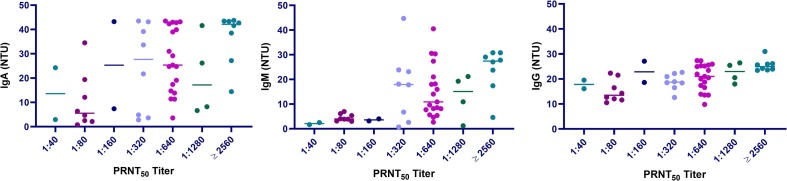
Considering the entire time frame (overall data), the diagnostic performances calculated by the area under the receiver operating characteristics curve (AUC) were 0.943 (95%CI: 0.910–0.976) for IgA, 0.856 (95%CI: 0.800–0.912) for IgM and 0.934 (95%CI: 0.899–0.970) for IgG. Performances (AUC) significantly differed for IgM with respect to IgA (χ2 = 9.52, p = 0.002) and IgG (χ2 = 6.92, p = 0.008). Diagnostic sensitivity and specificity, reported in Table 1 , were assessed considering overall data or two different periods (<12 days and ≥12 days) and calculated using both the manufacturer threshold (11 NTU) and the best cutoff, derived from Youden index. Findings from overall data showed that IgG outperformed IgA and IgM. Instead, specificities were elevated for all assays and, in particular, for IgA and IgG the upper 95% confidence limits were equal or above 99.9%. Marked differences in performances were obtained for the two time-frames. As expected, when the time frame <12 days was considered, the diagnostic sensitivity of the three assays was very limited and the best performances were found for IgA with a sensitivity equal to 65%, thus confirming our previously reported findings, as in most patients increased antibody levels should be detected only after 6–7 days post symptom onset (PSO) [7], [8], [9]. Considering samples collected ≥12 days after symptom onset, sensitivities were significantly higher, being IgG values above 90%. After cutoff optimization, a relevant improvement was achieved in both time-frame, especially for IgA and IgM. Positive and negative likelihood ratios were also calculated, and the best results were found for IgG. In addition, we have evaluate the neutralizing activity against SARS-CoV-2 as it is considered the gold standard for assessing the immune response. Results for neutralization PRNT50 values were reported in Supplementary Fig. 1. The highest correlation was found with IgM (rho = 0.582; p < 0.001), followed by IgG (rho = 0.573; p < 0.001) and IgA (rho = 0.480; p < 0.001). These results confirm previously published data by our group and by Perera et al., who reported a significant correlation between PRNT50 and IgM ELISA results [10], [11].
Table 1.
Clinical performances of Novalisa SARS-CoV-2 (COVID-19) IgA, IgM and IgG, obtained using as thresholds the value 11 NovaTec units (NTU) (claimed by manufacture’s inserts COVA940_20200416_EH, COVM0940_20200416_EH and COVG0940_20200416_EH) and the best cut-off defined by Youden index (obtained with the Overall data).
| Assay | Threshold | Time-frame | Sensitivity§ (95%CI) | Specificity§ (95%CI) | PLR (95%CI) | NLR (95%CI) |
|---|---|---|---|---|---|---|
| SARS-CoV-2 IgA | 11 NTU | <12 d | 44.8 (26.4–64.3) | 100.0 (91.6–100.0) | 38.7 (2.4–626.2) | 0.56 (0.40–0.77) |
| ≥12 d | 75.7 (66.3–83.6) | 64.9 (4.1–1023.4) | 0.25 (0.18–0.35) | |||
| Overall | 69.0 (60.3–76.8) | 59.2 (3.7–933.7) | 0.32 (0.24–0.41) | |||
| 6 NTU | <12 d | 65.5 (45.7–82.1) | 97.6 (87.4–99.9) | 27.5 (3.9–194.3) | 0.35 (0.21–0.58) | |
| ≥ 12 d | 84.5 (76.0–90.9) | 35.5 (5.1–246.4) | 0.16 (0.10–0.25) | |||
| Overall | 80.6 (72.7–87.0) | 33.9 (4.9–235.2) | 0.20 (0.14–0.28) | |||
| SARS-CoV-2 IgM | 11 NTU | <12 d | 27.6 (12.7–47.2) | 97.6 (87.4–99.9) | 8.1 (1.53–43.2) | 0.74 (0.59–0.94) |
| ≥12 d | 53.4 (43.3–63.3) | 15.3 (3.1–74.4) | 0.48 (0.39–0.60) | |||
| Overall | 48.8 (39.9–57.8) | 20.5 (2.9–143.4) | 0.52 (0.44–0.62) | |||
| 5 NTU | <12 d | 48.3 (29.4–67.5) | 88.1 (74.4–96.0) | 4.1 (1.6–10.0) | 0.59 (0.41–0.85) | |
| ≥12 d | 79.6 (70.5–86.9) | 6.7 (2.9–15.3) | 0.23 (0.16–0.34) | |||
| Overall | 72.9 (64.3–80.3) | 6.1 (2.7–14.0) | 0.31 (0.23–0.42) | |||
| SARS-CoV-2 IgG | 11 NTU | <12 d | 44.8 (26.4–64.3) | 100.0 (91.6–100.0) | 38.7 (2.4–626.2) | 0.56 (0.40–0.77) |
| ≥12 d | 90.3 (82.9–95.2) | 77.3 (4.9–1217.2) | 0.10 (0.06–0.18) | |||
| Overall | 79.8 (71.9–86.4) | 68.5 (4.4–1078.6) | 0.21 (0.15–0.29) | |||
| 9 NTU | <12 d | 48.3 (29.4–67.5) | 97.6 (87.4–99.9) | 20.3 (2.8–145.8) | 0.53 (0.37–0.76) | |
| ≥12 d | 94.2 (87.8–97.8) | 39.5 (5.7–274.4) | 0.06 (0.03–0.13) | |||
| Overall | 83.7 (76.2–89.6) | 35.2 (5.1–244.2) | 0.17 (0.11–0.25) |
PLR = positive likelihood ratio; NLR = negative likelihood ratio; d = days.
Subjects included in the analyses: Overall, n = 171; time frame < 12 d, n = 71 (positive and negative to SARS-CoV-2 rRT-PCT); time frame ≥ d, n = 145 (positive and negative to SARS-CoV-2 rRT-PCR).
Sensitivities and specificities values are expressed as percentages.
In conclusion, the Novalisa SARS-CoV-2 (COVID-19) immunoassay provides excellent analytical and clinical performances, especially for IgG. Our findings on PRNT50 demonstrate that neutralization titers are positively correlated with immunoassays results, even if the strength of the associations is limited. Improvement of the assay design, particularly an enhancement of the dynamic range, might increase the association of Ab levels results with neutralization titers, ameliorating the clinical utility of the assay.
Author contributions
All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Funding
None declared.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We acknowledge Technogenetics s.r.l Milan, Italy for kindly supplying reagents without any influence on study design and data analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cca.2020.08.024.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Comparison of plaque reduction neutralization test (PRNT) titers and IgA, IgM and IgG immunoassay results.
References
- 1.European Center for Disease Prevention and Control, Coronavirus Disease 2019 (COVID-19) in the EU/EEA and the UK –Eleventh Update. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-rapid-risk-assessment-20200810.pdf (accessed August 12, 2020).
- 2.World Health Organization, Public health surveillance for COVID-19: interim guidance. https://www.who.int/publications/i/item/who-2019-nCoV-surveillanceguidance-2020.7 (accessed August 12, 2020).
- 3.Peeling R.W., Wedderburn C.J., Garcia P.J., Boeras D., Fongwen N., Nkengasong J., Sall A., Tanuri A., Heymann D.L. Serology testing in the COVID-19 pandemic response. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30517-X. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Q.-X. Long, B.-Z. Liu, H.-J. Deng, G.-C. Wu, K. Deng, Y.-K. Chen, P. Liao, J.F. Qiu, Y. Lin, X.F. Cai XF, D.Q. Wang, Y. Hu, J.H. Ren, N. Tang, Y.Y. Xu, L.H. Yu, Z. Mo, F. Gong, X.L. Zhang, W.G. Tian, L. Hu, X.X. Zhang, J.L. Xiang, H.X. Du, H.W. Liu, C.H. Lang, X.H. Luo, S.B. Wu, X.P. Cui, Z. Zhou, M.M. Zhu, J. Wang, C.J. Xue, X.F. Li, L. Wang, Z.J. Li, K. Wang, C.C. Niu, Q.J. Yang, X.J. Tang, Y. Zhang, X.M. Liu, J.J. Li, D.C. Zhang, F. Zhang, P. Liu, J. Yuan, Q. Li, J.L. Hu, J. Chen, A.L. Huang, Antibody responses to SARS-CoV-2 in patients with COVID-19, Nat. Med. 26 (2020) 845–848. [DOI] [PubMed]
- 5.World Health Organization, Clinical Management of COVID-19, Interim Guidance. https://www.who.int/publications/i/item/clinical-management-of-covid-19 (accessed August 12, 2020).
- 6.Basso D., Aita A., Navaglia F., Franchin E., Fioretto P., Moz S., Bozzato D., Zambon C.-F., Martin B., Dal Prà C., Crisanti A., Plebani M. SARS-CoV-2 RNA identification in nasopharyngeal swabs: issues in pre-analytics. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0749. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 7.Plebani M., Padoan A., Negrini D., Carpinteri B., Sciacovelli L. Diagnostic performances and thresholds: the key to harmonization in serological SARS-CoV-2 assays? Clin. Chim. Acta. 2020;509:1–7. doi: 10.1016/j.cca.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padoan A., Sciacovelli L., Basso D., Negrini D., Zuin S., Cosma C., Faggian D., Matricardi P., Plebani M. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: A longitudinal study. Clin. Chim. Acta. 2020;507:164–166. doi: 10.1016/j.cca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padoan A., Cosma C., Sciacovelli L., Faggian D., Plebani M. Analytical performances of a chemiluminescence immunoassay for SARS-CoV-2 IgM/IgG and antibody kinetics. Clin. Chem. Lab. Med. 2020;58:1081–1088. doi: 10.1515/cclm-2020-0443. [DOI] [PubMed] [Google Scholar]
- 10.Perera R.A.P.M., Mok C.K.P., Tsang O.T.Y., Lv H., Ko R.L.W., Wu N.C., Yuan M., Leung W.S., Chan J.M.C., Chik T.S.H., Choi C.Y.C., Leung K., Chan K.H., Chan K.C.K., Li K.C., Wu J.T., Wilson I.A., Monto A.S., Poon L.L.M., Peiris M. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Eurosurveillance. 2020;25:2000421. doi: 10.2807/1560-7917.ES.2020.25.16.2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padoan A., Bonfante F., Sciacovelli L., Cosma C., Basso D., Plebani M. Evaluation of an ELISA for SARS-CoV-2 antibody testing: clinical performances and correlation with plaque reduction neutralization titer. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-1096. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]