Abstract
Objective
To study the differences between disease activity score 28 (DAS28) index and an ultrasound (US) approach using index echographic disease activity score (ECODAS).
Methods
This was a cross-sectional study in patients diagnosed with rheumatoid arthritis (RA). Demographic, clinical, and laboratory data were collected. We created a US index (ECODAS) evaluating the joints with synovitis using gray scale US (GSUS) and power Doppler US (PDUS) and calculated the formula of DAS28 index with both variables substituting tender joint for GSUS and swollen joint for PDUS (ECODAS1) and vice versa (ECODAS2).
Results
A total of 58 patients (65.5% women and 34.5% men) were included in the study. There was no significant difference between the 2 US indexes. We obtained a Pearson’s correlation coefficient (Pearson’s r) of 0.56 (p<0.00001) between DAS28 and ECODAS1 and of 0.57 (p<0.00001) between DAS28 and ECODAS2, respectively. However, for patients with a high disease activity [DAS28>5.1, tender joint count (TJC, high)], the correlation was poor (0.18) and ECODAS indexes were significantly lower (p=0.001). The correlation increased (0.86, p<0.001) when we excluded the tender joints and the joints with GS-positive synovitis in both the scores.
Conclusion
US reduces the bias in the evaluation of patients with RA with a high value in DAS28 index. We found a clear difference between DAS and ECODAS when TJC was high. The results suggest that joint tenderness reported by the patient is not a good reflection of inflammation. More studies are needed to find a new combined clinical and sonographic index that would better assess the disease activity in patients with RA.
Keywords: Ultrasonography, rheumatoid arthritis, index
Introduction
Rheumatoid arthritis (RA) is a debilitating disease that affects approximately 0.5%–1% of the population (1). Nowadays, the disease outcome has changed because of the development of disease-modifying drugs, especially biological therapies, but there is a percentage of patients who do not respond to the existing treatments. In addition, rheumatologists are considering tapering down the treatment more frequently because of either safety concerns or economic problems. Therefore, it is fundamental to correctly assess the disease activity to make evidence-based therapeutic decisions.
When assessing the disease activity, pain can be found on clinical examination, without the signs of swelling and with normal serum inflammatory parameters [C reactive protein (CRP), erythrocyte sedimentation rate (ESR)]. At present, disease activity in RA is measured by the disease activity score 28 (DAS28), which is a clinical and laboratory composite score. When calculating this score, the value given to the tender joints is higher than that given to the swollen joints. The use of ultrasound (US) in rheumatology has allowed for a better joint evaluation since it is more sensitive than clinical evaluation for synovitis detection (2). Moreover, it was demonstrated that the presence of pain does not correlate with that of synovitis observed during the US examination (3). We believe that in patients with chronic pain without synovitis, the DAS28 index is not a good tool for disease assessment.
The DAS28 index often does not reflect the actual situation of the patient (clinical remission or pain without inflammation on clinical examination) due to the bias of the bias incurred by variables such as the number of tender joints and patient global assessment and the subjectivity of physical examination, with high interobserver variability (4–6). Our study objective was to evaluate the correlation between clinical and US examination of all the joints included in the 28 joints count, creating an echographic disease activity score (ECODAS) without clinical variables as tender and swollen joint count (SJC).
Methods
Patients
We included patients with RA according to ACR/EULAR 2010 criteria (7). Patients were recruited consecutively from November 2014 to December 2015 in the outpatient clinic of the rheumatology unit. The patients could receive any type of treatment at the time of US evaluation. We did not include any patient with comorbidities, such as fibromyalgia or other pain conditions.
The study was approved by the Ethics Committee of Research (ECR) of the Vall d’Hebron Hospital in September 2014, and an informed consent was obtained from all the patients.
Clinical examination
Swollen joints, tender joints, and global assessment of health on a visual analog scale were assessed by a rheumatologist (RC) who was blinded to the ultrasonographical results. The ESR and CRP were obtained from routine follow-up blood tests for each patient.
Ultrasonography assessment
Ultrasonography was performed by a rheumatologist specialized in musculoskeletal ultrasonography (JJdA) who was blinded to other clinical information. The joints evaluated were the ones that comprise the DAS28 score (8). A General Electric Logiq 9 US device (General Electric Company; Boston, MA, USA) with a high frequency (8–13 MHz) linear transducer was used for the US evaluation. Power Doppler (PD) settings were standardized for the detection of synovial blood flow by adjusting color gain, pulse repetition, and flow optimization parameters according to a previous study (9), and finally were: pulse repetition frequency (PRF), 0.8; frequency, 10.0 MHz; gain, 27.5; and wall filter, 102. We evaluated the number of joints with synovitis in gray scale US (GSUS) and the number of joints with PDUS signal. GS and PD settings (medium dynamic range, medium persistence, medium frame rate, low wall filter, 0.8 Hz PRF) were identical throughout the examinations. Room temperature was maintained at 25°C. The patients were positioned comfortably, and the examinations were then started after 10 minutes of stabilization of the pulse rate. The scanning technique of each joint was standardized and fixed as follows: longitudinal and transverse scan of shoulders (posterior recess for gleno humeral joint, anterior recess for bursitis and bicipital tendon sheath), elbows (anterior, lateral, and posterior recesses), wrists (radioulnar, radiocarpal, and intercarpal), hands [dorsal and volar aspects of 1–5 metacarpophalangeal joint (MCP) and 1–5 proximal interphalangeal joint (PIP) joints], and knees (suprapatelar and parapatelar recesses). Scanning was performed in this order to optimize the time. Each joint was examined following the EULAR guidelines and recommendations. When assessing each joint, we also included tendon evaluation and noted the worst score of both for the indexes.
We defined ECODAS, a score calculated using the formula of DAS28 score, by substituting the clinical variables with the sonographic ones. We considered modifying DAS28 with US parameters similar to Damjanov et al (10). PDUS is the most similar US variable to SJC (11–13); therefore, synovitis by GS can be used as tender joint count (TJC). To decide how to use the US examination in the ECODAS score, we evaluated 2 different approaches. In the first approach, the GSUS was used as an equivalent for the TJC and the PDUS as an equivalent for the SJC. In the second approach, GSUS and PDUS were swapped, obtaining 2 scores, ECODAS1 and ECODAS2. We also analyzed the agreement between TJC and GSUS and between SJC and PDUS in a subgroup of patients (48) for which we had the corresponding data.
Statistical analysis
Statistical analyses were calculated using R version 3.3.2. Normally distributed continuous data were summarized by mean and standard deviation (SD). Any value of p<0.05 was considered statistically significant. Differences between the 2 groups were examined using Student’s t-test. The agreement between clinical and US evaluation of synovitis was assessed by kappa (k) statistics, unweighted for dichotomous scoring (e.g., the presence/absence of synovitis). A k-value less than 0.4 was considered poor; between 0.4 and 0.75, moderate; and more than 0.75, excellent (14). The correlation was evaluated using the Pearson’s coefficient.
Results
We included 58 patients with RA, and 16 patients were receiving biological treatment: 4, adalimumab; 2, etanercept; 3, tocilizumab; 1, infliximab; 2, certolizumab; 2, golimumab; 1, abatacept; and 1, rituximab. Among the patients receiving the biological therapy, 3 were on monotherapy, 2 treated with etanercept and 1 with adalimumab. Others received the biological treatment in combination with synthetic disease-modifying drugs: methotrexate, leflunomide, or hydroxychloroquine. Moreover, 28 patients were receiving monotherapy with methotrexate, 2 patients with leflunomide, 9 receiving combined therapy with methotrexate and leflunomide, 2 receiving triple therapy with methotrexate, leflunomide, and sulfasalazine, and 1 receiving triple therapy with methotrexate, leflunomide, and hydroxychloroquine. We included patients with high, moderate, and low disease activity. Demographic, clinical, and laboratory characteristics of the patients are shown in Table 1.
Table 1.
Clinical and laboratory characteristics of patients, and sonographic evaluation of the joints.
| Age, mean (range) years | 61.6 (27–84) |
| Sex, no. female/male | 1/9 |
| Duration of disease, years | 9.93 (1–31) |
| ESR, mm/hour | 29.2 (2–89) |
| CRP level, mg/dL | 0.87 (0.02–5.61) |
| SJC | 2.53 (0–10) |
| TJC | 7.24 (0–28) |
| Patient’s global assessment by VAS | 59.09 (8–100) |
| Examiner’s global assessment by VAS | 44.12 (4–95) |
| DAS28-ESR, mean±SD | 4.73±1.21 |
| CDAI, mean±SD | 16.26±10.23 |
| SDAI, mean±SD | 12.98±9.1 |
| GSUS, mean±SD | 5.12±4.25 |
| PDUS, mean±SD | 2.63±3.17 |
| ECODAS1, mean±SD | 4.48±1.10 |
| ECODAS2, mean±SD | 4.24±1.13 |
| CDAIE, mean±SD | 13.54±9.1 |
| SDAIE, mean±SD | 10.24±8.38 |
CDAI: clinical disease activity index; CDAIE: ultrasound clinical disease activity index; CRP: reactive C protein; DAS28: disease activity score; ESR: erythrocyte sedimentation rate; ECODAS: ultrasound disease activity score; GSUS: gray scale ultrasound; PDUS: power Doppler ultrasound; SD: standard deviation; SDAI: simplified disease activity index; SDAIE: ultrasound simplified disease activity index; SJC: swollen joint count; TJC: tender joint count; VAS: visual analog scale.
Assessment of disease activity
The mean of TJC and SJC was 7.24 and 2.53, respectively. The mean DAS28 in our cohort was 4.73 (1.21) that corresponds to a moderate disease activity. In addition, 2 patients (3.45%) were in remission (DAS28≤2.6), 4 (6.9%) had low disease activity (2.6<DAS28≤3.2), 29 (50%) had moderate disease activity (3.2<DAS28≤5.1), and 23 (39.65%) had high disease activity (DAS28>5).
US evaluation of disease activity
The mean number of joints with synovitis in GS and PD is summarized in Table 1. The mean for the 2 scores was calculated as stated in Methods.
Agreement between clinical and sonographic assessment of the presence or absence of synovitis
We first assessed the agreement between the TJC (out of 28 from the DAS28 score) and the GSUS (the same joints that were assessed as part of DAS28). While doing this for every patient, we found there was a poor agreement (k<0.4) in 83.33% of the patients (40 of 48 patients), there was a moderate agreement (0.4≤k<0.75) in 14.58% of the patients (7 of 48 patients), and an excellent agreement (k>0.75) in 2.08% of the patients (1 of 48 patients); the average agreement was 79%. Next, we evaluated the agreement between TJC and PDUS, between SJC and GSUS, and between SJC and PDUS. The average agreement between swollen joints and PDUS was 89.5%. The detailed results are presented in Table 2 showing a poor agreement overall between tender joints and synovitis detected by US, either in GS or with Doppler signal. There is a moderate agreement between swollen joints and synovitis detected by US, mostly in the small joints of the hands.
Table 2.
Agreement per patient and per joint.
| Level of agreement | TJC-GSUS | TJC-PDUS | SJC-GSUS | SJC-PDUS | ||||
|---|---|---|---|---|---|---|---|---|
| Poor (k<0.4) | 83.33 (40/48) | 83.33 (40/48) | 66.66% (32/48) | 66.66% (32/48) | ||||
| Moderate (0.4≤k<0.75) | 14.58% (7/48) | 8.33% (4/48) | 25% (12/48) | 27.08% (13/38) | ||||
| Excellent (k≥0.75) | 2.09% (1/48) | 8.33% (4/48) | 8.33% (4/48) | 6.25% (3/48) | ||||
|
| ||||||||
| Joint (right and left) | TJC-GSUS | TJC-PDUS | SJC-GSUS | SJC-PDUS | ||||
|
| ||||||||
| Shoulder | 0.098 | 0.093 | 0.12 | 0.09 | −0.07 | 0 | −0.06 | 0 |
| Elbow | 0.216 | 0.28 | 0.18 | 0.19 | 0.21 | 0.28 | 0.53 | −0.03 |
| Right wrist | 0.2 | 0.22 | 0.18 | 0.13 | 0.38 | 0.31 | 0.32 | 0.28 |
| Right 1 MCP | 0.07 | 0.24 | 0.06 | 0.3 | 0.55 | 0.35 | 0.33 | 0.38 |
| Right 2 MCP | 0.159 | −0.108 | 0.27 | −0.19 | 0.53 | 0.41 | 0.62 | 0.45 |
| Right 3 MCP | 0.06 | 0.315 | −0.03 | 0.29 | 0.24 | 0.7 | 0.45 | 0.66 |
| Right 4 MCP | 0.11 | 0.24 | −0.06 | 0.34 | 0.3 | 0 | −0.02 | 0 |
| Right 5 MCP | 0.5 | −0.108 | 0.43 | −0.03 | 0.48 | −0.03 | 0.63 | −0.02 |
| Right 1 PIP | −0.125 | 0.03 | −0.07 | −0.03 | −0.03 | 0 | −0.02 | 0 |
| Right 2 PIP | 0.25 | 0.089 | 0.06 | −0.01 | 0.37 | 0.22 | 0.65 | 0.37 |
| Right 3 PIP | 0.22 | 0.46 | 0.2 | 0.19 | 0.66 | 0.49 | 0.86 | 0.38 |
| Right 4 PIP | 0.142 | 0.33 | 0.07 | 0.35 | −0.03 | 0.25 | 0.65 | 0.65 |
| Right 5 PIP | 0.148 | 0.148 | 0.14 | 0 | 0.54 | 0 | 0.65 | 0 |
| Right knee | 0.08 | 0.107 | 0.08 | 0.26 | 0.32 | 0.19 | 0.5 | 0.34 |
GSUS: gray scale ultrasound; MCP: metacarpophalangeal joint; PIP: proximal interphalangeal joint; PDUS: power Doppler ultrasound; SJC: swollen joint count; TJC: tender joint count.
The next step was to evaluate the agreement between clinical and US examination for every joint. The results are presented in Table 2.
When analyzing the concordance between the clinical (TJC) and US (GSUS and PDUS) evaluation in patients with high discordance between the number of tender and swollen joints (>5), the agreement coefficient was lower in these patients than the patients with a lower discordance (p=0.01 for TJC-GSUS and 0.001 for TJC-PDUS, respectively). The patients with high discordance also had higher Patient’s Global Assessment by visual analog scale (Table 3).
Table 3.
k agreement coefficient for each joint in patients with high discordance between tender and swollen joints versus low discordance.
| Joint (right and left) | TJC-GSUS, TJC-SJC≤5 | TJC-GSUS, TJC-SJC>5 | TJC-PDUS, TJC-SJC≤5 | TJC-PDUS, TJC-SJC>5 | ||||
|---|---|---|---|---|---|---|---|---|
| Shoulder | 0.1775 | 0.091 | 0.099 | 0.2 | 0.1836 | 0.294 | 0.099 | 0 |
| Elbow | 0.388 | −0.049 | 0.0769 | 0.333 | 0.3093 | 0 | 0 | 0.158 |
| Wrist | 0.168 | 0.276 | 0.3333 | 0 | 0.1724 | 0.276 | 0.2631 | −0.111 |
| 1 MCP | 0.3548 | 0.459 | −0.1891 | −0.037 | 0.4285 | 0.714 | −0.1282 | −0.12 |
| 2 MCP | 0.228 | −0.114 | −0.0285 | −0.059 | 0.3816 | −0.10 | 0.1219 | −0.127 |
| 3 MCP | −0.0909 | 0.636 | 0.1232 | 0.077 | −0.0476 | 0.590 | 0.0588 | 0.077 |
| 4 MCP | 0 | −0.043 | −0.2173 | 0.259 | 0 | 0 | −0.1162 | 0.259 |
| 5 MCP | 0.52 | −0.103 | 0.4782 | 0 | 0.6321 | −0.049 | 0.2558 | 0 |
| 1 PIP | −0.081 | −0.043 | −0.2173 | 0 | −0.081 | −0.043 | 0 | 0 |
| 2 PIP | 0.3684 | −0.053 | 0.0724 | 0.111 | 0.3684 | −0.049 | −0.1267 | 0.043 |
| 3 PIP | 0.389 | 0.529 | 0.027 | 0.375 | 0.3885 | 0.365 | 0.0769 | 0 |
| 4 PIP | 0.15 | 0.784 | 0.1578 | −0.037 | 0.2156 | 0.652 | 0 | 0.2 |
| 5 PIP | 0.434 | 0.652 | 0 | −0.111 | 0.4754 | 0 | 0 | 0 |
| Knee | 0.1111 | 0.125 | −0.0158 | 0.015 | 0.2156 | −0.043 | 0.0985 | 0.310 |
GSUS: gray scale ultrasound; MCP: metacarpophalangeal joint; PIP: proximal interphalangeal joint; PDUS: power Doppler ultrasound; SJC: swollen joint count; TJC: tender joint count.
Relationship between clinical and US examination of the joints
We found a moderate correlation between DAS28 and ECODAS1, with a Pearson’s coefficient of 0.56 (r2=0.32, p<0.00001) as well as between DAS28 and ECODAS2, with a Pearson’s coefficient of 0.57 (p<0.00001). Since there is no significant difference between the 2 US scores (p=0.25) and there was a greater agreement between the swollen joints and the joints with synovitis with PD, we decided to use ECODAS1 score for further analysis. ECODAS1 was calculated by substituting TJC with GS-positive joints and SJC with PD-positive joints; hence, we thought this score would be a better surrogate for the DAS28 score because of the relation between clinical inflammation and PD evaluation.
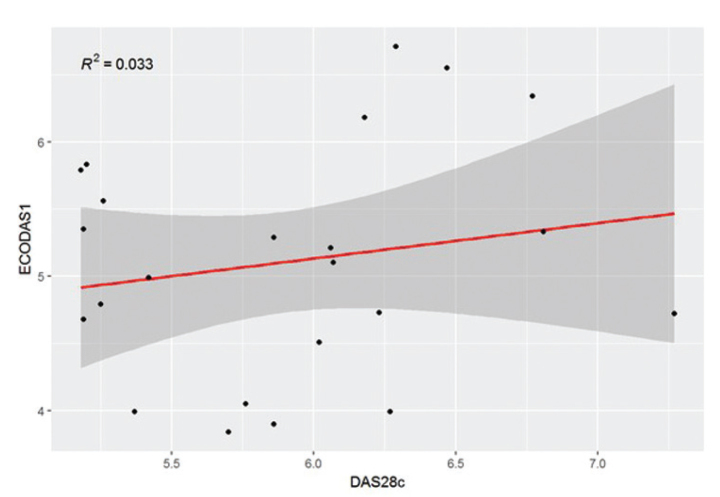
When comparing DAS28 with ECODAS1, we did not find any significant difference. Interestingly, after stratifying the patients by disease activity, in the group of patients with high disease activity, n=23, (DAS28>5.1) there was a significant difference between DAS28 and ECODAS1 (p=0007). Moreover, the correlation between the 2 scores in this subgroup of patients was poor, with a Pearson’s coefficient of 0.18 (Figure 1). The mean difference between TJC and SJC was 10.4 (range between 0 and 26) in patients with high disease activity and 6 (range from 7 to 14) for the rest of the patients (p<0.05).
Figure 1.
There is a poor correlation between the clinical and ultrasound scores in patients with high disease activity.
Because we found a poor agreement between the tender joints and US-detected synovitis, we also calculated other scores used for disease activity assessment: simplified disease activity index and clinical disease activity index and their US equivalents. We found a moderate correlation between both the scores, with a Pearson’s correlation of 0.51 and 0.4, respectively.
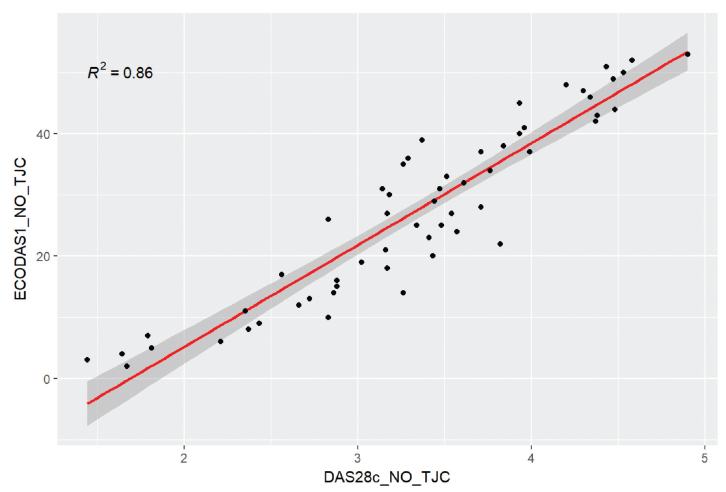
Given that we consider that pain overestimates DAS28, we decided to eliminate this variable and calculate a variant of DAS28 and ECODAS1 excluding the number of tender joints and the number of joints with GS-positive synovitis, respectively. The correlation coefficient between the 2 calculated scores was significantly better, 0.86 (p<0.001) (Figure 2).
Figure 2.
The correlation coefficient between the 2 calculated scores (DAS28 excluding tender joints and ECODAS1 excluding GS positive joints) was high (0.86).
Discussion
The correct assessment of disease activity in patients with RA is fundamental for offering adequate treatment to the patients. DAS28 score is the most frequently used tool for disease activity evaluation. Concerns about DAS28 reliability for disease activity assessment have been present for a long time and a lot of work has been carried out trying to find US-based scores to allow a more accurate evaluation of synovitis.
There are several US scores (15–21) using different number of joints with moderate correlation with clinical scores. We have decided to explore a new approach with a mixed index similar to the Damjanov et al. (10), eliminating the possible subjective parameters such as TJC. It was demonstrated that these reduced scores reflected therapeutic response; therefore, they could be used as a good tool for disease monitoring, but no consensus exists yet on what number of or which joints should be evaluated by US. In our study, we found a disagreement between the tender joints and synovitis detected by US in GS as well as PD. These findings support already published data that found no correlation between tender joints and US findings of synovitis (22). In contrast, we found a moderate agreement between the swollen joints and synovitis detected by US, in both GS and PD, mostly in the MCP joints and less in the PIPs. There was no agreement for the big joints, which suggests that clinical assessment of synovitis in these joints is trickier, possibly because the joints are profound, and it might be difficult to assess small effusions and low-grade synovitis.
Damjanov et al. (10) tried an approach similar to ours. They showed that US DAS had a stronger correlation with the historical and the near-future damage indexes detected by X-ray, magnetic resonance imaging, and US, and it better anticipated the probability of future joint damage than DAS28. Using this as a reference, we calculated ECODAS1 and 2, replacing TJC with GS synovitis and SJC with PD synovitis and vice versa. We found that the 2 options were equivalent. This could be because both GS and PD are the expressions of synovitis, and there was no significant difference between the number of joints with synovitis detected by GS and PD in our cohort. We decided to use ECODAS1 in the rest of the analysis owing to the relation between swollen joints and PD synovitis because we are aware that the presence of PD (a signal of intense vascularity) signal represents active inflammation, which is a known predictor for the development of erosions (23, 24) and probably reflects better inflammatory level than GS.
There was a moderate correlation (R2=0.32, p<0.05) between DAS28 score and ECODAS1. However, when analyzing the subset of patients with high disease activity there was no statistically significant correlation between both indexes (R2=0.03). Moreover, there was no statistically significant difference between the clinical score DAS28 and the US score ECODAS1, and DAS28 was significantly higher than ECODAS1 in patients with high disease activity (defined by DAS28>5.1). For this subgroup of patients, the mean difference between TJC and SJC was 10.43, significantly higher compared to the rest of the patients. Several studies found that DAS28 overestimates the disease activity in patients with fibromyalgia (6) because of a higher number of tender joints and a higher score for pain (25–27). The number of tender joints accounts double the number of swollen joints in the DAS28 score (28). We found that the agreement between clinical and US examination is lower when there is a high discordance between the TJC and SJC. In patients with RA, there are several non-inflammatory causes of pain, such as chronification of pain, development of central sensitization, and conditioned pain modulation (29, 30). All these causes lead to an increase of DAS28 because 2 parameters that reflect pain are used for its calculation: the TJC and the patient’s assessment of disease. When we calculated DAS28 and ECODAS1 after eliminating the pain component, the correlation between the 2 scores was higher (R2=0.86, p<0.001). A recent study has demonstrated that a high discordance between tender and swollen joints and between the patients’ and physician’s assessment of the disease may reduce the likelihood of remission in RA and psoriatic arthritis (31).
Therefore, our results suggest that DAS28 has a subjective component, pain, which overestimates the disease activity and can lead to unnecessary changes in the treatment. A composite score that would include objective measures, such as US parameters combined with clinical and biological ones, should be developed for a better assessment of the disease activity in patients with RA.
Of course, our study has limitations: the number of patients is low, and there is a skew in the disease activity toward high activity; nonetheless, among the patients with high disease activity, there was a high discordance between the clinical and US evaluation. We did not calculate the sample size before the study, but when we performed statistical analysis, the results were powerful to support the main objective. A larger sample size is needed to confirm the results. Another limitation of our study is the calculation of ECODAS1, substituting TJC by joints with synovitis in GS and SJC by joints with synovitis with PD signal because there is no equivalence between these parameters. However, this option is an objective method of evaluating the joints that does not take into the account the subjective component of pain.
In conclusion, our study found a clear difference between the clinical examination and US evaluation in patients with a high difference between the number of TJC and SJC. US is an objective method that can reduce overestimation of pain bias and can be used in combination with clinical and laboratory parameters to improve evaluation of inflammation in RA, creating a composite score.
The field of inflammatory arthritis requires the development of better tools to improve patient management, and ultrasound, which is relatively cheap and widely available, allowing bedside evaluations, should be among these tools.
Main Points.
Clinical scores overestimate the pain component in a relevant group of patients.
Mixed US indexes evaluated better inflammatory component than clinical scores.
Use ultrasonography in patients with other pain conditions.
Footnotes
Content of this journal is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Ethics Committee Approval: Ethics Committee Approval was received for this study from the Ethics Committee of Research (ECR) of the Vall d’Hebron Hospital.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - J.J.A.O.; Design - J.J.A.O., R.C.; Supervision - R.C., G.A.S.; Materials - R.C., G.A.S., M.B.B., A.S.F., S.F., S.C.B., B.R.D., J.J.A.O.; Data Collection and/or Processing - R.C., G.A.S.; Analysis and/or Interpretation - G.A.S., J.J.A.O.; Literature Search - G.A.S., J.J.A.O.; Writing Manuscript - G.A.S.; Critical Review - R.C., G.A.S., M.B.B., A.S.F., S.F., S.C.B., B.R.D., J.J.A.O.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002;4:S265–72. doi: 10.1186/ar578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szkudlarek M, Narvestad E, Klarlund M, Court-Payen M, Thomsen HS, Østergaard M. Ultrasonography of the metatarsophalangeal joints in rheumatoid arthritis: Comparison with magnetic resonance imaging, conventional radiography, and clinical examination. Arthritis Rheum. 2004;50:2103–12. doi: 10.1002/art.20333. [DOI] [PubMed] [Google Scholar]
- 3.Rees JD, Pilcher J, Heron C, Kiely PD. A comparison of clinical vs ultrasound determined synovitis in rheumatoid arthritis utilizing gray-scale, power Doppler and the intravenous microbubble contrast agent ‘Sono-Vue’. Rheumatology (Oxford) 2007;46:454–9. doi: 10.1093/rheumatology/kel256. [DOI] [PubMed] [Google Scholar]
- 4.Cheung PP, Gossec L, Mak A, March L. Reliability of joint count assessment in rheumatoid arthritis: A systematic literature review. Semin Arthritis Rheum. 2014;43:721–9. doi: 10.1016/j.semarthrit.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Jensen Hansen IM, Asmussen Andreasen R, van Bui Hansen MN, Emamifar A. The reliability of disease activity score in 28 joints-C-reactive protein might be overestimated in a subgroup of rheumatoid arthritis patients, when the score is solely based on subjective parameters: A cross-sectional, exploratory study. J Clin Rheumatol. 2017;23:102–6. doi: 10.1097/RHU.0000000000000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leeb BF, Andel I, Sautner J, Nothnagl T, Rintelen B. The DAS28 in rheumatoid arthritis and fibromyalgia patients. Rheumatology (Oxford) 2004;43:1504–7. doi: 10.1093/rheumatology/keh322. [DOI] [PubMed] [Google Scholar]
- 7.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs HA, Brooks RH, Callahan LF, Pincus T. A simplified twenty-eight-joint quantitative articular index in rheumatoid arthritis. Arthritis Rheum. 1989;32:531–7. doi: 10.1002/anr.1780320504. [DOI] [PubMed] [Google Scholar]
- 9.Kamishima T, Tanimura K, Henmi M, Narita A, Sakamoto F, Terae S, et al. Power Doppler ultrasound of rheumatoid synovitis: Quantification of vascular signal and analysis of interobserver variability. Skeletal Radiol. 2009;38:467–72. doi: 10.1007/s00256-009-0665-2. [DOI] [PubMed] [Google Scholar]
- 10.Damjanov N, Radunovic G, Prodanovic S, Vukovic V, Milic V, Simic Pasalic K, et al. Construct validity and reliability of ultrasound disease activity score in assessing joint inflammation in RA: Comparison with DAS-28. Rheumatology (Oxford) 2012;51:120–8. doi: 10.1093/rheumatology/ker255. [DOI] [PubMed] [Google Scholar]
- 11.Walther M, Harms H, Krenn V, Radke S, Faehndrich TP, Gohlke F. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44:331–8. doi: 10.1002/1529-0131(200102)44:2<331::AID-ANR50>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Szkudlarek M, Court-Payen M, Strandberg C, Klarlund M, Klausen T, Ostergaard M. Power Doppler ultrasonography for assessment of synovitis in the metacarpophalangeal joints of patients with rheumatoid arthritis: A comparison with dynamic magnetic resonance imaging. Arthritis Rheum. 2001;44:2018–23. doi: 10.1002/1529-0131(200109)44:9<2018::AID-ART350>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 13.Terslev L, Torp-Pedersen S, Savnik A, von der Recke P, Qvistgaard E, Danneskiold-Samsøe B, et al. Doppler ultrasound and magnetic resonance imaging of synovial inflammation of the hand in rheumatoid arthritis: A comparative study. Arthritis Rheum. 2003;48:2434–41. doi: 10.1002/art.11245. [DOI] [PubMed] [Google Scholar]
- 14.Wongpakaran N, Wongpakaran T, Wedding D, Gwet KL. A comparison of Cohen’s Kappa and Gwet’s AC1 when calculating inter-rater reliability coefficients: A study conducted with personality disorder samples. BMC Med Res Methodol. 2013;13:61. doi: 10.1186/1471-2288-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Backhaus M, Ohrndorf S, Kellner H, Strunk J, Backhaus TM, Hartung W, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: A pilot project. Arthritis Rheum. 2009;61:1194–201. doi: 10.1002/art.24646. [DOI] [PubMed] [Google Scholar]
- 16.Naredo E, Gamero F, Bonilla G, Uson J, Carmona L, Laffon A. Ultrasonographic assessment of inflammatory activity in rheumatoid arthritis: Comparison of extended versus reduced joint evaluation. Clin Exp Rheumatol. 2005;23:881–4. [PubMed] [Google Scholar]
- 17.Perricone C, Ceccarelli F, Modesti M, Vavala C, Di Franco M, Valesini G, et al. The 6-joint ultrasonographic assessment: A valid, sensitive-to-change and feasible method for evaluating joint inflammation in RA. Rheumatology (Oxford) 2012;51:866–73. doi: 10.1093/rheumatology/ker405. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimi R, Ihata A, Kunishita Y, Kishimoto D, Kamiyama R, Minegishi K, et al. A novel 8-joint ultrasound score is useful in daily practice for rheumatoid arthritis. Mod Rheumatol. 2015;25:379–85. doi: 10.3109/14397595.2014.974305. [DOI] [PubMed] [Google Scholar]
- 19.Hammer HB, Kvien TK. Comparisons of 7- to 78-joint ultrasonography scores: All different joint combinations show equal response to adalimumab treatment in patients with rheumatoid arthritis. Arthritis Res Ther. 2011;13:R78. doi: 10.1186/ar3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartung W, Kellner H, Strunk J, Sattler H, Schmidt WA, Ehrenstein B, et al. Development and evaluation of a novel ultrasound score for large joints in rheumatoid arthritis: One year of experience in daily clinical practice. Arthritis Care Res (Hoboken) 2012;64:675–82. doi: 10.1002/acr.21574. [DOI] [PubMed] [Google Scholar]
- 21.Seymour MW, Kelly S, Beals CR, Malice MP, Bolognese JA, Dardzinski BJ, et al. Ultrasound of metacarpophalangeal joints is a sensitive and reliable endpoint for drug therapies in rheumatoid arthritis: Results of a randomized, two-center placebo-controlled study. Arthritis Res Ther. 2012;14:R198. doi: 10.1186/ar4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirata A, Ogura T, Hayashi N, Takenaka S, Ito H, Mizushina K, et al. Concordance of patient-reported joint symptoms, physician-examined arthritic signs, and ultrasound-detected synovitis in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2017;69:801–6. doi: 10.1002/acr.23006. [DOI] [PubMed] [Google Scholar]
- 23.Taylor PC, Steuer A, Gruber J, Cosgrove DO, Blomley MJ, Marsters PA, et al. Comparison of ultrasonographic assessment of synovitis and joint vascularity with radiographic evaluation in a randomized, placebo-controlled study of infliximab therapy in early rheumatoid arthritis. Arthritis Rheum. 2004;50:1107–16. doi: 10.1002/art.20123. [DOI] [PubMed] [Google Scholar]
- 24.Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–67. doi: 10.1002/art.23945. [DOI] [PubMed] [Google Scholar]
- 25.Mian AN, Chaabo K, Wajed J, Subesinghe S, Gullick NJ, Kirkham B, et al. Rheumatoid arthritis patients with fibromyalgic clinical features have significantly less synovitis as defined by power Doppler ultrasound. BMC Musculoskelet Disord. 2016;17:404. doi: 10.1186/s12891-016-1258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Silva Chakr RM, Brenol JC, Behar M, Mendonça JA, Kohem CL, Monticielo OA, et al. Is ultrasound a better target than clinical disease activity scores in rheumatoid arthritis with fibromyalgia? A case-control study. PLoS One. 2015;10:e0118620. doi: 10.1371/journal.pone.0118620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranzolin A, Brenol JC, Bredemeier M, Guarienti J, Rizzatti M, Feldman D, et al. Association of concomitant fibromyalgia with worse disease activity score in 28 joints, health assessment questionnaire, and short form 36 scores in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61:794–800. doi: 10.1002/art.24430. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter L, Norton S, Nikiphorou E, Kiely P, Walsh DA, Dixey J, et al. Validation of methods for converting the original Disease Activity Score (DAS) to the DAS28. Rheumatol Int. 2018;38:2297–305. doi: 10.1007/s00296-018-4184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyden SD, Hossain IN, Wohlfahrt A, Lee YC. Non-inflammatory causes of pain in patients with rheumatoid arthritis. Curr Rheumatol Rep. 2016;18:30. doi: 10.1007/s11926-016-0581-0. [DOI] [PubMed] [Google Scholar]
- 30.Meeus M, Vervisch S, De Clerck LS, Moorkens G, Hans G, Nijs J. Central sensitization in patients with rheumatoid arthritis: A systematic literature review. Semin Arthritis Rheum. 2012;41:556–67. doi: 10.1016/j.semarthrit.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Michelsen B, Kristianslund EK, Hammer HB, Fagerli KM, Lie E, Wierød A, et al. Discordance between tender and swollen joint count as well as patient’s and evaluator’s global assessment may reduce likelihood of remission in patients with rheumatoid arthritis and psoriatic arthritis: Data from the prospective multicentre NOR-DMARD study. Ann Rheum Dis. 2017;76:708–11. doi: 10.1136/annrheumdis-2016-21028. [DOI] [PubMed] [Google Scholar]