Introduction
The COVID-19 pandemic has disrupted the way that clinical care is delivered and research is conducted. Clinical encounters have been postponed or transitioned to telehealth, research laboratories throughout the world have shut down, and many clinical research studies have been suspended. At the same time, there has never been such an urgent need to understand, prevent, and find effective treatments for a disease. At the time of this writing, SARS-CoV-2, the virus that causes COVID-19, has infected more than seven million people around the world and caused the deaths of almost half a million people, less than six months after it was first identified (1).
As a new virus, there is limited data from which to inform our advice to patients and how we provide care, especially to those with rheumatic diseases, many of whom take immunosuppressive drugs. Since traditional methods of conducting research are unavailable, physicians and scientists must find alternative ways to conduct research. Fortunately, the increased engagement of users on the internet and social media during the pandemic (2) brings opportunities to engage in research through novel strategies and methods.
Before the COVID-19 pandemic, rheumatologists’ use of social media was steadily increasing. Survey data from 233 European rheumatologists confirmed that 71% were using social media professionally, with the majority using it for communicating with friends/colleagues and receiving news, and clinical and research updates (3). In addition, rheumatologists have been using social media for medical education, global professional networking, research, and coordinated advocacy (4–6).
This social media engagement by rheumatologists was instrumental in the formation of the COVID-19 Global Rheumatology Alliance (C19-GRA). One tweet from one rheumatologist precipitated the development of an international grassroots organization with the goal of exploring the impact of the COVID-19 pandemic on the rheumatology patient community. Since its inception in March 2020, the C19-GRA has grown to over 400 members, 300 sponsoring organizations, and over 10,000 mailing list subscribers. Physicians have contributed thousands of cases to a physician-reported registry, and patients have contributed their own data to a patient-reported registry. Both projects have and will continue to improve our understanding of COVID-19 within this potentially vulnerable population.
In addition to coordinating large, international registries, the C19-GRA also developed teams that critically review the existing literature, synthesize the information, and disseminate and publish the best available evidence about the care of patients with rheumatic disease.
In this paper, we describe how we leveraged the internet and social media platforms to engage both physicians and patients in parallel and collaborative research projects to study the effects of the COVID-19 pandemic on people with rheumatic disease. We outline and discuss novel ways in which we conduct our projects, including their design, dissemination, analysis, publication, and rapid return of results to global stakeholders.
A brief history
As the COVID-19 pandemic began to affect Europe and North America, a group of gastroenterologists developed SECURE-IBD, a physician-reported registry of patients with inflammatory bowel disease that contracted COVID-19 (7). On March 11, 2020, Dr. Leonard Calabrese, a rheumatologist from the Cleveland Clinic, noted these accomplishments on Twitter and asked if a similar effort was underway for rheumatology. Within a day, a group of fifteen rheumatologists and researchers coordinated through Twitter, then email, and eventually moved to Slack and formally established the C19-GRA.
An initial step was establishing an online presence. A website, rheum-covid.org, and an organizational Twitter account, @rheum_covid, were established. A Steering Committee was formed for project management, policy, and governance.
Physician-reported registry
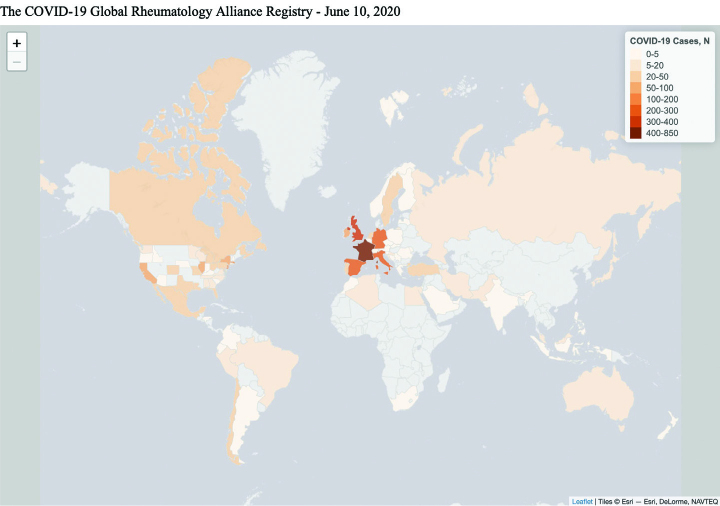
The goal of the physician-reported registry is to collect data on patients with rheumatic disease who have contracted COVID-19, identify risk factors for disease complications, and inform best practices. Physicians from across the globe can access the registry from our website and enter information about any patient with rheumatic disease and COVID-19 disease, whether they are hospitalized, seen in the clinic, or even through a virtual telehealth visit. For the development of the physician registry, we coordinated with a team at the University of California, San Francisco, to launch a REDCap-based, physician-entered registry that went live by March 24, 2020. REDCap is a browser-based, metadata-driven electronic data capture software and workflow methodology for designing clinical and translational research databases (8, 9). A parallel physician registry was also established for Europe by the European League Against Rheumatism (EULAR), which abides by the General Data Protection Regulation (GDPR), legislation that places additional protection of personal data in those living in Europe. The intent is for data from both registries to be shared and analyzed together. As of June 10, 2020, physicians from over 40 countries have contributed to both the Global and EULAR registries, with a combined total of more than 3,000 cases (Figure 1).
Figure 1.
Number of cases contributed to the physician registry per country, and per US state.
Patient experience survey
To have a first-hand understanding of the patient experience during the pandemic, the Patient Experience Survey was developed and launched on April 3, 2020 (10). This international, anonymous survey, is available in 9 languages and mirrors many data elements in the physician-reported registry. Any adult or parent of a child with a rheumatic disease is eligible to participate, whether or not they were diagnosed with COVID-19, by accessing the survey on our website. In addition to patient demographics and details about COVID-19 diagnosis, as applicable, the survey also collects data about mental and physical health, experiences with medication shortages, effects of the pandemic on employment and schooling, and other patient-reported outcomes. We used Qualtrics, a commercial survey platform that quickly and easily generates mobile responsive surveys and coordinated with teams at Boston Children’s Hospital, Beth Israel Deaconess Medical Center, and McMaster University for data analysis.
To ensure participation by a wide range of people in the Patient Experience Survey, we partnered with patient support organizations that could encourage their members to participate. Over 100 patient organizations globally have pledged their support of the C19-GRA efforts and disseminated information about the Patient Experience Survey to their members. In the spirit of collaboration, we also planned to share aggregate data from members belonging to those organizations back to their respective organizations. To help recruit patients for our project, teams of patient partners helped to create a marketing plan, including custom graphics and ads. Patient partners were instrumental in developing emails that were sent to leaders of global patients organizations to engage them with the survey. The patient partners also created a Communication Tool Kit to guide patient organizations in their recruitment strategy. As of June 14, 2020, the Patient Experience Survey has had more than 13,000 individuals contribute data, representing more than 90 countries.
Communicating within the C19-GRA
Once the C19-GRA appeared to be gaining membership and momentum, email became an inefficient mode of communication so a Slack workspace was established. Based on an early model of internet communication--internet relay chat--Slack is a business communication platform with chat rooms organized by topic, private groups, and direct messaging integrating with multiple document sharing formats (slack.com).
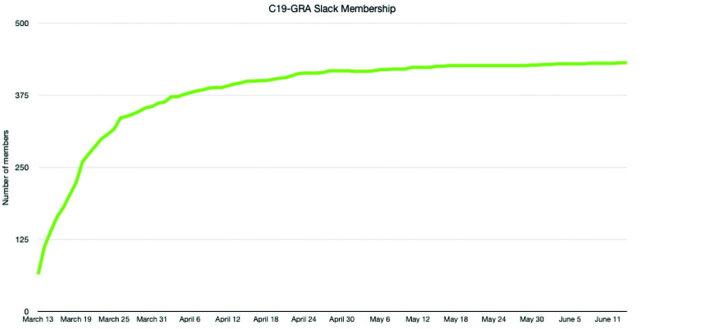
After one week, the Slack group had over 200 participants, and currently has over 400 members. Medical trainees of all levels and backgrounds-epidemiologists, clinicians, patients, parents of children with rheumatic disease, and leaders of patients organizations- from around the world were invited to participate. Members have sent over 38,000 messages and shared over 1,300 files on Slack (Figure 2). Access to Slack to multiple stakeholders democratized research participation on a global scale.
Figure 2.
C19-GRA Membership growth in Slack.
Communicating with patients, physicians, and organizations
Website
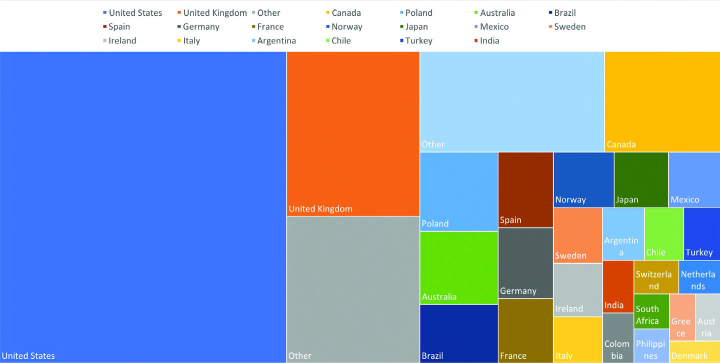
The rheum-covid.org website was initially created to describe the project, seek support from rheumatology organizations, and promote the registries. It is the main gateway to both the physician-reported and patient-reported registries and provides information regarding ethical approval for our projects. Organizations that have confirmed official support of the C19-GRA are also featured on our website. Official supporters were asked to “broadcast the news about our registries to your organization, in hopes that as many patients can be added to the registry as quickly as possible.” At the time of this writing, 330 organizations from all areas of the globe are included as official supporters. As the C19-GRA registries data were analyzed and disseminated, links to all of the published manuscripts and media reports about the C19-GRA were created. As of June 5, the website has received 189,000 views from users in 163 countries (Figure 3).
Figure 3.
Proportion of rheum-covid.org website views from the top 28 countries.
The @rheum_covid Twitter account was created on March 14, 2020. Initially, the purpose of the Twitter account was to recruit individuals interested in assisting with the project, such as helping to translate the registry forms into different languages, or joining the Patient Board, as well as to seek support from rheumatology organizations from around the world. The account has also been used to disseminate data and links to published articles, giving rapid access to information during the pandemic. The physician-reported registry launched on March 24, with the first tweet reporting summary statistics reporting on COVID-19 outcomes among patients with rheumatic disease occurring 7 days later. The Twitter account was also useful to recruit patients with rheumatic disease for the Patient Experience Survey. The number of followers of the twitter account has grown to over 5,000 in three months (Figure 4).
Figure 4.
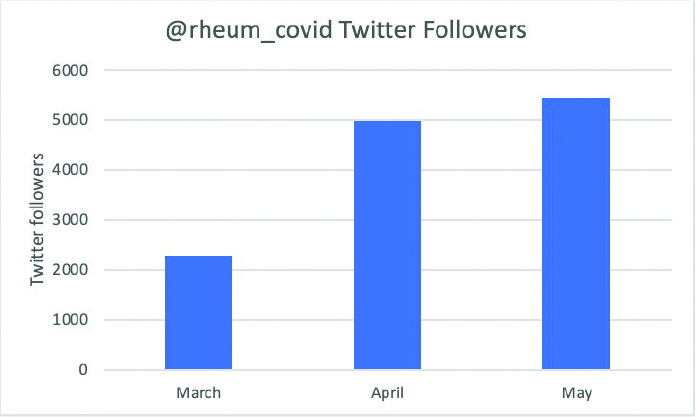
New Twitter followers of @rheum_covid within the first 3 months of the C19-GRA.
From March 14 until May 31, 2020, the Twitter account has disseminated over 200 tweets that received a total of 7,593 likes and 4,144 retweets. The most engaging tweet during this time was regarding an early result from our project which revealed the lack of efficacy of hydroxychloroquine in fully protecting patients against COVID-19, which received hundreds of likes and retweets (Figure 5).
Figure 5.

Tweet from the C19-GRA addressing the lack of efficacy of hydroxychloroquine in protecting patients against COVID-19.
Newsletters
Users interested in the C19-GRA were invited to subscribe to a mailing list to receive general updates from the C19-GRA. In addition, patients who completed the Patient Experience Survey were also invited to subscribe to a separate mailing list that provides updates from the patient survey as well as information regarding future patient surveys. More than 2,000 people have signed up for the general C19-GRA newsletter (Figure 6), whereas the patient survey mailing list contains over 7,000 addresses The percentage of users that have opened each of the sent emails ranged from 54–64%. These open rates are better than the average email open rates for government and nonprofit emails, at 31% and 25%, respectively (11).
Figure 6.
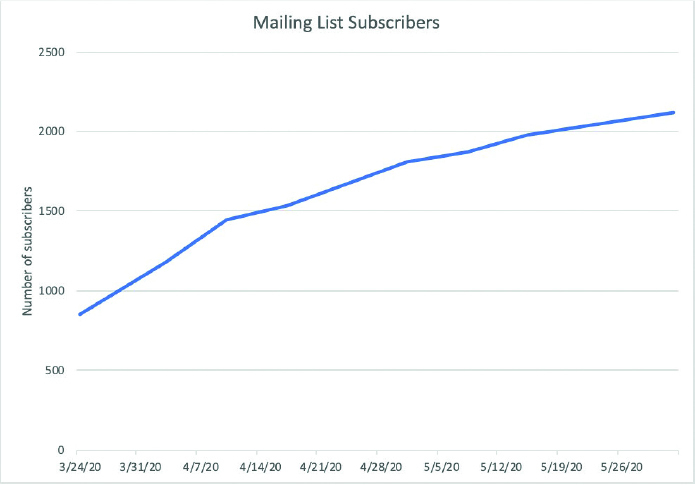
Number of total subscribers to the C19-GRA general mailing list over time.
Outputs to date
To date, the C19-GRA has published nine articles in the peer-reviewed literature; six advocacy or editorial pieces (10, 12–16), and three papers with primary data about patient outcomes (17–19). A key finding has been that in people with rheumatic disease who develop COVID-19 moderate to high dose glucocorticoids were associated with a higher risk of hospitalization for COVID-19, while biologic therapies, NSAIDs and conventional disease modifying antirheumatic drugs, namely antimalarials like hydroxychloroquine, were not associated with a higher risk of hospitalization for COVID-19. There have been at least ten mentions in journal articles, and upwards of 60 mentions in rheumatology or mainstream media. Currently, the physician-reported registry data is planned to be used for multiple additional data analyses about key COVID-19 outcomes for people with rheumatic disease and for analysis of outcomes for patients with specific diseases. The Patient Experience Survey data is currently being analyzed to determine risk factors for the development of COVID-19 in adults and children with rheumatic disease, explore medication shortages, such as those that occurred with the use of antimalarials, and to understand the mental and physical toll that the pandemic has had on patients and their caregivers.
Conclusions and future directions
Despite the challenges and social distancing brought on by the COVID-19 pandemic, the rheumatology community has been able to come together virtually, collect and analyze data, rapidly generate knowledge and disseminate it widely to improve the care of patients with rheumatic disease. Social media platforms have allowed patients, physicians, researchers, and other stakeholders to collaborate in an ambitious project to understand the impact of the COVID-19 pandemic in those with rheumatic diseases. In a few short months, the C19-GRA has grown from a tweet to an organization with over 400 members and governance policies, and which has produced 10 articles in peer-reviewed journals, as well as multiple messages through social media platforms that enabled patients and providers to get very early information about the outcomes of COVID-19 in people with immune-mediated diseases. As we continue to live through the COVID-19 pandemic, we will be forced to consider the long-term application of social media and online platforms to medical research. Our model can serve as a foundation for future global collaborative efforts to advance medical knowledge and rapidly return research results to patients and the physicians that care for them.
Footnotes
Disclaimer: The views expressed here are those of the authors and participating members of the COVID-19 Global Rheumatology Alliance and do not necessarily represent the views of the American College of Rheumatology, the European League Against Rheumatism (EULAR), or any other organization.
Conflict of Interest: J.S.H. is supported by grants from the Rheumatology Research Foundation and Childhood Arthritis and Rheumatology Research Alliance, and he has performed consulting for Novartis unrelated to this work (<$10,000). P.S. reports honorarium for doing social media for American College of Rheumatology journals (<$10,000). S.B. reports non-branded marketing campaigns for Novartis (<$10,000). J.W.L. is supported by a grant from the National Institutes of Health. P.M.M. has received consulting/speaker’s fees from Abbvie, BMS, Celgene, Eli Lilly, Janssen, MSD, Novartis, Pfizer, Roche and UCB, all unrelated to this manuscript (all < $10,000). P.M.M. is supported by the National Institute for Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre (BRC). Z.S.W. is supported by grants from the National Institutes of Health and he has performed consulting for Teva Pharmaceuticals and Gilead Sciences unrelated to this project. P.C.R. reports personal consulting and/or speaking fees from Abbvie, Eli Lilly, Janssen, Novartis, Pfizer and UCB and travel assistance from Roche (all < $10,000). J.Y. is supported by grants from the National Institutes of Health, Centers for Disease Control and Agency for Healthcare Research and Quality and she has performed consulting for Eli Lilly and Astra Zeneca unrelated to this project. R.G. reports personal and/or speaking fees from Abbvie, Janssen, Novartis, Pfizer, Cornerstones and travel assistance from Pfizer (all < $10,000). E.S. is a Board Member of the Canadian Arthritis Patient Alliance, a patient run, volunteer based organization whose activities are largely supported by independent grants from pharmaceutical companies.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.World Health Organization. WHO coronavirus disease (COVID-19) dashboard. [cited 2020 June 6]. Available from: https://covid19.who.int.
- 2.Koeze E, Poppe N. The virus changed the way we internet. New York Times [Internet] 2020 Apr 7; [cited 2020 June 13]. Available from: https://www.nytimes.com/interactive/2020/04/07/technology/coronavirus-internet-use.html. [Google Scholar]
- 3.Nikiphorou E, Studenic P, Ammitzbøll CG, Canavan M, Jani M, Ospelt C, et al. Social media use among young rheumatologists and basic scientists: Results of an international survey by the Emerging EULAR Network (EMEUNET) Ann Rheum Dis. 2017;76:712–5. doi: 10.1136/annrheumdis-2016-209718. [DOI] [PubMed] [Google Scholar]
- 4.Childers L. Social media connects, informs rheumatologists. The Rheumatologist [Internet] 2018. Apr 26, Available from: https://www.the-rheumatologist.org/article/social-media-connects-informs-rheumatologists/
- 5.Collins C, Campos J, Isabelle A, Jayatilleke A, Sufka P. AB1385 #rheumjc: 3-year analysis of a twitter-based rheumatology journal club. Ann Rheum Dis. 2018;77:1777. doi: 10.1136/annrheumdis-2018-eular.3486. [DOI] [Google Scholar]
- 6.Amigues I, Sufka P, Bhana S, Campos J, Collins C. #Rheumjc: Impact of invited authors on a Twitter based Rheumatology Journal Club [abstract] Arthritis Rheumatol. 2016;68(suppl 10) [cited 2020 June 18]. Available from: https://acrabstracts.org/abstract/rheumjc-impact-of-invited-authors-on-a-twitter-based-rheumatology-journal-club/ [Google Scholar]
- 7.Brenner E, Ungaro R, Gearry R, Kaplan G, Kissous-Hunt M, Lewis J, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: Results from an International Registry Gastroenterology. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.032. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. REDCap consortium: Building an international community of software partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sirotich E, Dillingham S, Grainger R, Hausmann JS COVID-19 Global Rheumatology Alliance Steering Committee. Capturing patient-reported outcomes during the COVID-19 pandemic: Development of the COVID-19 Global Rheumatology Alliance Patient Experience Survey. Arthritis Care Res (Hoboken) 2020;72:871–3. doi: 10.1002/acr.24257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campaign Monitor. Ultimate email marketing benchmarks for 2020: By industry and day [Internet] [cited 2020 June 14]. Available from: https://www.campaignmonitor.com/resources/guides/email-marketing-benchmarks/
- 12.Kim AH, Sparks JA, Liew JW, Putman MS, Berenbaum F, Duarte-García A, et al. A rush to judgment? Rapid reporting and dissemination of results and its consequences regarding the use of hydroxychloroquine for COVID-19. Ann Intern Med. 2020;172:819–21. doi: 10.7326/M20-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson PC, Yazdany J. The COVID-19 Global Rheumatology Alliance: Collecting data in a pandemic. Nat Rev Rheumatol. 2020;16:293–4. doi: 10.1038/s41584-020-0418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yazdany J, Kim AHJ. Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: What every clinician should know. Ann Intern Med. 2020;172:754–5. doi: 10.7326/M20-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graef ER, Liew JW, Putman MS, Simard JF, Sirotich E, Berenbaum F, et al. Festina lente: Hydroxychloroquine, COVID-19 and the role of the rheumatologist. Ann Rheum Dis. 2020;79:734–6. doi: 10.1136/annrheumdis-2020-217480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace ZS, Bhana S, Hausmann JS, Robinson PC, Sufka P, Sirotich E, et al. The Rheumatology Community responds to the COVID-19 pandemic: The establishment of the COVID-19 Global Rheumatology Alliance. Rheumatology (Oxford) 2020;59:1204–6. doi: 10.1093/rheumatology/keaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianfrancesco MA, Hyrich KL, Gossec L, Strangfeld A, Carmona L, Mateus EF, et al. Rheumatic disease and COVID-19: Initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol. 2020;2:e250–e253. doi: 10.1016/S2665-9913(20)30095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konig MF, Kim AH, Scheetz MH, Graef ER, Liew JW, Simard J, et al. Baseline use of hydroxychloroquine in systemic lupus erythematosus does not preclude SARS-CoV-2 infection and severe COVID-19. Ann Rheum Dis. 2020 May 7; doi: 10.1136/annrheumdis-2020-217690. doi: 10.1136/annrheumdis-2020-217690. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: Data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–66. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]