Abstract
Objective
This study aimed to characterize the process of disease progression in the early stage of rapidly progressive osteoarthritis of the hip (RPOH) and clarify its association with potential pathological factors of RPOH.
Methods
This monocentric retrospective study included 41 female patients who met the criteria for RPOH, chondrolysis >2 mm during 12 months from the onset of hip pain based on a series of radiographs and computed tomography. This study also included 9 female patients with osteoarthritis secondary to developmental dysplasia of the hip (DDH) who demonstrated chondrolysis >2 mm during 12 months from the onset of hip pain. Cortical thickness index (CTI) correlated with bone mineral density of the hip, pelvic tilt, and serum concentrations of matrix metalloproteinase (MMP)-3 were analyzed.
Results
RPOH was classified into two types based on the absence (type 1, n=17) and presence (type 2, n=24) of subsequent femoral head destruction within 12 months after the onset of hip pain. MMP-3 significantly increased in RPOH type 2 compared with type 1 and DDH. Increased posterior pelvic tilt was found in RPOH type 2 compared with DDH. Logistic regression and receiver operating characteristic curve analyses indicated that MMP-3 may be associated with differentiation between RPOH types 1 and 2. No difference was found in the CTI between the RPOH types and DDH.
Conclusion
Disease progression of RPOH during 12 months after the onset of hip pain could be classified into two distinct types based on the absence (type 1) and presence (type 2) of femoral head destruction in association with MMP-3 and pelvic tilt as biological and mechanical factors, respectively. MMP-3 may be helpful to differentiate these two types in the early stage of RPOH.
Keywords: Arthritis, classification, diagnosis, hip joint, osteoarthritis
Introduction
Osteoarthritis (OA) is characterized by destruction of joint cartilage and subchondral bone with degradation of the extracellular matrix. Rapidly progressive OA of the hip (RPOH), also known as rapidly destructive arthritis/osteoarthritis/coxopathy, is an unusual subset of OA. RPOH has been defined as chondrolysis >2 mm in 1 year with no evidence of other forms of rapidly progressive arthropathy including antecedent OA, osteonecrosis, neuropathy, infection, or inflammatory disease (1). RPOH commonly occurs in elderly women and causes severe disability (2). Although the pathogenesis of RPOH remains unclarified, potential causes of RPOH include subchondral insufficiency fracture (SIF) resulting from osteoporosis (3, 4), increasing posterior pelvic tilt as a mechanical factor (5), and high serum levels of matrix metalloproteinase (MMP)-3 and MMP-9 as biological factors (6).
Although RPOH develops >2 mm of joint space narrowing in 1 year, the process of disease progression in the early stage of RPOH is still equivocal. Several studies of case series have suggested gradual joint destruction in RPOH. Subsequent to rapid joint space narrowing observed in the early phase of RPOH, the femoral head and acetabulum are destroyed within the subsequent 6–24 months. Thereafter, some patients with RPOH develop massive deformation of the femoral head and acetabulum in the hips (7, 8). Another case series has indicated that RPOH may be classified into two types (9). While some patients with RPOH show only joint space narrowing with no bone loss, others demonstrate massive femoral head destruction within 6 to 18 months after initial presentation. Rapid progression of this disease makes it difficult to obtain sequential radiographs in its early stage (7), which is likely to cause discrepancy between those observations of disease progression. In addition, no information is available about association between the disease progression of RPOH and its potential causes.
The current diagnostic criteria (chondrolysis >2 mm per year) require patient follow-ups for at least 12 months. In addition, no previous study has demonstrated any specific marker in the early stage of RPOH that can predict subsequent bone destruction of the hip joint. This study aimed to characterize the process of RPOH progression by sequential radiological data in its early stage and investigate its association with the proposed pathological factors, MMP-3, pelvic tilt, and osteoporosis.
Methods
Patients and their demographic, radiographical, and hematological data
This monocentric retrospective study was approved by the Ethics Committee of Kobe City Medical Center General Hospital (approval no: k190516). Informed consent was not sought due to the retrospective nature of the study. This study enrolled female patients with sufficient clinical records including the onset of hip pain, age, and body mass index (BMI) at the onset, a series of radiographs taken at intervals of every 2–3 months during the period of >12 months from the onset of hip pain, and hematological data including MMP-3. Serum samples were collected by venous puncture from each patient at the first visit to our hospital. Because every patient was referred to our hospital by local clinics, the duration between the onset of hip pain and blood tests was different in different patients. The serum concentration of MMP-3 was determined by latex turbidimetric immunoassay. This study excluded male patients because RPOH occurs mainly in elderly females, and the reference intervals of MMP-3 are different between males (36.9–121 ng/mL) and females (17.3–59.7 ng/mL). Of a consecutive series of patients with hip pain from 2012 through 2018, we found the hip joints of 41 patients met the diagnostic criteria of RPOH; chondrolysis >2 mm in 1 year (1). In each case, the disease was unilateral without evidence of antecedent OA, osteonecrosis, neuropathy, infection, or inflammatory disease including rheumatoid arthritis. We also found 9 female patients with OA secondary to developmental dysplasia of the hip (DDH) showing chondrolysis >2 mm in 1 year with insufficient coverage of the femoral head by the acetabulum.
Radiological parameters
The cortical thickness index (CTI) is defined as the ratio of the femoral diaphyseal diameter minus the intramedullary canal diameter to the femoral diaphyseal diameter (10). These diameters were measured 10 cm below the midpoint of the lesser trochanter. There was significant correlation between CTI and the bone mineral density of the hip (11, 12). Pelvic tilt was estimated by the ratio between the vertical and the horizontal diameters of the pelvic foramen on the supine anteroposterior radiograph (13). These parameters were measured on the initial radiograph at the onset of hip pain using a PACS (picture archiving and communication system) workstation. Computed tomography (CT) was taken at intervals of every 2–3 months and used to evaluate bone destruction in the hip joint. Magnetic resonance imaging (MRI) was employed to rule out other diagnoses.
Statistical analysis
The data were expressed as the mean±standard error (SE). The data were compared between two groups using Mann-Whitney U test, while they were compared between three groups using a one-way analysis of variance (ANOVA) accompanied by a post hoc Bonferroni correction for multiple comparisons. Logistic regression analysis was used to identify significant variables for characterization of RPOH. Receiver operating characteristic (ROC) curves were performed and cutoff values for high specificity or high sensitivity were identified for MMP-3. Statistical analyses were conducted in SPSS for Windows, Version 25 (IBM Corp.; Armonk, NY, USA). The level of significance was set at p<0.05.
Results
Classification of RPOH based on femoral head destruction
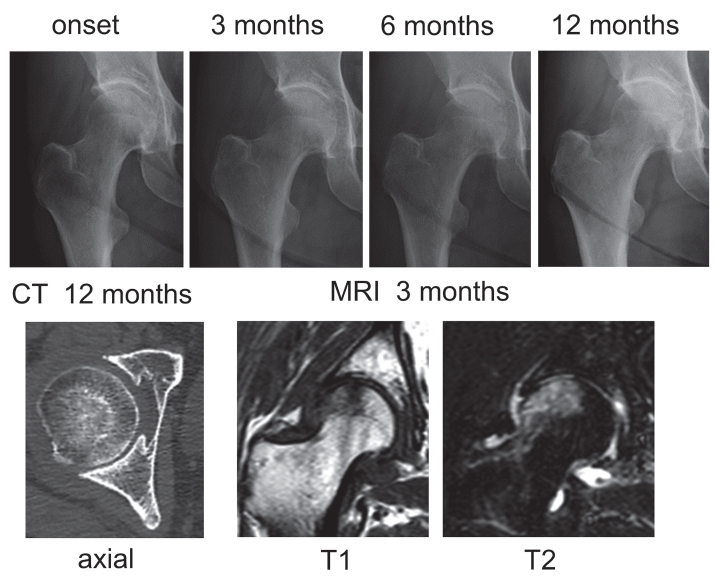
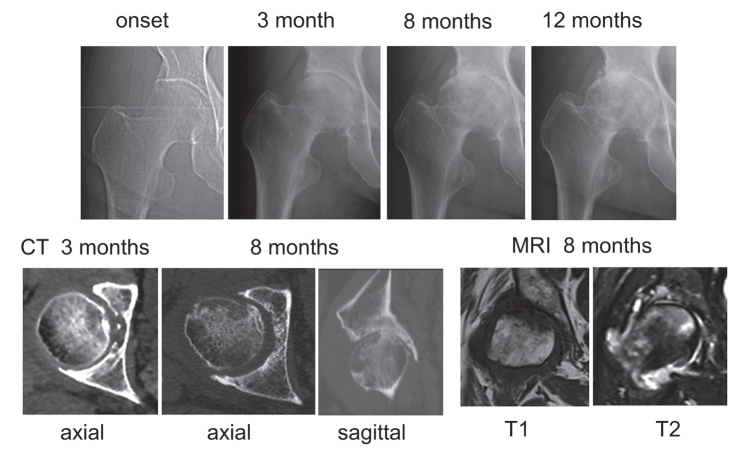
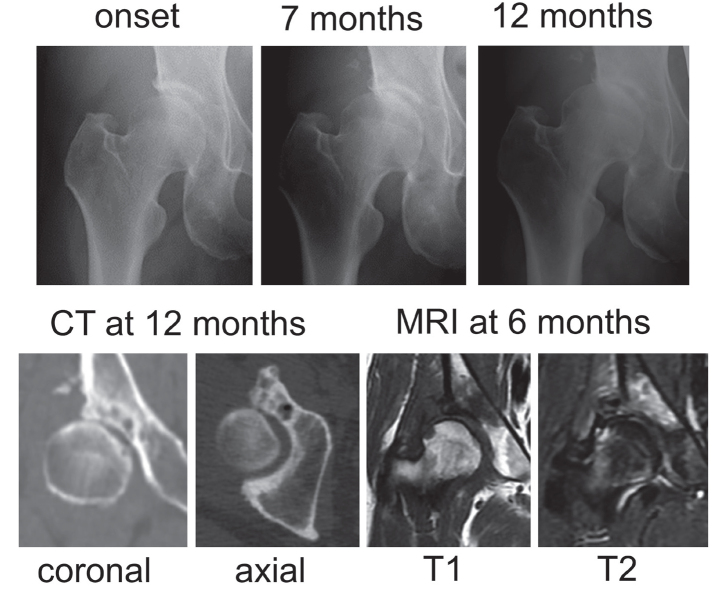
Of 41 hips with RPOH, 17 hips showed only joint space narrowing with no femoral head destruction within 12 months after the onset of hip pain (Figure 1), which was classified as RPOH type 1. Following rapid joint space narrowing, femoral head destruction was observed on CT within 12 months after the onset of hip pain in 24 hips (Figure 2), which was classified as RPOH type 2. Femoral head destruction in RPOH type 2 was found for the first time on a CT taken at 4 months and more after the onset of hip pain. Bone destruction started in the anterior portion of the femoral head in every patient with RPOH type 2 as shown on the axial CT view in Figure 2. In addition, 9 hips with DDH showed joint space narrowing at the pace of >2 mm per year after the onset of pain (Figure 3). MRI demonstrated a subchondral area of low signal intensity on T1-weighted spin-echo images and inhomogeneous high intensity on fat-suppressed T2-weighted spin-echo images in the superolateral portion of the femoral head in RPOH types 1 and 2 and DDH. Subchondral cyst-like lesions in the acetabulum showed low intensity on T1-weighted images and high intensity on fat-suppressed T2-weighted images in some patients with RPOH as well as DDH.
Figure 1.
Rapidly progressive osteoarthritis of the hip (RPOH) type 1. Right hip joint showing chondrolysis >2 mm/year on a series of radiographs without femoral head destruction on computed tomography (CT) at 12 months after the onset. MRI: magnetic resonance imaging.
Figure 2.
RPOH type 2. Right hip joint demonstrating partial destruction of the anterior portion in the femoral head on CT at 8 months after the onset following chondrolysis.
Figure 3.
Osteoarthritis secondary to developmental dysplasia of the hip. Right hip joint showing chondrolysis >2 mm/year on a series of radiographs without femoral head destruction on CT at 12 months after the onset.
Comparison of radiological and hematological parameters among RPOH and DDH within 12 months after the onset of hip pain (Table 1)
Table 1.
Comparisons of demographic, radiographic, and hematologic data within 12 months after the onset of hip pain among patients with rapidly progressive osteoarthritis of the hip and developmental dysplasia of the hip.
| RPOH | DDH (n=9) | p by ANOVA | p by Bonferroni test | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| type 1 (n=17) | type 2 (n=24) | types 1–2 | type 1- DDH | type 2-DDH | |||
| Age (years) | 73.9±2.2 | 73.9±1.8 | 62.9±3.8 | 0.010 | 1.000 | 0.019 | 0.012 |
| Body mass index (kg/m2) | 23.6±1.0 | 22.5±0.7 | 22.6±1.3 | 0.661 | 1.000 | 1.000 | 1.000 |
| Cortical thickness index | 0.528±0.016 | 0.530±0.012 | 0.546±0.023 | 0.790 | 1.000 | 1.000 | 1.000 |
| Pelvic tilt parameter | 0.557±0.026 | 0.492±0.025 | 0.649±0.021 | 0.002 | 0.190 | 0.142 | 0.002 |
| Duration between the onset of hip pain and blood test (months) | 4.88±0.76 | 5.92±0.75 | 5.89±1.23 | 0.621 | 1.000 | 1.000 | 1.000 |
| Matrix metalloproteinase-3 (ng/mL) | 64.7±6.5 | 141.8±22.8 | 34.2±4.8 | 0.001 | 0.011 | 1.000 | 0.004 |
Values are expressed as mean±SE.
ANOVA: one-way analysis of variance; RPOH: rapidly progressive osteoarthritis of the hip; DDH: developmental dysplasia of the hip
We compared the possible pathological factors of RPOH (osteoporosis, pelvic tilt, and MMP-3) among RPOH types 1 and 2 and DDH. No difference was found in CTI, which is associated with bone mineral density of the hip (12) between these groups. The pelvic tilt parameter, which was determined as described above, was significantly smaller in RPOH type 2 compared to that in DDH. Although the difference was not statistically different, the parameter tended to be smaller in RPOH type 2 than that in RPOH type 1. Serum levels of MMP-3 significantly increased in RPOH type 2 compared with RPOH type 1 and DDH.
Identification of determinants for RPOH type 2
Because the results from Table 1 indicated that RPOH type 2 may be distinct from RPOH type 1 and DDH, we further analyzed determinant variables for RPOH type 2 among patients with the hip demonstrating chondrolysis >2 mm in 1 year. From logistic regression analysis using the data on pelvic tilt and MMP-3 obtained from RPOH types 1 and 2 and DDH, the lower pelvic tilt parameter and higher MMP-3 levels were found to be significant determinants of RPOH type 2 (Table 2). Logistic regression analysis with the data from RPOH types 1 and 2 revealed that MMP-3 was a useful determinant for RPOH type 2 (Table 2).
Table 2.
Logistic regression analysis for determinant variables of RPOH type 2 within 12 months after the onset of hip pain.
| Data from | Determinants | OR | [95% CI] | p |
|---|---|---|---|---|
| RPOH types 1 and 2 and DDH | pelvic tilt parameter | 2.225 X 10−5 | 4.221 X 10−7-0.120 | 0.009 |
| MMP-3 | 1.036 | 1.011–1.062 | 0.005 | |
| RPOH types 1 and 2 | MMP-3 | 1.026 | 1.001–1.050 | 0.038 |
RPOH: rapidly progressive osteoarthritis of the hip; DDH: developmental dysplasia of the hip; OR: odds ratio; CI: confidence interval; MMP-3: matrix metalloproteinase-3
Differentiation of RPOH type 2 from type 1 before demonstration of femoral head destruction
Because femoral head destruction in RPOH type 2 was observed on CT for the first time at 4 months and more after the onset of hip pain, we further investigated the possibility of early differentiation between RPOH types using MMP-3 before demonstration of femoral head destruction. MMP-3 was measured within 4 months after the onset of hip pain in 11 of 17 patients with RPOH type 1 and 13 of 24 patients with RPOH type 2. MMP-3 levels in RPOH type 2 (140.3±27.0 ng/mL) were significantly higher (p=0.002) than those in RPOH type 1 (60.7±8.4 ng/mL). Logistic regression and ROC analyses using the MMP-3 data suggested that MMP-3 could differentiate RPOH type 2 from type 1 at the time before demonstration of femoral head destruction by CT (Table 3).
Table 3.
Logistic regression and receiver operating characteristic curve analyses for MMP-3 of RPOH type 2 within 4 months after the onset of hip pain.
| p | OR | [95% CI] | ||||
|---|---|---|---|---|---|---|
| Logistic regression analysis | <0.001 | 1.057 | 1.007–1.109 | |||
| AUC | p | [95% CI] | Cutoff | Specificity | Sensitivity | |
|
| ||||||
| Receiver operating characteristic curve analysis | 0.881 | 0.002 | 0.749–1.000 | 79.6 ng/mL | 0.818 | 0.769 |
RPOH: rapidly progressive osteoarthritis of the hip; AUC: area under the curve; CI: confidence interval; MMP-3: matrix metalloproteinase-3
Discussion
There is consensus that the first manifestation of RPOH is rapid chondrolysis with progressive joint space narrowing. Subsequent destruction of the femoral head and acetabulum may be observed in some patients with RPOH within 12 months following initial presentation (7, 9). Probably due to lack of sequential clinical findings in its early stage, it has been controversial whether these patients show the late stage of RPOH as the result of gradual joint destruction (7, 8) or it is another entity of the disease distinct from RPOH without bone destruction (9). Based on the present study, RPOH could be classified into two distinct types based on femoral head destruction in the early stage during 12 months after the onset of hip pain. In addition, no study has stratified this disease by possible pathogenetic factors. This study is the first to demonstrate association of femoral head destruction in RPOH with MMP-3 and pelvic tilt using the hematological and radiological data within 12 months after the onset of hip pain.
This study has demonstrated that femoral head destruction within 12 months after the onset of hip pain was associated with higher serum levels of MMP-3 in RPOH. Previous biochemical studies suggest that synovial fibroblasts from patients with RPOH may secrete MMP-3 (6, 14). MMP-3 expression is regulated by proinflammatory cytokines (15). T cells from the affected femoral head as well as synovial membrane of patients with RPOH can produce interleukin (IL)-6 and IL-lα. In addition, T cells from peripheral blood mononuclear cells in patients with RPOH yield tumor necrosis factor α (TNFα) (16). Although the mechanism leading to rapid joint destruction in RPOH is not fully clarified, histological and in vitro studies have shown the presence of mature and activated osteoclasts in the synovium of RPOH with femoral head destruction over a short period of time, compatible with RPOH type 2 in the present study, but not in the OA synovium (17). A combination of IL-1 and TNFα can induce the differentiation of osteoclasts (18). Alternative combination of TNFα and IL-6 induces osteoclast-like cells with in vitro and in vivo bone-resorptive activity (19). Collectively, several combinations of proinflammatory cytokines, which are likely to coexist in the hips with RPOH (16), can induce the differentiation of bone-resorbing cells. This raises the possibility that MMP-3 induction and osteoclastogenesis involve a common pathway stimulating proinflammatory cytokine production. Elucidation of the pathway may help develop preventive treatment for joint destruction in RPOH.
The sagittal spino-pelvic alignment could affect development of hip diseases (20). Some hip joints with normal acetabular coverage may develop OA in elderly individuals because lumbar degenerative kyphosis can increase posterior pelvic tilt resulting in a decrease in anterior acetabular coverage of the femoral head (5, 21). In the previous investigation on the sagittal spino-pelvic alignment between female patients with RPOH and OA, the patients with RPOH have shown a significant increase in posterior pelvic tilt in combination with lower lumbar lordosis, lumbar range of motion and sacral slope compared with those with OA (22). Similarly, posterior pelvic tilt was greater in RPOH type 2 than DDH and was inclined to be greater than type 1 in the present study. A decrease in anterior acetabular coverage of the femoral head by posterior pelvic tilt increases stress concentration in the weight bearing area of the hip joint (5, 21). Therefore, increased posterior pelvic tilt may work as a mechanical factor and increase stress concentration in the anterior portion of the femoral head leading to bone destruction in RPOH type 2.
There are several limitations to the present study. First, this was a retrospective study with some selection bias. Second, this study investigated a small number of female subjects with the absence of males and a healthy control. However, the number of patients investigated for RPOH markers in previous studies has ranged from 12 to 20 (6, 23–25). In addition, it may be difficult to recruit a large number of patients with a complete set of data within 12 months after disease onset. Third, this study was a cross-sectional design and MMP-3 in each subject was determined at a single time point (the first visit to our hospital). Time course of MMP-3 remains unclear in each patient with RPOH during the disease progression from the onset. Fourth, the spinal and pelvic alignment was not assessed with whole spine and lower extremity radiographs. Fifth, we only evaluated CTI without bone mineral density measurement at the proximal femur.
In conclusion, the process of RPOH progression during 12 months after the onset of hip pain could be classified into two types based on the absence (type 1) and presence (type 2) of femoral head destruction in association with MMP-3 and pelvic tilt. A prospective study that includes a higher number of patients in the early stage of RPOH is needed to verify the predictive role of MMP-3 and pelvic tilt in subsequent bone destruction in patients with RPOH. Early differentiation of RPOH type 2 from type 1 by MMP-3 and pelvic tilt in its early stage could help development of effective intervention to prevent subsequent bone destruction.
Main Points.
Disease progression of rapidly progressive osteoarthritis of the hip (RPOH) during the first 12 months after the onset is classified into two types based on the absence and presence of subsequent femoral head destruction.
RPOH with femoral head destruction within 12 months after the onset is associated with increased matrix metalloproteinase-3 (MMP-3) and increased posterior pelvic tilt.
MMP-3 may predict subsequent femoral head destruction at the time before its initiation.
Footnotes
Content of this journal is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Kobe City Medical Center General Hospital (Decision Number: k190516; Decision Date: February 24, 2018).
Informed Consent: Informed consent was not obtained due to the retrospective nature of the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - T.Y.; Design - T.Y., K.M., T.H., Y.T., T.S., S.O., S.F., E.O.; Supervision - T.Y.; Resources - T.Y.; Materials - T.Y., K.M., T.H., Y.T., T.S., S.O., S.F., E.O.; Data Collection and/or Processing - T.Y., K.M., T.H., Y.T., T.S., S.O., S.F., E.O.; Analysis and/or Interpretation - T.Y.; Literature Search - T.Y., K.M., T.H., Y.T., T.S., S.O., S.F., E.O.; Writing Manuscript - T.Y.; Critical Review - T.Y., K.M., T.H., Y.T., T.S., S.O., S.F., E.O.; Other - K.M., T.H., Y.T., T.S., S.O., S.F., E.O.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received financial support from the Japan Hip Joint Foundation.
References
- 1.Lequesne M. [Rapid destructive coxarthritis]. Rhumatologie. 1970;2:51–63. (In French). [PubMed] [Google Scholar]
- 2.Bock GW, Garcia A, Weisman MH, Major PA, Lyttle D, Haghighi P, et al. Rapidly destructive hip disease: Clinical and imaging abnormalities. Radiology. 1993;186:461–6. doi: 10.1148/radiology.186.2.8421751. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T, Bullough PG. The role of subchondral insufficiency fracture in rapid destruction of the hip joint: A preliminary report. Arthritis Rheum. 2000;43:2423–7. doi: 10.1002/1529-0131(200011)43:11<2423::AID-ANR8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe W, Itoi E, Yamada S. Early MRI findings of rapidly destructive coxarthrosis. Skelet Radiol. 2002;3:35–8. doi: 10.1007/s00256-001-0445-0. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe W, Sato K, Itoi E, Yang K, Watanabe H. Posterior pelvic tilt in patients with decreased lumbar lordosis decreases acetabular femoral head covering. Orthopedics. 2002;25:321–4. doi: 10.3928/0147-7447-20020301-16. [DOI] [PubMed] [Google Scholar]
- 6.Masuhara K, Nakai T, Yamaguchi K, Yamasaki S, Sasaguri Y. Significant increases in serum and plasma concentrations of matrix metalloproteinases 3 and 9 in patients with rapidly destructive osteoarthritis of the hip. Arthritis Rheum. 2002;46:2625–31. doi: 10.1002/art.10547. [DOI] [PubMed] [Google Scholar]
- 7.Sugano N, Ohzono K, Nishii T, Sakai T, Haraguchi K, Yoshikawa H, et al. Early MRI findings of rapidly destructive coxopathy. Magn Reson Imaging. 2001;19:47–50. doi: 10.1016/S0730-725X(01)00221-1. [DOI] [PubMed] [Google Scholar]
- 8.Zazgyva A, Gurzu S, Gergely I, Jung I, Roman CO, Pop TS. Clinico-radiological diagnosis and grading of rapidly progressive osteoarthritis of the hip. Medicine (Baltimore) 2017;96:e6395. doi: 10.1097/MD.0000000000006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pivec R, Johnson AJ, Harwin SF, Mont MA. Differentiation, diagnosis, and treatment of osteoarthritis, osteonecrosis, and rapidly progressive osteoarthritis. Orthopedics. 2013;36:118–25. doi: 10.3928/01477447-20130122-04. [DOI] [PubMed] [Google Scholar]
- 10.Yeung Y, Chiu KY, Yau WP, Tang WM, Cheung WY, Ng TP. Assessment of the proximal femoral morphology using plain radiograph-can it predict the bone quality? J Arthroplasty. 2006;21:508–13. doi: 10.1016/j.arth.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 11.Baumgärtner R, Heeren N, Quast D, Babst R, Brunner A. Is the cortical thickness index a valid parameter to assess bone mineral density in geriatric patients with hip fractures? Arch Orthop Trauma Surg. 2015;135:805–10. doi: 10.1007/s00402-015-2202-1. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen BN, Hoshino H, Togawa D, Matsuyama Y. Cortical thickness index of the proximal femur: A radiographic parameter for preliminary assessment of bone mineral density and osteoporosis status in the age 50 years and over population. Clin Orthop Surg. 2018;10:149–56. doi: 10.4055/cios.2018.10.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishihara S, Sugano N, Nishii T, Ohzono K, Yoshikawa H. Measurements of pelvic flexion angle using three-dimensional computed tomography. Clin Orthop Relat Res. 2003;411:140–51. doi: 10.1097/01.blo.0000069891.31220.fd. [DOI] [PubMed] [Google Scholar]
- 14.Masuhara K, Bak Lee S, Nakai T, Sugano N, Ochi T, Sasaguri Y. Matrix metalloproteinases in patients with osteoarthritis of the hip. Int Orthop. 2000;24:92–6. doi: 10.1007/s002640000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauviel A. Cytokine regulation of metalloproteinase gene expression. J Cell Biochem. 1993;53:288–95. doi: 10.1002/jcb.240530404. [DOI] [PubMed] [Google Scholar]
- 16.Tamai M, Sagawa K, Kawabata R, Inoue A, Itoh K. Production of IL-6 by T cells from the femoral head of patients with rapidly destructive coxopathy (RDC) Clin Exp Immunol. 1996;103:506–13. doi: 10.1111/j.1365-2249.1996.tb08309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa K, Mawatari M, Komine M, Shigematsu M, Kitajima M, Kukita A, et al. Mature and activated osteoclasts exist in the synovium of rapidly destructive coxarthrosis. J Bone Miner Metab. 2007;25:354–60. doi: 10.1007/s00774-007-0761-0. [DOI] [PubMed] [Google Scholar]
- 18.Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, et al. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med. 2005;202:589–95. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokota K, Sato K, Miyazaki T, Kitaura H, Kayama H, Miyoshi F, et al. Combination of tumor necrosis factor α and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheumatol. 2014;66:121–9. doi: 10.1002/art.38218. [DOI] [PubMed] [Google Scholar]
- 20.Offierski CM, MacNab I. Hip-spine syndrome. Spine. 1983;8:316–21. doi: 10.1097/00007632-198304000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimoto H, Sato S, Masuda T, Kanno T, Shundo M, Hyakumachi T, et al. Spinopelvic alignment in patients with osteoarthrosis of the hip: A radiographic comparison to patients with low back pain. Spine. 2005;30:1650–7. doi: 10.1097/01.brs.0000169446.69758.fa. [DOI] [PubMed] [Google Scholar]
- 22.Morimoto T, Kitajima M, Tsukamoto M, Yoshihara T, Sonohata M, Mawatari M. Sagittal spino-pelvic alignment in rapidly destructive coxarthrosis. Eur Spine J. 2018;27:475–81. doi: 10.1007/s00586-017-5282-5. [DOI] [PubMed] [Google Scholar]
- 23.Abe H, Sakai T, Ogawa T, Takao M, Nishii T, Nakamura N, et al. Characteristics of bone turnover markers in rapidly destructive coxopathy. J Bone Miner Metab. 2017;35:412–8. doi: 10.1007/s00774-016-0769-4. [DOI] [PubMed] [Google Scholar]
- 24.Garnero P, Conrozier T, Christgau S, Mathieu P, Delmas PD, Vignon E. Urinary type II collagen C-telopeptide levels are increased in patients with rapidly destructive hip osteoarthritis. Ann Rheum Dis. 2003;62:939–43. doi: 10.1136/ard.62.10.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger CE, Kroner A, Stiegler H, Leitha T, Engel A. Elevated levels of serum type I collagen C-telopeptide in patients with rapidly destructive osteoarthritis of the hip. Int Orthop. 2005;29:1–5. doi: 10.1007/s00264-004-0608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]