Clinical Implications.
-
•
Coronavirus disease 2019 may present with nasal and ocular manifestations that could be confused with allergic rhinoconjunctivits at the onset. The 2 clinical scenarios present some differences that allow one to clearly distinguish the 2 diseases even during the allergic seasonal period.
Coronavirus disease 2019 (COVID-19) is characterized by various clinical conditions, ranging from mild symptoms to severe pneumonia and death. Nasal and ocular manifestations are included among the possible presentations of COVID-19. Recent articles have suggested that anosmia, with minimal or absent nasal obstruction and rhinorrhea, could be highly predictive of COVID-19,1 , 2 whereas ocular involvement is variably reported in the literature.3 , 4 Other upper respiratory tract infections and allergic rhinoconjunctivitis (ARC) may present some similar symptoms to COVID-19 and should be included in the differential diagnosis. In Italy, the COVID-19 outbreak has coincided with the beginning of the main allergenic pollens season, complicating the diagnosis. ARC affects about 25% of the Italian population5; therefore, a large proportion of patients with COVID-19 could present ARC manifestations at the same time, making it difficult to differentiate the origin of these symptoms. This is also confirmed by the fact that Google Trends shows Italian queries for “is it allergy or coronavirus?” peaked in the period March 16 to April 11. The objective of this study was to describe sinonasal and ocular manifestations and their impact on quality of life at the onset of COVID-19 among patients who also suffer from ARC.
All patients diagnosed with COVID-19 in our hospital were retrospectively studied via telephone interview. Inclusion criteria were laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in symptomatic patients and previous diagnosis of ARC. Patients who were either unable to answer or unreachable by phone were excluded from the study. Written informed consent was obtained. The study was approved by the Local Ethics Committee (no. 2488). Medical history was collected for each patient. Sinonasal and ocular manifestations reported in the 2 weeks before COVID-19 diagnosis and disease-related quality of life were studied with a validated questionnaire: The Mini Rhinoconjunctivitis Quality of Life Questionnaire (MiniRQLQ).6 The questionnaire evaluated a series of 14 signs and symptoms on a scale from 0, indicating “Not troubled,” to 6, indicating “Extremely troubled” (total score, 0-84). Information regarding the day of onset of each symptom was collected. The MiniRQLQ was then used for investigating sinonasal and ocular manifestations and disease-related quality of life of classic ARC in those same patients by asking them to describe their last allergic symptomatic period before the COVID-19 outbreak in Italy (February 21, 2020). Patients were asked to compare the 2 clinical manifestations by defining them as “identical,” “similar,” “different,” or “completely different.” Furthermore, we compared MiniRQLQ scores and the presence of taste and/or smell dysfunction between the included COVID-19–allergic patients and a control group of 182 COVID-19 nonallergic patients, which were enrolled in a previous study,1 matched for age and sex. Smell and taste dysfunctions were investigated by asking the patients to answer the question “Did you experience any reduction or loss of smell and/or taste at the onset of COVID-19?” Possible answers were “yes” or “no.” Differences in the MiniRQLQ scores relative to ARC and to the onset of COVID-19 were described as mean, SD, and 95% CI. A P value of less than .05 was considered as significant.
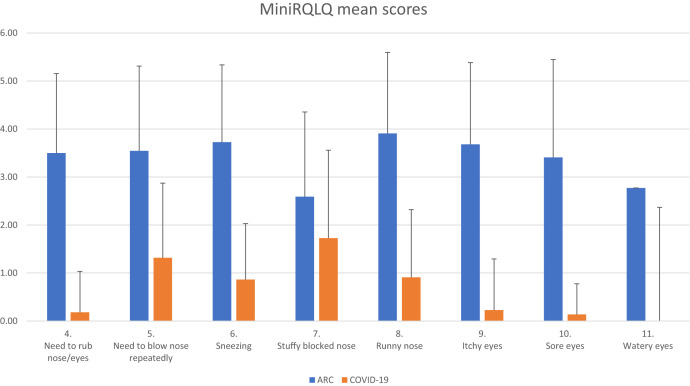
Among 204 patients with COVID-19, 22 (10.8%) fulfilled the inclusion criteria, as also affected by ARC. Median age was 51 years (range, 23-72 years). All clinical characteristics, general symptoms at the onset, and comorbidities are reported in Table I . The MiniRQLQ median scores relative to the last ARC symptomatic period before the COVID-19 outbreak and at the onset of COVID-19 were 39.5 (range, 8-60) and 15 (range, 1-35), respectively. The mean difference was 22.5 (P < .001; 95% CI, 15.94-29.05). The great majority of patients reported that their last allergic manifestation was dated 2019 or earlier. The most significant score differences were found for items from 4 to 11 (Figure 1 ). The mean difference in the total scores of items from 4 to 11 was 21.8 (P < .001; 95% CI, 18.13-25.41). Clinical manifestations of ARC compared with those of COVID-19 at the onset were defined as “completely different” by 15 (68.2%) patients, “different” by 3 (13.6%), and “similar” by 4 (18.2%). The 2 clinical presentations were never reported as “identical.” The median MiniRQLQ score in the control group of 182 COVID-19 nonallergic patients was 11 (range, 0-52). No difference was found with respect to COVID-19 sinonasal manifestations in patients with allergy (P = .288). No difference was found between the prevalence of taste and/or smell dysfunction in COVID-19–allergic patients (14 of 22; 63.6%) and the control group of COVID-19 nonallergic patients (102 of 182; 56%; P = .649).
Table I.
Demographic characteristics of patients with allergies
| Characteristic | Median | Range |
|---|---|---|
| Age (y) | 51 | 23-72 |
| N | % | |
|---|---|---|
| Sex | ||
| Female | 10 | 45.5 |
| Male | 12 | 54.5 |
| Smoking history | ||
| No | 14 | 63.6 |
| Yes | 8 | 36.3 |
| Known exposure to source of SARS-CoV-2 transmission within the previous 14 d | ||
| No | 10 | 45.5 |
| Yes | 12 | 54.5 |
| General symptoms at the onset | ||
| Fever | 18 | 81.8 |
| Dry cough | 15 | 68.1 |
| Taste and/or smell dysfunction | 14 | 63.6 |
| Headache | 8 | 36.3 |
| Fatigue | 7 | 31.8 |
| Myalgia or arthralgia | 5 | 22.7 |
| Dyspnea | 4 | 18.2 |
| Nausea or vomiting | 1 | 4.5 |
| Diarrhea | 1 | 4.5 |
| Rhinitis at the onset of COVID-19 | 3 | 13.6 |
| Conjunctivitis at the onset of COVID-19 | 1 | 4.5 |
| Comorbidities | ||
| Dyslipidemia | 6 | 27.3 |
| Obesity (BMI ≥ 30.0 kg/m2) | 4 | 18.2 |
| Asthma | 3 | 13.6 |
| Cardiovascular disease | 3 | 13.6 |
| Hypertension | 3 | 13.6 |
| COPD | 1 | 4.5 |
| Diabetes | 1 | 4.5 |
BMI, Body mass index; COPD, chronic obstructive pulmonary disease.
Figure 1.
Mean scores for MiniRQLQ items 4 to 11 during ARC and at the onset of COVID-19.
COVID-19 could present some overlap in symptoms with seasonal allergies, creating diagnostic doubts at the onset. Our study showed that MiniRQLQ scores differ significantly between ARC and COVID-19 in the same patients. Patients with allergy are very familiar with their ARC symptoms and can precisely anticipate the sequence of events related to their allergies in most cases. To our knowledge, this is the first study comparing sinonasal and ocular COVID-19 manifestations and ARC. Taste and smell dysfunctions were also investigated to test the hypothesis that patients with a diagnosis of ARC seem to be more affected by olfactory dysfunction when contracting COVID-19, as raised by some authors.7 In our experience, no differences in taste and smell dysfunction were reported among patients with COVID-19 with or without a diagnosis of ARC. Recently, it was hypothesized that asthma, ARC, and chronic rhinosinusitis with nasal polyps could have a protective effect against SARS-CoV-2 infection or its severity.8 These diseases are characterized by a pronounced type 2 immune reaction and a deficit in IFNs, which might downregulate the expression of angiotensin-converting enzyme inhibitors, which is used by SARS-CoV-2 to enter human cells.9 In our series of 204 patients, the prevalence of ARC was only 10.8%, which is well below the prevalence of ARC in the Italian population (25%).5 Further studies are needed to establish causal relationships. Limitations to this study include a relatively small sample size and the absence of objective and detailed sinonasal and ocular examinations, owing to the logistical challenges to examining these patients at this time. The analysis included self-reported clinical manifestations related to the last allergic event before COVID-19 outbreak, with the possibility of recall bias.
This study demonstrated that COVID-19 manifestations at the onset were clearly different with respect to ARC. Patients with allergy are very familiar with ARC symptoms and are able to distinguish them from COVID-19 rhinoconjunctival manifestations in most cases.
Footnotes
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Mercante G., Ferreli F., De Virgilio A., Gaino F., Di Bari M., Colombo G. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. 2020;146:723–728. doi: 10.1001/jamaoto.2020.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A. Olfactory and gustatory dysfunction as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 2020. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf Available from: [Google Scholar]
- 4.Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Marco R., Cappa V., Accordini S., Rava M., Antonicelli L., Bortolami O. Trends in the prevalence of asthma and allergic rhinitis in Italy between 1991 and 2010. Eur Respir J. 2012;39:883–892. doi: 10.1183/09031936.00061611. [DOI] [PubMed] [Google Scholar]
- 6.Juniper E.F., Thompson A.K., Ferrie P.J., Roberts J.N. Development and validation of the Mini Rhinoconjunctivitis Quality of Life Questionnaire. Clin Exp Allergy. 2000;30:132–140. doi: 10.1046/j.1365-2222.2000.00668.x. [DOI] [PubMed] [Google Scholar]
- 7.Gengler I., Wang J.C., Speth M.M., Sedaghat A.R. Sinonasal pathophysiology of SARS-CoV-2 and COVID 19: a systematic review of the current evidence. Laryngoscope Investig Otoaryngol. 2020;5:354–359. doi: 10.1002/lio2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jian L., Yi W., Zhang N., Weiping W., Krysko O., Song W. Perspective: COVID-19, implications of nasal diseases and consequences for their management. J Allergy Clin Immunol. 2020;146:67–69. doi: 10.1016/j.jaci.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson D.J., Busse W.W., Bacharier L.B., Kattan M., O’Connor G.T., Wood R.A. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146:203–206.e3. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]