Abstract
Many factors can influence perceptions of successful aging (SA), including social isolation and poor physical health. We hypothesized that social support attenuates the negative effect of plasma D-dimer, a correlate of HIV and aging, on SA. Participants included 230 adults (134 people with HIV; PWH, 96 HIV−), ages 36–65, segregated into age cohorts with up to five yearly visits. Multilevel modeling examined longitudinal within-person associations between D-dimer, social support, and SA. Social support moderated the relationship between D-dimer and SA and was significant among PWH and older individuals (ages 56–65), but not HIV− or younger cohorts. This association was significant only at extreme levels of social support, with significant decreases in social support potentiating the negative impact of D-dimer on SA and significant increases in social support facilitating increased SA. Despite declining health, high social support may improve SA in PWH and older adults, and low support may be especially problematic for older adults.
Keywords: AIDS, Aging, Well-being, Health, Inflammation
INTRODUCTION
Aging has traditionally been viewed as a progressive decline in physical, cognitive, and psychosocial functioning, and aging research has primarily focused on characterizing and identifying ways of ameliorating these declines. Contemporary views of aging have shifted focus from a process of decline to one of growth (Rowe & Kahn, 1997), with considerations of aging as also marked by continued neuroplasticity, neuro-regeneration, and positive psychological traits such as optimism, resilience, wisdom, and increased social engagement (Jeste & Palmer, 2013). This shift from notions of decline and illness to the maintenance and promotion of well-being has led to the concept of “successful aging” (SA).
Definitions of SA classically comprise of components such as low probability of disease and disease-related disability, high cognitive and physical functioning, and active engagement with life (Rowe & Kahn, 1997) and often do not ask older adults themselves of how they feel in their aging procecss. Although physical health and functional abilities play an important role in aging, there is evidence to also suggest that older adults continue to report aging “successfully” despite poor physical health status (Montross et al., 2006; Reichstadt, Sengupta, Depp, Palinkas, & Jeste, 2010) and that traditional definitions of objective SA may be too restrictive (Stewart, Auais, Belanger, & Philips, 2019; Strawbridge, Wallhagen, & Cohen, 2002). Thus, SA may be considered a separate construct from the absence of disease or freedom from disability, and instead focused on self-rated well-being, positive psychological factors, and psychosocial functioning (Depp and Jeste, 2006; Jeste et al., 2012). Cross-sectionally, self-ratings of SA have been associated with higher ratings of physical and social functioning, better emotional health, increased energy/vitality, and greater resiliency (Montross et al., 2006). Longitudinally, SA predicts decreased likelihood of transitioning into long-term care (Nosraty et al., 2017) and mortality independent of disease and disability in older adults (Idler & Benyamini, 1997). Ratings of SA have also been linked to age-related leukocyte telomere length (Huang et al., 2019). For these reasons, subjective SA rates may be an important construct to examine in older adults.
While SA is important for all aging adults, in recent years, interest in understanding and promoting SA among people with HIV (PWH) has been gaining momentum (Rooney et al., 2019; Rueda, Law, & Rourke, 2014; Vance et al., 2019; Wallace et al., 2017). Following the introduction of combination antiretroviral therapy in 1996, many PWH are now approaching older adulthood and have life expectancies approximating those among individuals of the general population (Antiretroviral Therapy Cohort, 2017). It is estimated that the proportion of PWH aged 50 years and older will increase from 28% in 2010 to 73% in the next ten years (Smit et al., 2015). Research has shown that SA is possible among older PWH (Malaspina et al., 2011; Moore et al., 2013; Rubtsova et al., 2019) and that higher perceptions of SA are linked to better health and health-related quality of life (Moore et al., 2018; Moore et al., 2014). Indeed, levels of various positive psychological factors and quality of life (mental and physical) among PWH who rate themselves as aging successfully are comparable to HIV− individuals (Moore et al., 2018).
Although SA has been robustly linked to improved health outcomes in the general population, little is known about the influence of psychosocial variables on the relationship of SA with indicators of health. One psychosocial variable that may contribute to improved SA in the context of poor health is social support. In particular, social support has been estimated to account for a substantial proportion of the variance in satisfaction with life ratings (Gow et al., 2007), has been directly linked to successful aging (Gow, Pattie, Whiteman, Whalley, & Deary, 2007; Howie, Troutman-Jordan, & Newman, 2014), and has positive effects on overall health and longevity in both older adults (Holt-Lunstad, Robles, & Sbarra, 2017; Seeman, Lusignolo, Albert, & Berkman, 2001) and among PWH (Earnshaw, Lang, Lippitt, Jin, & Chaudoir, 2015). Taken together, it is of interest to examine the effect of social support on the relationship between SA and indicators of poor health in the context of HIV infection.
Among the plethora of objective biomarkers of poor health is D-dimer, a fibrin degradation product and marker of coagulation activation and chronic inflammation. We are particularly interested in D-dimer because its circulating concentrations are both age sensitive (Carr, McKinney, & McDonagh, 1989; Harper, Theakston, Ahmed, & Ockelford, 2007) and have been associated with increased mortality both in healthy adult populations (Di Castelnuovo et al., 2013) as well as to PWH (Calmy et al., 2009). Specifically, among a panel of coagulation and inflammatory biomarkers tested in participants of the Strategies for Management of Antiretroviral Therapy (SMART) study (El-Sadr et al., 2006), D-dimer was found to be the most predictive biomarker of overall mortality among PWH (Kuller et al., 2008). Given associations between SA, social support and health, this study was motivated to investigate the moderation of the relationship between SA and D-dimer by social support.
This study utilized a longitudinal, within-person research design, with yearly visits over a period of up to five years, to investigate the influence of social support on the intra-individual relationship between SA and D-dimer. We hypothesized that social support would attenuate the negative effect of D-dimer on SA. Specifically, we hypothesized that, on visits where individuals reported greater social support, D-dimer would have less negative influence on SA. In addition, we aimed to explore whether this moderated effect was driven by HIV and/or age. Therefore, both PWH and HIV− groups, as well as different aged cohorts, regardless of HIV serostatus, were included in the study.
METHODS
Participants
Two hundred and thirty individuals (134 PWH and 96 HIV-seronegative) were drawn from a longitudinal cohort study of aging at the University of California, San Diego (UC San Diego; the Multi-Dimensional Successful Aging Among HIV-Infected Adults study; Moore et al., 2018; Rooney et al., 2019). All participants were between 36 and 65 years of age at baseline and were recruited into age cohorts by decade (i.e., ages 36–45, 46–55, and 56–65 years). Eligibility criteria for participation included the ability to communicate in English and possession of the capacity to provide informed consent. Exclusionary criteria were a history of head injury with loss of consciousness greater than 30 minutes or known neurological complications, seizure disorder, stroke with neurological or neuropsychiatric sequelae, psychotic disorder, non-HIV neurological disorder (e.g., dementia), the presence of a severe learning disability (e.g., Wide Range Achievement Test, Fourth Edition Reading score <70), or positive urine toxicology for illicit substances (excluding marijuana) on the day of testing. Informed consent was obtained from all individual participants included in the study and the study was approved by the UC San Diego Institutional Review Board.
Measures
In addition to standard sociodemographic and psychiatric information at baseline, participants completed the following measures at each yearly study visit:
Self-Rated Successful Aging (SRSA).
The degree to which participants reported perceiving themselves as aging successfully was assessed using a single self-report question on a scale of Self-Rated Successful Aging. Participants responded to the question, “Using your own definition, where would you rate yourself in terms of “successful aging?” on a 10-point Likert scale with anchors ranging from 1 – “Least Successful” to 10- “Most successful.” This single-item questionnaire has been shown to be associated with having more close friends, greater resilience, and better every day functioning and health-related quality of life (Montross et al., 2006). In fact, in a sample of participants classified as aging successfully based on published, researcher-defined criteria of SA (Phelan & Larson, 2002), 92% of individuals rated themselves at least a 7/10 on the SRSA (Montross et al., 2006). This researcher-defined SA criteria was multi-dimentional and encompassed the following objective and subjective ratings of SA: (1) independent living, (2) positive adaptation, (3) active engagement with life, (4) mastery/growth), (5) and life satisfaction/well-being, (6) freedom from disability, (7) and absence of disease. The SRSA item is also correlated with a separate aging measure (“I am aging well”; Montross et al., 2006).
Social Support.
Social support was measured using the 4-item Social Interaction subscale of the Duke Social Support Index (DSSI; Koenig et al., 1993). The DSSI is a well-validated measure that has been used extensively in both cross-sectional and longitudinal studies among aging populations and in the context of chronic illness. Participants responded to questions assessing the number of persons in their area they could depend on or that they felt close to (possible 1 to 3 points), as well as the frequency of their engagement in social interactions over the past week (possible 1 to 8 points for each of the remaining three items). These four items of the Social Interaction Subscale were summed to yield a score ranging from 4 to 27, with higher numbers indicating greater social support.
D-dimer.
Peripheral blood samples were collected by routine phlebotomy and D-dimer assays measured by immunoturbidimetric assay (SEKISUI Diagnostics; Lexington, MA) in duplicate in ethylenediaminetetraacetic acid (EDTA)-treated plasma. Measurements were repeated if the coefficient of variation was greater than 20% or if the measurement was greater than four standard deviations from the mean. Higher values are traditionally associated with increased degree of intravascular coagulation and fibrinolysis as well as increased age (Carr et al., 1989; Harper et al., 2007).
Data Analysis
As the data were longitudinal and collected across multiple years, the data conformed to a multilevel structure with observations nested within individuals. Thus, multilevel modeling was used to examine the associations between SA, social support, and D-dimer. Using multilevel models, self-rated SA was regressed on social support, D-dimer, and their interaction to obtain within-person estimates of effects. Relevant covariate controls were included at both within-person (age and time) and the between-person (age, sex, ethnicity, education, and employment) levels. To further examine effects by HIV serostatus and age cohort, post-hoc multigroup analyses were carried out. Additionally, the Johnson-Neyman procedure (Palmer O. Johnson & Fay, 1950; Palmer Oliver Johnson & Neyman, 1936) was used to determine the specific regions at which social support significantly moderated the relationship between D-dimer and SA.
All analyses were conducted using Mplus v7.4 with robust maximum likelihood estimation (MLR) to produce valid statistical inferences in the presence of non-normality of data and data that are missing at random (Muthén & Muthén, 1998–2015).
RESULTS
Participants had up to five yearly visits (M= 1.7, SD= 1.3). Descriptive statistics of the sample and summaries of study variables at baseline are presented separately by HIV status in Table 1. Table 1 additionally presents the results of tests examining differences between PWH and HIV− participants, where applicable. Paired samples t-tests and Wilcoxon tests were used for continuous variables and Chi-square tests were used for categorical variables. Per study design, PWH and HIV− participants were comparable in age (on average M= 51.0, SD= 8.5 and M= 51.1, SD= 7.6 years, respectively). They were also comparable by ethnicity, with the majority of participants being White, non-Hispanic (PWH= 53%; HIV−= 68%). PWH and HIV− persons differed on other demographic characteristics, with greater prevalence of males (75% vs. 68%; p< .001), fewer years of education (14.4 vs. 15.0 years; p= .04), and less employment (32% vs. 77%; p< .001) in PWH. There were no differences between groups on D-dimer concentrations or total score on the DSSI, but HIV− individuals reported higher SRSA than PWH.
Table 1.
Baseline Participant Characteristics by HIV Serostatus
| HIV+ (n=134) | HIV− (n=96) | p | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 51.0 (8.5) | 51.1 (7.6) | .45 |
| Sex (male) | 105 (75) | 67 (68) | <.001 |
| Race/Ethnicity | -- | -- | -- |
| White | 74 (53) | 67 (68) | .78 |
| Black | 23 (16) | 13 (13) | -- |
| Hispanic | 28 (20) | 17 (17) | -- |
| Asian | 2 (2) | 0 (0) | -- |
| Other | 8 (6) | 1 (1) | -- |
| Education (years) | 14.4 (2.7) | 15.0 (2.3) | .04 |
| Employment | 45 (32) | 76 (77) | <.001 |
| HIV Characteristics | |||
| Historical AIDS diagnosis | 75 (53) | -- | -- |
| Detectable viral load | 10 (7) | -- | -- |
| Current CD4 count (cells/mm3) | 666 [613–717] | -- | -- |
| Nadir CD4 (cells/mm3) | 257 [232–311] | -- | -- |
| CD4/CD8 Ratio | .9 [.8–1.0] | -- | -- |
| Estimated duration living with HIV (months) | 14.4 (9.9) | -- | -- |
| On Antiretroviral Therapy | 135 (96) | -- | -- |
| Log D-dimer (µg/L) | 576.9 (432.1) | 519.2 (310.4) | .86 |
| Duke Social Support Index (DSSI) | 8.2 (1.9) | 8.5 (1.7) | .13 |
| Self-Rated Successful Aging (SA) | 7.2 (2.2) | 7.9 (1.5) | <.001 |
Values are presented in mean (SD) or N (%);
DSSI score range is between 4 and 27 (with higher numbers indicate greater social support);
SA score range is between 1 (least successful) and 10 (most successful)
The first column in Table 2 presents the results of multilevel models that evaluated the effects of social support and D-dimer on measures of SA, controlling for appropriate covariates. Within-individual covariates (age and visit number) were chosen based on demographic variables that changed from visit to visit. Between-level covariates (age, sex, ethnicity, education, and employment status) were chosen based on their association with the dependent variable (i.e., SRSA). Social support moderated the relationship between D-dimer and SRSA (β= 2.06, p= .009). This was such that, when a participant reported increases in social support above their average levels across all study visits, the negative association between SA and D-dimer was attenuated. In planned follow-up analyses by HIV serostatus, the moderation of the effect of D-dimer on SRSA by social support was significant only among PWH (β = 2.11, p= .05; Table 2). By age cohort, the moderation effect on SRSA was significant only among older individuals (ages 56–65: β = 3.32, p< .001; Table 3).
Table 2.
Perceptions of Aging on D-dimer and DSSI by total sample and HIV serostatus
| Total Sample | HIV+ | HIV− | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | B | SE | p | |
| Within Level | |||||||||
| D-dimer | −.40 | .84 | .63 | −1.05 | 1.09 | .34 | .69 | 1.21 | .57 |
| DSSI total | .08 | .11 | .51 | .04 | .15 | .81 | .11 | .14 | .46 |
| D-dimer*DSSI | 2.06 | .79 | .009 | 2.11 | 1.07 | .05 | 5.08 | 2.87 | .08 |
| Age | −.50 | .18 | .006 | −.69 | .26 | .007 | −.12 | .25 | .65 |
| Visit | .04 | .02 | .007 | .07 | .02 | .002 | .00 | .02 | .99 |
| Between Level | |||||||||
| Age | .01 | .02 | .34 | .03 | .02 | .18 | −.01 | .02 | .67 |
| Sex | −.19 | .27 | .47 | −.06 | .48 | .91 | −.48 | .30 | .11 |
| Ethnicity | .62 | .27 | .02 | .87 | .43 | .04 | .47 | .28 | .10 |
| Education | −.01 | .05 | .90 | −.04 | .08 | .65 | .03 | .06 | .62 |
| Employment | .77 | .24 | .001 | .68 | .34 | .05 | .65 | .33 | .05 |
DSSI = Duke Social Support Index
Table 3.
Perception of aging on D-dimer and DSSI by age cohort
| 36–45 | 46–55 | 56–65 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | B | SE | p | |
| Within Level | |||||||||
| D-dimer | −1.78 | 1.93 | .36 | 1.13 | 1.46 | .44 | −.26 | .88 | .77 |
| DSSI total | .06 | .15 | .70 | −.34 | .17 | .04 | .58 | .18 | .001 |
| D-dimer*DSSI | 1.43 | 3.68 | .70 | 1.34 | 2.00 | .50 | 3.32 | .51 | <.001 |
| Age | −1.19 | .41 | .003 | .02 | .32 | .96 | −.44 | .32 | .17 |
| Visit | .09 | .03 | .01 | .00 | .03 | .95 | .05 | .03 | .10 |
| Between Level | |||||||||
| Age | −.01 | .06 | .92 | −.03 | .09 | .75 | .08 | .07 | .23 |
| Sex | −.39 | .37 | .29 | −.65 | .52 | .21 | .49 | .47 | .29 |
| Ethnicity | .79 | .40 | .05 | .42 | .47 | .37 | .35 | .53 | .50 |
| Education | −.10 | .06 | .10 | .04 | .10 | .66 | .08 | .11 | .46 |
| Employment | 1.09 | .41 | .008 | .90 | .41 | .03 | .39 | .39 | .31 |
DSSI = Duke Social Support Index
The Johnson-Neyman procedure was used to determine regions along the range of social support with significant moderation in the relationship between D-dimer and SA. Results for the combined (PWH and HIV) sample are presented in Figure 1 where the DSSI mean was 8.3 and standard deviation was 1.8. D-dimer was significantly associated with SA at low levels of social support (1.6 standard deviation units below the combined sample mean, corresponding to a raw total score of 5.5) and at high levels of social support (1.1 standard deviation units above the mean, corresponding to a raw total score of 10.3). In particular, at low levels of social support, the relationship between SA and D-dimer was negative, such that increases in D-dimer level above an individual’s mean scores across visits were associated with lower ratings of SA. Conversely, at high levels of social support, elevated levels of D-dimer were associated with increases in SA.
Figure 1.
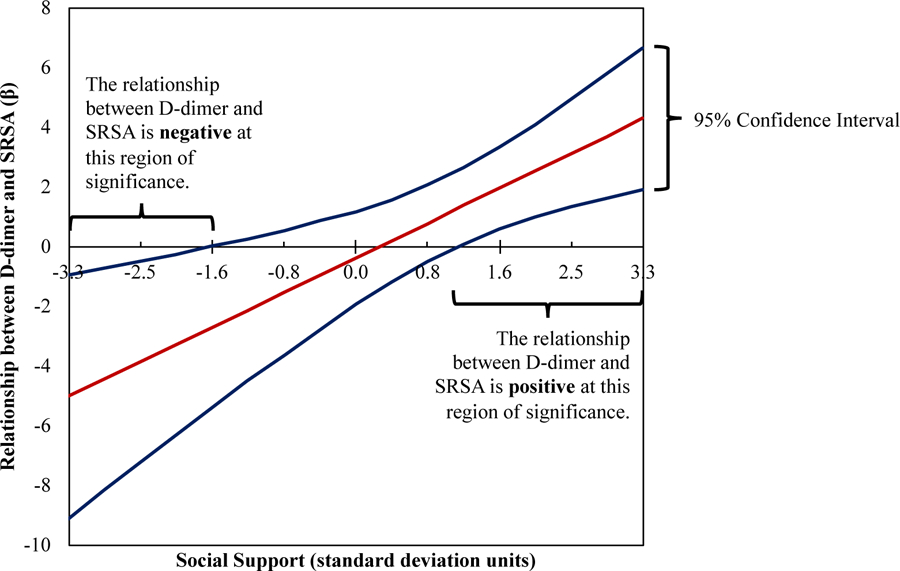
Social support moderates the relationship between D-dimer and SRSA at significant regions when the blue confidence lines both cross the x-intercept. In PWH and HIV−, at high levels of social support (obtaining a score of 10.30 or more on the Duke Social Support Index, DSSI; score range 4–27), D-dimer and SRSA have a positive relationship. At low levels of social support (obtaining a score of 5.49 or less on the DSSI), D-dimer and SRSA have a negative relationship (interaction p= .009). The within level analysis was corrected for age and number of visits.
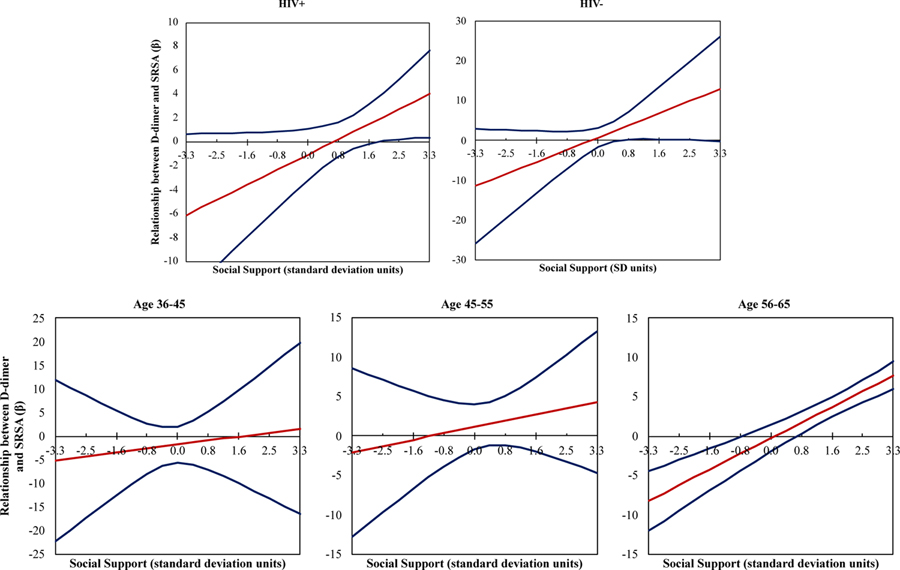
The same method of identifying the region of significance was utilized among PWH (DSSI M=8.2, SD= 1.9; Figure 2 top). Similar to in a combined sample, the relationship between SA and D-dimer was significant and positive at high levels of social support (1.6 standard deviation units above the mean, corresponding to a raw total score of 11.1), such that increases in D-dimer levels were associated with increases in SA. The negative association between D-dimer and SA at low levels of social support was not observed in the PWH only sample.
Figure 2.
Social support moderates the relationship between D-dimer and SRSA in PWH and older adults. Within PWH, at high levels of social support (obtaining a score of 11.24 or more on the Duke Social Support Index, DSSI), D-dimer and SRSA have a positive relationship (interaction p= .05). Within older adults (age 56–65), at high levels of social support (obtaining a score of 9.62 or more on the DSSI), D-dimer and SRSA have a positive relationship and at low levels of social support (obtaining a score of 6.68 or less on the DSSI), D-dimer and SRSA have a negative relationship (interaction p< .001). The within level analysis was corrected for age and number of visits.
The region of significance analyses was also applied to the combined PWH and HIV− sample by age cohorts (Figure 2 bottom). Among the oldest individuals (aged 56–65 years at baseline; DSSI M= 8.4, SD= 1.8), the relationship between SA and D-dimer was significant at both low levels of social support (0.8 standard deviation units below the mean, corresponding to a raw total score of 6.9) and at high levels of social support (0.77 standard deviation units above the mean, corresponding to a raw total score of 9.7), mirroring findings among the combined PWH and HIV− sample. Among these older individuals (aged 56–65 years at baseline), at low levels of social support, increases in D-dimer levels were associated with lower ratings of SA while, at high levels of social support, increases in D-dimer above an individual’s mean were associated with increases in SA. Social support did not moderate the relationship between D-dimer and SA in the younger cohorts.
DISCUSSION
SA has been associated with physical health; however, little is known about the influence of social support on the relationship of SA with objective biomarkers of health, such as D-dimer. In the context of a longitudinal study of aging and HIV, we examined the moderation of within-person relationships between self-reported ratings of SA and D-dimer by social support. Consistent with our hypotheses, we found social support to moderate within-person relationships between SA and D-dimer, with increases in social support above an individual’s average level across all study visits attenuating the negative impact of D-dimer on SA. In follow-up analyses, we found nuance in the ways in which social support may act to influence this intra-individual relationship. In particular, we found that the influence of social support was conditional upon the levels of support, with significant decreases in social support potentiating the negative impact of D-dimer on SA and significant increases in social support facilitating increased SA, even in the context of high D-dimer. These bifurcated effects were largely seen among PWH and among older individuals regardless of HIV serostatus (aged 56 to 65 years old at baseline). Thus, our study findings highlight the importance of recognizing the potential for large fluctuations in social support in the context of HIV and aging to greatly influence SA.
This study’s findings are consistent with a considerable body of literature that documents the negative effects of poor social support on objective and subjective indicators of health (Reblin & Uchino, 2008). Our findings of potentiated negative associations between D-dimer and SA at low levels of social support, primarily among older adults, suggest that low social support may have a more detrimental effect in the context of poor health among older individuals. Unexpectedly, we found that high levels of social support might permit flourishing, even in the context of indications of poor physical health. In persons with high levels of social support, as D-dimer increased, their self-ratings of SA also increased. Upon detailed examination by HIV status and age cohorts, this positive D-dimer-SA relationship seemed to be driven exclusively by PWH and the older age cohort (ages 56–65 at baseline) but not by HIV− group and younger individuals.
These findings are interesting because intuitively, one would predict these more vulnerable populations to continue to feel less successful as they decline physically. Especially in the context of living with HIV, where it would be expected that greater susceptibility to physical illness, accelerated physical decline, social stigma, discrimination, and survivors’ guilt might lead persons to feel less successful as they physically become less well (Emlet, 2016; Khoury et al., 2017; Meanley et al., 2019). Yet, the data revealed a strong reverse relationship. This may be due to a survivorship effect, where persons living with HIV into their older age may also have more positive perspectives on aging. Indeed, it has been established that PWH do place greater value on the quality of life and life satisfaction rather than physical health and chronological age (Fazeli, Montoya, McDavid, & Moore, 2018). Perhaps this is especially true among individuals with large social networks that may help reinforce the importance of life quality. The literature also supports the idea that PWH report similar levels of optimism and personal mastery as HIV− individuals (Moore et al., 2013) and resilience may play a key role in the aging process for this population (Fumaz et al., 2015). These specific positive psychological factors may be especially prevalent in PWH with high levels of social support as these characteristics may be factors that help to maintains a positive outlook on aging despite disease progression. Future studies are needed to determine potential positive psychosocial factors (i.e., resilience, grit) driving the D-Dimer/SA relationship in the context of high levels of social support.
There is previous evidence that supports the positive relationship between physical decline and SA in older age. In a case-controlled study of SA at UC San Diego [Successful Aging Evaluation (SAGE) study], researchers found that, in a large community-based sample of individuals across the entire adult lifespan selected using random digit dialing, older age was associated with higher SA despite decreased physical health and cognitive function (Jeste et al., 2013). In the current study, this relationship did not hold at low levels of social support. In fact, when compared to younger individuals, older adults with poor support viewed their aging to be less successful as a function of poorer health. In addition, our moderation results were consistent with a separate, large, cross-sectional study of older adults by our group, where Moore et al. (2015) used moderated mediation analyses to examine the role of perceived stress on physical health functioning and SA. The authors reported that, when compared to low social support, perceived stress was less influential in mediating the relationship between physical functioning and SA in a high social support context. Contrary to that finding, we observed not only a change in magnitude, but also in the direction of association between an indicator of physical health and SA at different levels of social support.
In considering a mechanism that links D-dimer and SA at the expected direction (increased D-dimer is associated with decreased SA, as observed in older adults (age 56–65) with low social support), we must accept that a component of SA must comprise of physical health. Although we argue that SA is possible outside of physical illness, it cannot be ignored that plasma D-dimer levels, a physical indicator of coagulation, inflammation, and older age, reflects a physical state of the individual. Perhaps in the context of poor social support, individuals may have less resources to buffer against the influence of poor health on SA, and therefore rate their aging process more based on their physical health status. On the contrary, in the context of higher social support in both PWH and older adults, we found D-dimer demonstrated to have a less negative impact on SA. In these circumstances, perhaps perceptions of SA is considered to be less physical and include more aspects of positive psychological factors. Furture investigations into how individual groups define SA is needed.
The present study builds upon the foundations of previous research on psychosocial aspects of SA in PWH and aging. To our knowledge this is the first study to investigate the longitudinal effects of social support on the relationship between SA and D-dimer, a biomarker associated with HIV and aging. However, there are limitations to our work. First, it should be noted that both the dependent variable (SA) and moderator (social support) are potentially affected by biases inherent to self-report. Additionally, our measures of SA and social support were abbreviated in length (1 item for SA and 4 items for Duke Social Support Index) and may not capture the range of each construct. Specifically, the social support index is limited in its assessment of the frequency of social interactions and is not inclusive of quality of those interactions. For these reasons, more comprehensive assessments of both these constructs will be beneficial for future research. Second, although D-dimer is an important biomarker associated with HIV (Kuller et al., 2008) and aging (Colombo, Furlan, & Colombo, 2014), we are well aware of its lack of specificity to general health in this population. Elevated D-dimer is reportedly associated with an array of other conditions including Hepatitis B/C, higher plasma HIV RNA levels, being off ART, increased levels of CRP, IL-6, and cystatin C, higher CD4+ counts, and lower high-density lipoprotein cholesterol (Borges et al., 2014). It is also a protein that fluctuates with various biological states and is not specific to HIV or age. In addition, we know that chronic inflammation and immune activation typically observed in older adults can be observed much earlier in PWH, and this concept of “inflammaging” is critical to address as it significantly affect quality of life (De Francesco et al., 2019; Nasi et al., 2017). Therefore, inclusion of additional chronic-illness, inflammatory, and age-related biomarkers, as well as HIV disease characteristics as indicators of physical health may strengthen findings and should be considered for future directions.
In summary, our findings suggest that high levels of social support may be protective against the deleterious effects of D-dimer on SA and low levels of social support may be a risk factor for greater impact of D-dimer on SA; furthermore PWH and older adults may be particularly sensitive to the extremes of social support. Our findings highlight the complex and potentially non-linear influence of social support on the relationships between physical health and subjective ratings of SA among PWH and aging adults. Empirically supported individual and group interventions for older adults, such as telephone support services and support groups, may promote social support, decrease isolation, and allow individuals to achieve SA even in the face of poor physical health (Gardiner, Geldenhuys, & Gott, 2018).
Acknowledgments
• All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (UCSD Human Research Protections Program IRB00000355, Project #120244) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
• The authors declare that they have no conflict of interest.
COMPLIANCE WITH ETHICAL STANDARDS
• Informed consent was obtained from all individual participants included in the study.
REFERENCES
- Antiretroviral Therapy Cohort, C. (2017). Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV, 4(8), e349–e356. doi: 10.1016/S2352-3018(17)30066-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmy A, Gayet-Ageron A, Montecucco F, Nguyen A, Mach F, Burger F, … Group, S. S. (2009). HIV increases markers of cardiovascular risk: results from a randomized, treatment interruption trial. AIDS, 23(8), 929–939. [DOI] [PubMed] [Google Scholar]
- Carr JM, McKinney M, & McDonagh J (1989). Diagnosis of disseminated intravascular coagulation. Role of D-dimer. Am J Clin Pathol, 91(3), 280–287. doi: 10.1093/ajcp/91.3.280 [DOI] [PubMed] [Google Scholar]
- Colombo G, Furlan L, & Colombo G (2014). Age-adjusted D-dimer cutoff levels and pulmonary embolism. JAMA, 312(5), 557. doi: 10.1001/jama.2014.7604 [DOI] [PubMed] [Google Scholar]
- De Francesco D, Wit FW, Burkle A, Oehlke S, Kootstra NA, Winston A, … the Co-morBidity in Relation to, A. C. (2019). Do people living with HIV experience greater age advancement than their HIV-negative counterparts? AIDS, 33(2), 259–268. doi: 10.1097/QAD.0000000000002063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castelnuovo A, de Curtis A, Costanzo S, Persichillo M, Olivieri M, Zito F, … Investigators, M. -S. P. (2013). Association of D-dimer levels with all-cause mortality in a healthy adult population: findings from the MOLI-SANI study. Haematologica, 98(9), 1476–1480. doi: 10.3324/haematol.2012.083410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw VA, Lang SM, Lippitt M, Jin H, & Chaudoir SR (2015). HIV stigma and physical health symptoms: do social support, adaptive coping, and/or identity centrality act as resilience resources? Aids and Behavior, 19(1), 41–49. doi: 10.1007/s10461-014-0758-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, … Rappoport C (2006). CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med, 355(22), 2283–2296. doi: 10.1056/NEJMoa062360 [DOI] [PubMed] [Google Scholar]
- Emlet CA (2016). Social, Economic, and Health Disparities Among LGBT Older Adults. Generations, 40(2), 16–22. [PMC free article] [PubMed] [Google Scholar]
- Fumaz CR, Ayestaran A, Perez-Alvarez N, Munoz-Moreno JA, Molto J, Ferrer MJ, & Clotet B (2015). Resilience, ageing, and quality of life in long-term diagnosed HIV-infected patients. Aids Care-Psychological and Socio-Medical Aspects of Aids/Hiv, 27(11), 1396–1403. doi: 10.1080/09540121.2015.1114989 [DOI] [PubMed] [Google Scholar]
- Gardiner C, Geldenhuys G, & Gott M (2018). Interventions to reduce social isolation and loneliness among older people: an integrative review. Health Soc Care Community, 26(2), 147–157. doi: 10.1111/hsc.12367 [DOI] [PubMed] [Google Scholar]
- Gow AJ, Pattie A, Whiteman MC, Whalley LJ, & Deary IJ (2007). Social support and successful aging: Investigating the relationships between lifetime cognitive change and life satisfaction. Journal of Individual Differences, 28(3), 103–115. [Google Scholar]
- Harper PL, Theakston E, Ahmed J, & Ockelford P (2007). D-dimer concentration increases with age reducing the clinical value of the D-dimer assay in the elderly. Intern Med J, 37(9), 607–613. doi: 10.1111/j.1445-5994.2007.01388.x [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Robles TF, & Sbarra DA (2017). Advancing social connection as a public health priority in the United States. Am Psychol, 72(6), 517–530. doi: 10.1037/amp0000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie LO, Troutman-Jordan M, & Newman AM (2014). Social support and successful aging in assisted living residents. Educational gerontology, 40(1), 61–70. [Google Scholar]
- Huang Y, Yim OS, Lai PS, Yu R, Chew SH, Gwee X, … Gouin JP (2019). Successful aging, cognitive function, socioeconomic status, and leukocyte telomere length. Psychoneuroendocrinology, 103, 180–187. doi: 10.1016/j.psyneuen.2019.01.015 [DOI] [PubMed] [Google Scholar]
- Idler EL, & Benyamini Y (1997). Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav, 38(1), 21–37. [PubMed] [Google Scholar]
- Johnson PO, & Fay LC (1950). The Johnson-Neyman technique, its theory and application. Psychometrika, 15(4), 349–367. doi: 10.1007/bf02288864 [DOI] [PubMed] [Google Scholar]
- Johnson PO, & Neyman J (1936). Tests of certain linear hypotheses and their application to some educational problems. Statistical research memoirs.
- Khoury AL, Morey MC, Wong TC, McNeil DL, Humphries B, Frankey K, … McKellar MS (2017). Diminished physical function in older HIV-infected adults in the Southeastern U.S. despite successful antiretroviral therapy. PLoS One, 12(6), e0179874. doi: 10.1371/journal.pone.0179874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, … Group, I. S. S. (2008). Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med, 5(10), e203. doi: 10.1371/journal.pmed.0050203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina L, Woods SP, Moore DJ, Depp C, Letendre SL, Jeste D, … Group, H. I. V. N. R. P. (2011). Successful cognitive aging in persons living with HIV infection. Journal of Neurovirology, 17(1), 110–119. doi: 10.1007/s13365-010-0008-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meanley SP, Stall RD, Hawk ME, Surkan PJ, Shoptaw SJ, Matthews DD, … Plankey MW (2019). Multifactorial discrimination, discrimination salience, and prevalent experiences of internalized homophobia in middle-aged and older MSM. Aging Ment Health, 1–8. doi: 10.1080/13607863.2019.1594161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montross LP, Depp C, Daly J, Reichstadt J, Golshan S, Moore D, … Jeste DV (2006). Correlates of self-rated successful aging among community-dwelling older adults. Am J Geriatr Psychiatry, 14(1), 43–51. doi: 10.1097/01.JGP.0000192489.43179.31 [DOI] [PubMed] [Google Scholar]
- Moore DJ, Fazeli PL, Moore RC, Woods SP, Letendre SL, Jeste DV, … Program, H. I. V. N. R. (2018). Positive Psychological Factors are Linked to Successful Cognitive Aging Among Older Persons Living with HIV/AIDS. Aids and Behavior, 22(5), 1551–1561. doi: 10.1007/s10461-017-2001-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Fazeli PL, Jeste DV, Moore DJ, Grant I, Woods SP, & Group, H. I. V. N. R. P. (2014). Successful cognitive aging and health-related quality of life in younger and older adults infected with HIV. Aids and Behavior, 18(6), 1186–1197. doi: 10.1007/s10461-014-0743-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Moore DJ, Thompson WK, Vahia IV, Grant I, & Jeste DV (2013). A case-controlled study of successful aging in older HIV-infected adults. J Clin Psychiatry, 74(5), e417–423. doi: 10.4088/JCP.12m08100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén B, & Muthén L (1998–2015). Mplus User’s Guide. Seventh Edition. In Muthén M (Ed.). [Google Scholar]
- Nasi M, De Biasi S, Gibellini L, Bianchini E, Pecorini S, Bacca V, … Cossarizza A (2017). Ageing and inflammation in patients with HIV infection. Clin Exp Immunol, 187(1), 44–52. doi: 10.1111/cei.12814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan EA, & Larson EB (2002). “Successful aging”--where next? Journal of the American Geriatrics Society, 50(7), 1306–1308. doi: 10.1046/j.1532-5415.2002.t01-1-50324.x [DOI] [PubMed] [Google Scholar]
- Reichstadt J, Sengupta G, Depp CA, Palinkas LA, & Jeste DV (2010). Older adults’ perspectives on successful aging: qualitative interviews. Am J Geriatr Psychiatry, 18(7), 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney AS, Moore RC, Paolillo EW, Gouaux B, Umlauf A, Letendre SL, … Program, H. I. V. N. R. (2019). Depression and aging with HIV: Associations with health-related quality of life and positive psychological factors. J Affect Disord, 251, 1–7. doi: 10.1016/j.jad.2019.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JW, & Kahn RL (1997). Successful aging. Gerontologist, 37(4), 433–440. [DOI] [PubMed] [Google Scholar]
- Rubtsova AA, Wingood GM, Ofotokun I, Gustafson D, Vance DE, Sharma A, … Holstad M (2019). Prevalence and Correlates of Self-Rated Successful Aging Among Older Women Living With HIV. J Acquir Immune Defic Syndr, 82 Suppl 2, S162–S169. doi: 10.1097/QAI.0000000000002175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda S, Law S, & Rourke SB (2014). Psychosocial, mental health, and behavioral issues of aging with HIV. Curr Opin HIV AIDS, 9(4), 325–331. doi: 10.1097/COH.0000000000000071 [DOI] [PubMed] [Google Scholar]
- Seeman TE, Lusignolo TM, Albert M, & Berkman L (2001). Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol, 20(4), 243–255. [DOI] [PubMed] [Google Scholar]
- Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem A, … cohort A. o. (2015). Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis, 15(7), 810–818. doi: 10.1016/S1473-3099(15)00056-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Auais M, Belanger E, & Philips SP (2019). Comparison of self-rated and objective successful ageing in an international cohort. Ageing & Society, 39(7), 1317–1334. [Google Scholar]
- Strawbridge WJ, Wallhagen MI, & Cohen RD (2002). Successful aging and well-being: self-rated compared with Rowe and Kahn. Gerontologist, 42(6), 727–733. doi: 10.1093/geront/42.6.727 [DOI] [PubMed] [Google Scholar]
- Vance DE, Blake BJ, Brennan-Ing M, DeMarco RF, Fazeli PL, & Relf MV (2019). Revisiting Successful Aging With HIV Through a Revised Biopsychosocial Model: An Update of the Literature. J Assoc Nurses AIDS Care, 30(1), 5–14. doi: 10.1097/JNC.0000000000000029 [DOI] [PubMed] [Google Scholar]
- Wallace LMK, Ferrara M, Brothers TD, Garlassi S, Kirkland SA, Theou O, … Guaraldi G (2017). Lower Frailty Is Associated with Successful Cognitive Aging Among Older Adults with HIV. Aids Research and Human Retroviruses, 33(2), 157–163. doi: 10.1089/aid.2016.0189 [DOI] [PMC free article] [PubMed] [Google Scholar]