Abstract
The development of drug addiction is associated with functional adaptations within the reward circuitry, within which the nucleus accumbens (NAc) is anatomically positioned as an interface between motivational salience and behavioral output. The functional output of NAc is profoundly altered after exposure to drugs of abuse, and some of the functional changes continue to evolve during drug abstinence, contributing to numerous emotional and motivational alterations related drug taking, seeking, and relapse. As in most brain regions, the functional output of NAc is critically dependent on the dynamic interaction between excitation and inhibition. One of the most prominent sources of inhibition within the NAc arises from fast-spiking interneurons (FSIs). Each NAc FSI innervates hundreds of principal neurons, and orchestrates population activity through its powerful and sustained feedforward inhibition. While the role of NAc FSIs in the context of drug addiction remains poorly understood, emerging evidence suggests that FSIs and FSI-mediated local circuits are key targets for drugs of abuse to tilt the functional output of NAc toward a motivational state favoring drug seeking and relapse. In this review, we discuss recent findings and our conceptualization about NAc FSI-mediated regulation of motivated and cocaine-induced behaviors. We hope that the conceptual framework proposed in this review may provide a useful guidance for ongoing and future studies to determine how FSIs influence the function of NAc and related reward circuits, ultimately leading to addictive behaviors.
Keywords: fast-spiking interneuron, cocaine, addiction, nucleus accumbens
Introduction
Drug addiction is a chronic brain disorder, characterized by a series of emotional and motivational states that drives compulsive drug craving, seeking, and taking. These addiction-related emotional and motivational states are thought to be partially mediated by alterations in the functional output of the nucleus accumbens (NAc) induced by repeated exposure to drugs of abuse1. As a key node of the dopamine (DA) reward circuit, the NAc receives and integrates dopaminergic afferents from the ventral tegmental area (VTA) and excitatory projections from limbic and paralimbic regions, including the basal lateral amygdala, the ventral subiculum, dorsal hippocampus2,3. In turn, the NAc transmits its functional output through principal medium spiny neurons (MSNs), the projection neurons in the NAc4, to downstream targets of the reward circuit, such as the ventral pallidum, VTA, and hypothalamus2,5. Previous studies have identified many critical adaptations in the membrane excitability and synaptic function of MSNs that contribute to the development and maintenance of drug-induced behaviors1,4,6. However, MSNs are only one major cell type embedded within the complex and intricate local circuit network of the NAc, through which their functional outputs are dynamically regulated by interneurons7. Although historically understudied, there is now increasing evidence indicating that fast-spiking interneurons (FSIs) represent a prominent interneuron subtype that regulates the activity of NAc MSNs in the development and maintenance of addiction-related behaviors.
NAc FSIs are medium-sized GABAergic neurons, which receive excitatory inputs from the same brain regions that project to MSNs, and form monosynaptic contacts with MSNs8–12. Through these interconnected circuits, FSIs fine tune the functional output of NAc MSNs by gating the initiation of action potential firing, regulating the pattern of action potential firing, as well as balancing synaptic excitation9,13,14. It has recently been recognized that FSIs and FSI-mediated regulation of NAc MSNs are also targeted by drugs of abuse, through which the overall functional output of NAc can be tilted. While related studies remain far from abundant and mostly focused on psychostimulants, FSIs are fast emerging as a key neuronal subtype bearing important circuit mechanisms underlying drug addiction. In this review, we hope to provide a timely summary of recent findings about cocaine-induced adaptations in NAc FSIs and formulate a conceptual framework to understand how FSIs influence drug-induced behaviors.
Physiology and morphology of NAc FSIs
Physiology
There are two major subtypes of inhibitory interneurons in the NAc, each of which constitute 1–2% of NAc neurons, and can be differentiated by their firing properties and the expression of their signature proteins: 1) FSIs that express parvalbumin (PV), and 2) persistently low threshold spiking (PLTS) interneurons that express somatostatin (SOM), neuropeptide Y (NPY), and neuronal nitric oxide synthase (nNOS)7. Other subtypes of interneurons have been discovered in the dorsal striatum7, but it remains unclear if those interneuron subtypes are also present in the NAc. Each interneuron subtype in the NAc is not homogeneous. It was recently discovered that a portion of NAc FSIs uniquely express cannabinoid receptor 1 (CB1)15. These CB1-expressing FSIs largely overlap with PV-expressing FSIs, but not completely, and may thus represent a functionally distinct FSI subpopulation in the NAc. For the purpose of simplicity, we will refer to FSIs as a uniform population hereafter, given the uniformity of their functional properties reported thus far in the literature8–12,15. It is important to note that the NAc can be subdivided into the core and shell subdivisions, each influencing behavior differently3. While the general distribution of FSIs appears to be similar in the core and shell10,15, studies comparing the biochemical and bihysical properties FSIs between the core versus shell are lacking, and therefore will not be differentiated in the following discussions.
One of the fundamental electrophysiological features of FSIs is their sustained, high action potential firing frequencies, which can exceed 200 Hz upon strong excitatory inputs7. Such high-frequency firing equips FSIs with a capacity to provide a powerful and sustained blanket of inhibition to principal neurons16. In addition, striatal and NAc FSIs display bursting firings in response to relatively weak excitatory inputs15,17, with the bursting pattern determined by FSI’s intrinsic membrane properties18, likely the unique combination of potassium and sodium channels19,20 (for review see ref. 13). Such firing patterns may provide temporally defined windows of inhibition to control the activation timing of principal neurons. Another unique electrophysiological property of FSIs is that they are electrically coonected by gap junctions15. These gap junctions allow FSIs to synchronize their activities with one another under various conditions21, and empower FSIs to regulate and synchronize large MSN ensembles. This unique property, common to FSIs throughout the brain13, may serve as a cellular mechanism in generating large-scale rhythmic activities (see below)22–24.
Morphology
Morphologically, striatal and NAc FSIs possess a highly branched, but compact dendritic arbors, usually 200–300-μm in size17,25. In addition, their dendrites are aspiny—i.e., lacking spines. Therefore, unlike MSNs, synapses on FSIs lack the compartmentalization, resulting in significant functional differences such as a broadened integration and filtering of excitatory inputs26,27 and reduced confinement of biochemical signaling28–30. These properties may enable FSIs to more equally integrate synaptic input from a wide range of sources, while also hindering the plasticity of specific individual synapses. These anatomic features imply that FSIs are built to encode information more broadly, rather than specific details that pertain to individual experiences.
The axonal arbors of striatal and NAc FSIs are also relatively restricted, usually projecting within a diameter of 400–600 μm17. These axonal arbors, however, are extremely dense, indicating that each FSI innervates a large number of MSNs within its local vicinity. As such, FSIs may control large ensembles of MSNs that are relatively spatially compact and functionally synchronous. Interestingly, the axonal arbors of FSIs typically extend beyond their dendrites, suggesting an anatomical possibility that FSIs deliver inhibition to MSNs that do not necessarily receive inputs from the same upstream excitatory inputs. As such, FSIs may coordinate competitive signaling from different upstream inputs conveying differing information. Taken together, these distinct physiological and morphological properties confer FSIs with unique abilities to regulate NAc MSNs, and fine tune their role in motivated and addictive behaviors.
Synaptic connectivity of NAc FSIs
FSIs are the main source of feedforward inhibition to nearby NAc MSNs8–10. Individual FSIs deliver unitary IPSCs to postsynaptic MSNs with amplitudes often beyond 1000 pA8,9. This synaptic strength renders FSI-mediated inhibition of MSNs exceedingly stronger than the inhibition arising from PLTS internurons10, as well as the lateral inhibition between MSNs8. Such a strong FSI-mediated inhibition results, in part, from the unique subcellular connectivity of FSI-to-MSN synapses. Electrophysiological studies show that FSI-MSN synaptic transmission exhibits a short delay of onset and fast activation kinetics8, suggesting that FSIs synapse on the proximal dendritic and somatic domains of MSNs, which is consistent with results from electron microscopic studies12. This proximal innervation partially immunes FSI-generated IPSCs from heavy dendritic filtering. It also positions FSI-mediated inhibition to effectively control the spiking output of MSNs rather than just suppressing excitatory inputs that arrive at distal dendrites. Such a connectivity of FSIs, in combination with their membrane properties that confer a short latency to fire action potentials upon excitation, allows FSIs to inhibit MSNs even before they fire the first action potential in response to the same wave of excitatory inputs9. This FSI-mediated inhibition is so strong that when even a single FSI is prevented from activation, the adjacent MSNs, which otherwise would not fire action potentials in response to low intensity excitatory inputs, start firing regularly8,9.
Pairwise recording in brain slices reveals that individual NAc FSIs innervate a large number of MSNs, forming functional synapses with approximately 50–60% of MSNs within their dendritic arbors8,9. At the populational level, optogenetic activation of all channelrhodopsin-expressing FSIs elicits IPSCs in dopamine D1- and D2-receptor expressing MSNs with similar intensity and connectivity10. However, it remains to be determined whether individual FSIs exhibit biased innervation of D1 versus D2 MSNs. Nonetheless, with the density of MSNs approximately 150,000 per mm3 in the NAc31, a single FSI is capable of innervating ~15,000 MSNs within its vicinity. This widespread innervation positions NAc FSIs as an organizer of functional ensembles, where they synchronize the activation dynamics of a large population of MSNs.
The FSI-mediated feedforward inhibition is driven by excitatory inputs. NAc FSIs receive monosynaptic excitatory inputs from the same limbic and paralimbic brains regions as MSNs, including the prefrontal cortex (PFC), dorsal and ventral hippocampus (dHPC, vHPC), basolateral amygdala (BLA), paraventricular thalamus (PVT), and ventral tegmental area (VTA)9–12. However, the synaptic strength of each of these inputs is substantially stronger in FSIs compared to neighboring MSNs8–11. In addition, excitatory synapses on NAc FSIs exhibit much faster activation kinetics8, and trigger action potential firing with much shorter delay compared to MSNs9. These synaptic properties are consistent with the unique synaptic arrangement of NAc FSIs. Specifically, FSIs in the striatum receive many synaptic contacts from a small number of individual afferent neurons along their proximal somatodendritic domains32. Similar synaptic arrangement is observed on NAc FSIs12. This contrasts strikingly with MSNs, which receive a few synaptic contacts from a large number of individual afferent neurons along the distal dendrites33. As such, it is unlikely that FSIs operate as input integrators by sampling diverse inputs and converting them into a single output, as MSNs are thought to do. Rather, FSIs more likely serve as a gatekeeper; upon receiving a specific input, they deliver powerful inhibition and effectively gate the activation of ensembles of MSNs. The dynamic ‘opening’ and ‘closing’ of these inhibitory gates may orchestrate the activation pattern of different MSN ensembles throughout the NAc, ultimately sculpting behaviors. With this in mind, we will discuss in the following sections how NAc FSIs may contribute to motivated behaviors and how drug-induced adaptations in FSIs may derail these motivated behaviors favoring drug seeking and drug taking behaviors.
Role of NAc FSIs in motivated behaviors
The NAc has been implicated in several aspects of motivated behaviors, including cue-associated drug craving and seeking3,5,34–37. Given the general inhibitory role of FSIs at the cellular level, it is reasonable to speculate that activation of FSIs suppresses such motivated behaviors. This speculation is supported by a recent study in which synchronous activation of NAc FSIs with in vivo optogenetics promotes conditioned aversion12. However, such a synchronous activation of FSIs throughout the NAc does not likely occur under physiological conditions, and is therefore, much more dynamic and complex during different behavioral performances. This is echoed by striatal FSIs, which display highly uncoordinated and unsynchronized firing patterns during the performance of a reward seeking maze task38. Furthermore, subsets of FSIs in the NAc display increased activities during contextually-conditioned reward seeking (e.g., conditioned place preference, or CPP)11,39, and specific inhibition of these FSIs disrupts the performance of CPP11. Similarly, FSIs in the NAc display ramping increases in their activities as rats approach rewards during a reward-searching maze task39. Therefore, rather than general inhibition, it is more likely that the dynamic activities of NAc FSIs are an essential component of the circuit mechanism that promotes motivated behaviors. Yet, an outstanding question is how FSIs promote motivated behaviors.
MSNs in the NAc and dorsal striatum are not a uniform population, but are physiologically, anatomically, and functionally heterogeneous. MSNs throughout the entire striatum can be divided into two major subpopulations, namely D1 MSNs and D2 MSNs40. NAc D1 and D2 MSNs have somewhat divergent projections and different functional roles in regulating behaviors41,42. Furthermore, within a given subpopulation of MSNs (e.g., D1 MSNs), there are differential projections that can have opposing behavioral roles43–45. These findings lead to the functional ensemble hypothesis, proposing that NAc MSNs are organized as individual functional groups, or ensembles, for different aspects of emotional and motivational behavior46. This hypothesis has been supported by in vivo electrophysiology and calcium imaging studies, which demonstrate that separate populations of MSNs in the NAc and the striatum encode the identities of different rewarding or aversive stimuli47–49, as well as fine aspects of motor outputs (e.g., forward acceleration, turn right, and rearing)50–52. Thus, a logical extension of the ensemble hypothesis is that for the proper execution of a specific motivated behavior, the activities of different MSN ensembles must be coordinated in a precise fashion, such that the MSN ensemble encoding the chosen behavior is activated while the ensembles encoding competing behaviors are suppressed. This scenario is supported by in vivo electrophysiological recordings, where NAc MSNs exhibit highly diverse activation patterns during the execution of motivated behaviors. For example, some populations of MSNs are activated during different phases of the behavior (e.g., seeking versus consumption), while other populations are quite53–60. Furthermore, preventing the activation of MSNs that are normally activated during a given motivated behavior impairs the execution of the behavior61,62. Similarly, activating the MSNs that are normally suppressed during a given motivated behavior also impairs the behavioral execution43,55. These findings suggest the existence of circuit mechanisms within the NAc that precisely orchestrate the activation patterns of different functional MSN ensembles for the proper execution of motivated behaviors. We propose that NAc FSIs are one of such circuit orchestrators coordinating the output of motivated behaviors (Fig. 1).
Figure 1. Proposed role of NAc FSIs in regulating behavior.
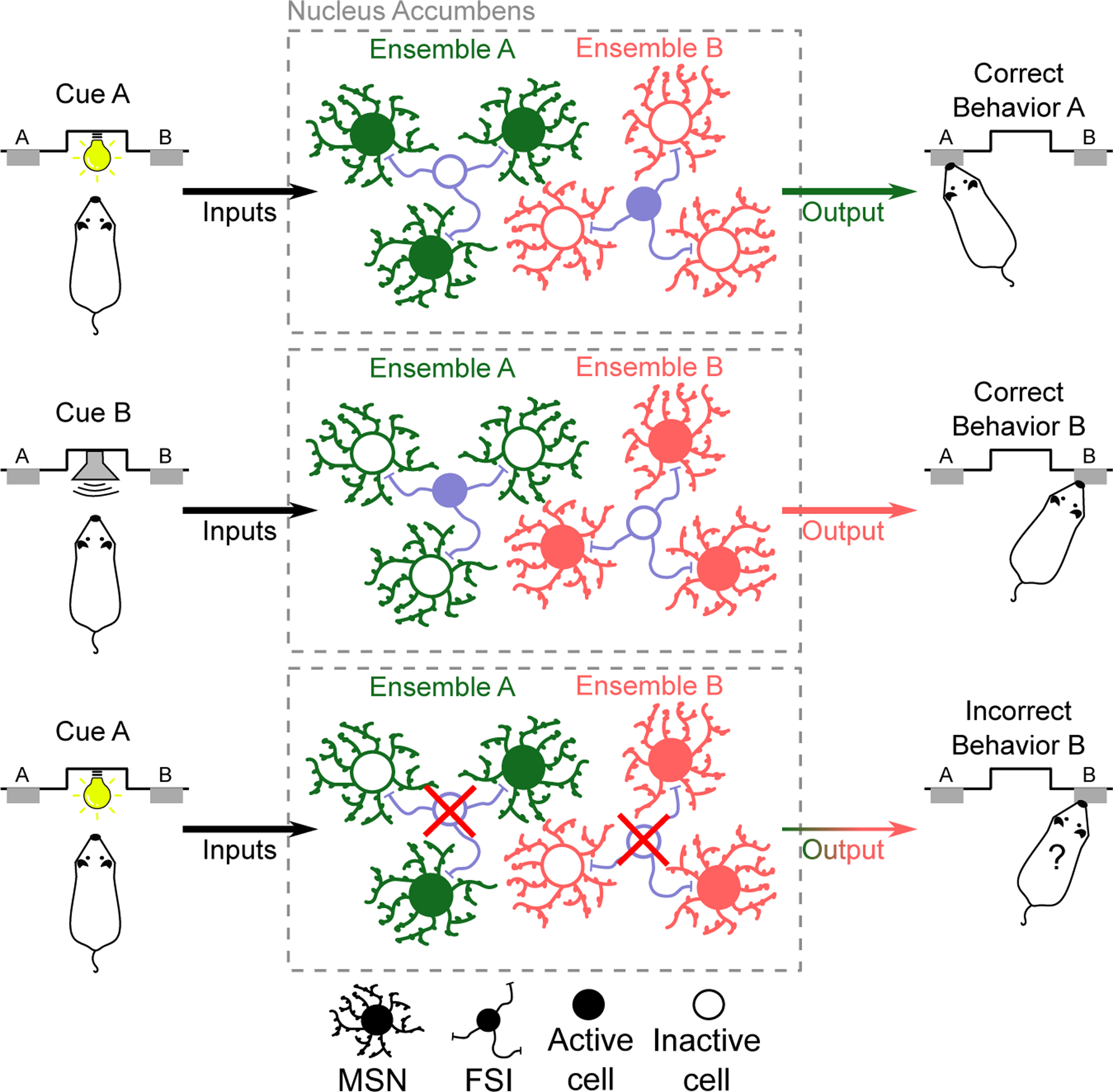
(A-C) Schematic depictions of our proposed model where FSIs serve to orchestrate the activity of functionally distinct MSN ensembles to select the appropriate behavior over others. Examples showing that when Cue ‘A’ is presented, inputs to the NAc excite MSNs forming ensemble ‘A’ while MSNs in ensemble ‘B’ are suppressed by excited FSIs, which ultimately results in the execution of the correct behavioral response (A). However, when Cue ‘B’ is presented, MSNs forming ensemble ‘B’ are activated while FSIs suppress MSNs in ensemble ‘A’, resulting in a different behavioral response that is not appropriate (B). When the function of FSIs is disrupted, MSNs in both ensemble ‘A’ and ensemble ‘B’ may be activated, resulting in a disorganized functional output of the NAc and execution of an inappropriate behavior (C).
Orchestrating Role of NAc FSIs
While direct evidence supporting the orchestrating role of NAc FSIs is still lacking, insights are accumulating. In the dorsal striatum, MSN ensembles appear to be organized in spatial clusters, such that MSNs encoding a given action are located close to one another50–52. The size of these spatial clusters is relatively compact, typically confined within several hundred micrometers. This size is approximately the same as what the local axonal arbors of a single FSI covers, which is typically 400–600 μm in diameter17. Furthermore, the activities between FSIs and MSNs are strongly correlated within this confined anatomical space63. This coincidence prompts a possibility that MSNs in a single functional NAc ensemble are innervated by the axonal arbors of a single or only a few FSIs. If this is the case, the activation and inactivation of MSNs within an ensemble may, at least in part, be coordinated by a single FSI. This notion is further supported by recent in vivo studies in the dorsal striatum and cortices, where the activity of FSIs functions to constrain the size of functional ensembles by preventing the activation of non-relevant principle neurons64–66. In addition, FSIs also regulate the activation magnitude and pattern of striatal and NAc MSN ensembles, therefore fine tuning the ensemble-mediated encoding processes11,63. In vivo, activation of FSIs precedes their adjacent MSNs, and remain activated through behavioral execution63, thus influencing the entire cascade of MSN-mediated behaviors. Furthermore, a recent study utilizing machine learning demonstrates that the activity of MSNs can be predicted from the activity of FSIs, indicating a controlling influence of FSIs over the intricate activity patterns of MSNs63. It is also worth noting that FSIs are connected by electrical synapses, which promote their synchronized activation and create multi-FSI-mediated ensembles. Indeed, small groups of striatal FSIs appear to encode specific behavioral features, forming distinct functional ensembles67.
The ensemble-selective inhibition by FSIs may critically contribute to a proper execution of intended behaviors by helping select and coordinate appropriate actions. This notion is supported by three separate observations. First, in a choice-based reward seeking task, in which the animals must choose one behavioral action over another, striatal FSIs display an increase in activities specifically at the moment the choice is made68. Furthermore, different sets of FSIs are activated upon making different choices, which may result in selective suppression of different MSN ensembles. Importantly, the activation of FSIs during the choice period coincides with the time locked suppression of MSNs that encode alternative behaviors68. These results suggest that ensemble-selective inhibition by FSIs is implicated in the online prioritization and selection of behavioral outputs. Second, in the performance of a delay task, in which the animal must wait over a delay period before performing a behavior, NAc FSIs exhibit increased activity sustained throughout the delay period until the behavior is executed69. On trials when the animal performs the behavior prematurely, relatively low activity levels are simultaneously detected in NAc FSIs. Furthermore, when such low activity levels are chemogenetically induced in NAc FSIs during the delay task, the rate of premature responding increases sharpely69. Thus, in addition to generally suppressing unintended behaviors, FSIs are part of the mechanisms that time the expression of intended behaviors. Third, during the development of habitual responding, overtraining renders striatal FSIs more excitable, resulting in generally decreased activities in most MSNs but enhanced gamma-frequency (30–100Hz) spiking in a small MSN population, while chemogenetic inhibition of FSIs prevents the expression of established habitual lever pressing70. On the surface, this finding indicates that striatal FSIs are essential for the expression of habitual motor responses. However, this finding can also be interpreted as that the strengthened FSI output may constrain most NAc MSN ensembles while favoring activation of specific MSN ensembles to produce rigid behaviors despite the changing outcomes. Thus, the FSI activity dynamics may play a role in shifting behavioral output to contribute to behavioral flexibility.
As mentioned earlier, MSNs in the NAc are heterogenous. It remains unclear how these heterougenous populations of MSNs coordinate their activities with each other to potentially form functional ensembles. For example, D1 and D2 MSNs in the striatum, in general, have opposing effects on motor behavior71, and thus may form separate ensembles. Yet, D1 and D2 MSNs are concurrently activated during action initiation72,73. Furthermore, in studies imaging D1 and D2 MSNs in separate animals, ensembles of both D1 and D2 MSNs were found to encode the same motor behaviors50,51. However, in the context of motivated behavior, the relationship of NAc D1 and D2 MSN activities is more nuanced74–77. Although it is possible that MSN ensembles encoding specific behaviors consist of both D1 and D2 MSNs, how these two types of MSNs are functionally bound remains to be explored. In the earlier Synaptic Connectivity section, we discussed that the overall population of FSIs in the NAc does not show biased inhibition of D1 versus D2 MSNs10. However, it is possible that individual FSIs form biased synaptic innervations on D1 versus D2 MSNs, such that specific FSIs provide inhibition to an ensemble of MSNs consisting only of D1 or D2 MSNs.
It is also important to note that different subdivisions of the NAc (i.e., shell versus core) regulate different aspects of motivated behavior3. At this point, there remain too few studies to depict conclusive differences that FSIs in the NAc shell versus core exert on motivated behaviors. However, at the local circuit level, FSIs in the shell versus core exhibit similar biophysical connectivity properties, prompting us to speculate that FSIs act as similar ensemble orchestrators in both the NAc shell and core. As such, the behavioral differences between shell and core FSIs are embedded in the generall different behavioral role of the shell versus core.
Taken together, we propose an orchestrating role of NAc FSIs. Rather than encoding specific behavioral response, NAc FSIs regulate motivated behaviors through selective and dynamic inhibition of encoding MSN ensembles. Selective inhibition entails a local circuit-based mechanism underlying behavioral prioritization versus suppression, while dynamic inhibition may participate in executive control over behavioral output. This proposed role also raises two outstanding questions for the field; 1) what other aspects of motivated behaviors are NAc FSIs involved in, and 2) whether the same or different sets of FSIs are involved in different aspects of motivated behaviors?
Additional mechanisms for FSIs in regulating motivated behavior
While the above sections focus on the role of FSIs in regulating NAc output, alternative mechanisms also exist through which FSIs regulate motivated behaviors. These alternative mechanisms are not necessarily exclusive of, but rather complementary to, the role of FSIs discussed above.
Rhythmic oscillation is a basic form of populational activities of the brain, during which activation and inactivation of neurons within the same or different brain regions are coupled in synchrony78–81. FSIs are involved in most types of such rhythmic oscillations78,82, among which they are particularly important in generating and maintaining high frequency gamma oscillations83. In the NAc, high-powered local field potentials are enriched in the gamma oscillation range84,85. During reward seeking, gamma oscillations switch between discrete high and low frequency bands in response to different behavioral details84. In addition to gamma oscillation, the NAc also exhibits theta oscillations, which are implicated in encoding the spatial locations of rewards and are governed by the FSI activity11,86. Interestingly, different MSN ensembles in the NAc exhibit different patterns of rhythms86, which appear to be tuned by different subgroups of FSIs that also exhibit different rhythms11. Therefore, FSIs may orchestrate different MSN ensembles by entraining them to different rhythmic oscillations. It was recently proposed that different activity oscillations within a brain region is critical for the routing of specific information, and potentially allows for the multiplexing of information87,88. As such, rhythmic oscillations may be a macroscopic readout of ensemble synchronization, which is regulated by NAc FSIs in processing and coordinating different informational flows for behavioral output.
FSIs throughout the brain, including the striatum and NAc, deliver inhibition partially by activating postsynaptic GABAA receptors13,89. However, activation of GABAA receptors does not always result in inhibition. If the resting membrane potential of the postsynaptic neuron is below the reversal potential for GABAA receptors (i.e., chloride conductance), activation of these receptors induces depolarization, resulting in a so-called shunting inhibition90. The reversal potential of GABAA receptors in NAc MSNs, including those directly activated by FSIs, is approximately −60 mV9,91, which is substantially depolarized compared to the resting membrane potential of −80mV in NAc MSNs92. Thus, when MSNs are at their resting membrane potentials, input from FSIs depolarizes, rather than suppresses, NAc MSNs9. This unique feature may have several implications in regulating the activity pattern of MSNs. First, MSNs in vivo fluctuate between two functional states: a relatively hyperpolarized, ‘down’ state (~−80 mV) where MSNs are largely silent, and a relatively more depolarized, ‘up’ state (~−55 mV), where MSNs actively fire action potentials upon excitatory inputs93,94. With the relatively depolarized reversal potential, FSI-to-MSN synapses may contribute to shifting MSNs to the functionally active upstate state. Given the large number of MSNs innervated by a single FSI, shunting inhibition from FSIs can also contribute to the synchronous down-up state transitions in MSNs observed in vivo94, promoting synchronized activation of MSNs within the same ensembles. It is important to indicate that this shunting effect of FSIs can be both excitatory and inhibitory, depending on the relative timing of inhibitory and excitatory inputs. If excitatory inputs arrive at postsynaptic MSNs while the GABAA receptors are open, GABAA receptors function to sustain the membrane potential toward their reversal potentials, hindering depolarization. Therefore, FSI activation exerts either excitatory or inhibitory effects on MSNs depending on the membrane potential condition of MSNs. These bidirectional effects may explain how FSI activation paradoxically promotes action potential firing in some populations of MSNs while suppressing firing of other MSNs in vivo70. In this case, FSIs may orchestrate the functional output of the NAc not only by suppressing the activation of MSN ensembles encoding competing behaviors, but also potentially by promoting the activation of MSNs encoding appropriate behavior.
Cocaine-induced adaptations in FSIs and behavioral consequences
By inhibiting monoamine reuptake, administration of cocaine acutely and greatly increases the level of dopamine in the NAc, an effect tought to initiate the motivated responses to cocaine95,96. After withdrawal from repeated use, re-exposure to cocaine-associated cues induces NAc dopamine transients, which are tightly linked to cue-induced drug seeking97,98. Dopamine as a neuromodulator does not directly evoke action potentials. As such, its effects on NAc-based behaviors are expected to be mediated by its cellular actions on the intrinsic membrane excitability of NAc neurons and excitatory and inhibitory synaptic inputs to these neuron99. Indeed, dopamine signaling has been found to regulate the excitability of FSIs in vitro and in vivo100,101, which may critically contribute to the altered activity patterns of MSN ensembles. The role of NAc dopamine in initiating and regulating motivated behaviors have been discussed by several excellent reviews, and thus will not be focused here98,102,103.
A defining cellular characteristics of addiction is that the maladaptive cellular changes are highly persistent and can promote drug relapse long after drug abstience102. It has long been known that addictive drugs induce functional adaptions in the reward circuits to promote addictive behaviors1,6,104. Within the NAc, exposure to cocaine induces a variety of adaptations in MSNs, including changes in the membrane excitability105,106, excitatory synapses1 and inhibitory synapses107, which are critical for the development of addiction-associated behaviors. These cellular adaptations in MSNs may collectively reshape the spiking output of NAc MSNs that correspond to certain addiction-associated behaviors108–111. However, given the indispensable role of FSIs in regulating the MSN functional output, it is surprising that there has not been a systematic discussion about how FSIs and FSI-embedded circuits are targeted by drugs of abuse, such as cocaine, to reshape NAc MSNs and NAc-based behaviors. Below, we discuss cocaine-induced adaptations in the NAc FSI-mediated feedforward circuit, and how these adaptations contribute to cocaine-induced behaviors.
Cocaine-induced adaptations at FSI-to-MSN inhibitory synapses
The core transmission within the NAc FSI-mediated feedforward circuit is mediated by FSI-to-MSN synapses, which can undergo both short- and long-term plasticity. As discussed above, a significant proportion of FSIs in the NAc express CB1 (~80%)15, rendering synaptic transmission susceptible to regulation by CB1-signaling. As such, a prominent form of short-term plasticity at FSI-to-MSN synapses is the depolarization-induced suppression of inhibition (DSI)112, where a brief depolarization of MSNs induces a postsynaptic release of endocannabinoids that inhibit presynaptic release from FSIs via CB19,15. Alterations in the magnitude of DSI at FSI-to-MSN synapses may effectively alter the feedforward inhibition and thus the ability of FSIs to regulate MSNs. However, DSI at FSI-to-MSN synapses remains largely intact after either short- or long-term withdrawal from repeated intraperitoneal (i.p.) injections or self-administration of cocaine9,15, suggesting that DSI, a widely expressed short-term form of presynaptic plasticity, is resistant to cocaine-induced adaptations.
In addition to short-term plasticity, FSIs throughout the brain also undergo experience-dependent long-term plasticity113, a prominent form of which at NAc FSI-to-MSN synapses is long-term depression (LTD)8. The induction of this LTD involves both CB1 and TRPV1 channels, similar, but not identical, to endocannabinoid-mediated LTD of excitatory and inhibitory synaptic transmission in the striatum114,115. Contrasting LTD is long-term potentiation (LTP), which is possibly present at FSI-to-MSN synapses as well, but remains to be experimentally tested. Either LTP or LTD, if induced at FSI-to-MSN synapses by drug experience, may persistently reshape the ability of FSIs to regulate the NAc output. However, the efficacy of the basal NAc FSI-to-MSN synaptic transmission remains largely intact after short- or long-term withdrawal from cocaine self-administration9. Specifically, the overall amplitude of transmission between individual, synaptically connected FSI-to-MSN pairs is not changed after cocaine self-administration. Furthermore, both the presynaptic release and postsynaptic responsiveness at FSI-to-MSN synapses also remains unchanged after withdrawal from cocaine. These results largely exclude the possibility that cocaine experience induces LTD- or LTP-like adaptations to alter the basal transmission efficacy of FSI-to-MSN synapses. However, when inhibitoy inputs to MSNs are collectively sampled without differentiating afferents, either increases116 or decreases107 in overall inhibitory inputs to NAc MSNs are detected after cocaine exposure under different experimental conditions. Thus, other sources of inhibitory input to MSNs, such as other interneuron subtypes117 or MSN collaterals118, may be altered after cocaine experience as well but in different manners. Taken together, at least for cocaine experience, FSI-to-MSN connections appear to be rigid and hardwired, and do not directly embed synaptic traces for drug experience.
Cocaine-induced adaptations in excitatory inputs to FSIs
While FSI-to-MSN synapses are not altered by cocaine experience, the functionality of the feedforward circuit can still be changed due to the alterations in other key components, such as the membrane excitability of MSNs and FSIs, as well as the excitatory synaptic inputs these neurons receive. There is a rich literature on how cocaine experience alters the synaptic inputs and membrane excitability of NAc MSNs1,6. Briefly, cocaine exposure increases the strength of excitatory synapses onto MSNs, with an initial increase in presynaptic release119,120 followed by postsynaptic strengthening after cocaine withdrawal121–123. This synaptic strengthening, however, is functionally balanced by a decrease in the membrane excitability of NAc MSNs92,124 through a synapse-membrane homeostatic crosstalk106. It is thought that the synaptic strengthening of MSNs occurs specifically at synapses that encode cocaine experience. An extreme example is the cocaine-induced generation of new, immature synapses, which functionally mature during withdrawal, and appear to encode critical aspects of cue-associated cocaine memories125–128. Such a general decrease in the membrane excitability excitability with selectively strengthening of cocaine-related synaptic input may set NAc MSNs to selectively respond to cocaine-associated stimuli and ‘ignore’ other stimuli, as implied by results from recent in vivo electrophysiological recordings110,129. However, to generate a precise picture about how the output of NAc is altered by cocaine experience, it is also critical to understand how cocaine experience reshapes NAc FSIs and FSI-mediated circuits.
In the NAc, FSIs receive excitatory projections from the same brain regions as MSNs. While most inputs to MSNs are strengthened after cocaine withdrawal123,130,131, such synaptic strengthening is projection-specific for NAc FSIs. Specifically, the excitatory projection from the basolateral amygdala (BLA) to FSIs is potentiated after cocaine self-administration through an increase in the presynaptic release probability, and remains elevated through long-term withdrawal9. In contrast, the projection from the medial prefrontal cortex (mPFC) to FSIs is not altered9, nor the projection from the ventral hippocampus10 (Fig. 2). When the overall excitatory input to FSIs is collectively assessed by measuring miniature excitatory postsynaptic currents, no overall changes are detected after cocaine exposure, suggesting a lack of presynaptic alteration in the majority of excitatory inputs15. On the other hand, cocaine exposure increases the membrane excitability of FSIs15. This increase renders all excitatory projections more effective in eliciting action potentials in NAc FSIs. It is worth noting that no changes are detected in basal levels of PV, levels of which reflect the basal FSI activity, after withdrawal from cocaine self-administration132. Therefore, cocaine-induced changes in the membrane excitability and excitatory synaptic inputs to FSIs do not seem to influence the tonic, basal activity of these neurons; rather, these changes may selectively increase the responsiveness of FSIs to excitatory synaptic inputs (e.g., BLA projections) that are strengthened by drug experience.
Figure 2. Long-term adaptations in the NAc FSI circuits induced by cocaine experience.
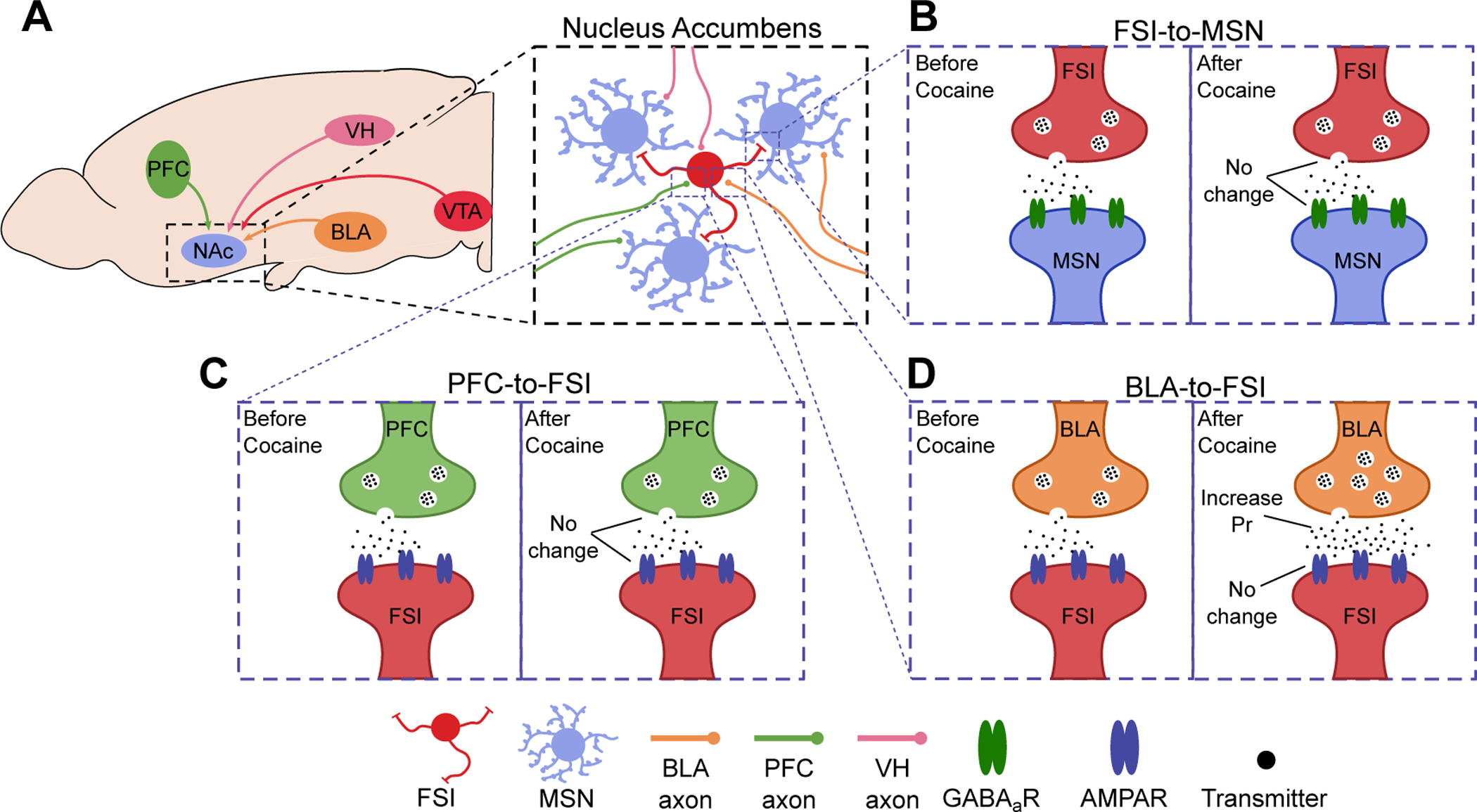
(A) Schematic diagrams showing the excitatory inputs to the NAc arising from the BLA, PFC, VH, VTA. These inputs target both MSNs and FSIs within the NAc. (B-D) Schematic diagrams showing baseline properties of different synapses within the NAc feedforward circuitry before and after cocaine self-administration training. These diagrams do not depict synaptic transmission while cocaine is present in the system. (B) At FSI-to-MSN synapses, there is no change in the strength of synaptic transmission, including no changes in presynaptic release probability or postsynaptic responsiveness, following withdrawal from cocaine self-administration. (C) At PFC-to-FSI synapses, there is no change in the presynaptic release probability or postsynaptic responsiveness following withdrawal from cocaine self-administration. (D) At BLA-to-FSI synapses, there is an increase in the presynaptic release probability following withdrawal from cocaine self-administration, while no change in postsynaptic responsiveness. The increase in presynaptic release persists throughout long-term withdrawal. The adaptations depicted here are reported in ref 9.
How do these cellular adaptations in FSIs contribute to cocaine-induced behaviors? While it remains unclear how the increased FSI membrane excitability affects behavioral output, Yu et al. recently demonstrate that potentiation of BLA synaptic input to FSIs promotes the acquisition of cocaine self-administration9. Specifically, when the BLA projection to FSIs is optogenetically potentiated in vivo prior to the self-administration training, mice exhibit an expedited rate of acquiring the cocaine self-administration task, as well as an increased accuracy of cue-conditioned responding9. However, after 5 days of training, mice with the BLA-to-FSI strengthening plateau at the same level of operant responding as control mice, suggesting that the effect of cocaine on BLA-to-FSI transmission facilitates the acquisition of cocaine self-administration, but does not affect the intensity of cocaine taking once the behavior is established.
In lieu of these results and the body of work suggesting that FSIs operate to orchestrate the output of NAc, we hypothesize that the pro-excitation effects of cocaine on FSIs helps sculpt the activation of MSN ensembles to promote cocaine seeking. During the learning and acquisition phase of an associative behavior, new neural ensembles emerge to encode the association, which involves neurons gaining or losing responsiveness to the stimulus, changes in the magnitude of the responsiveness, and changes in the synchrony and reproducibility of response patterns133,134. We speculate that during the acquisition of cocaine self-administration, FSIs operate to suppress MSNs that are not related to cocaine, while allowing the activation of MSN ensembles that encode cocaine-related information. The potentiation of synaptic inputs to FSIs may occur in a synapse-specific manner, which alters how FSIs deliver inhibition to different MSN ensembles. For example, BLA inputs may be selectively strengthened to the FSIs that provide inhibition to non-encoding MSNs. Indeed, potentiation of the BLA input does not appear to be uniform across all FSIs9. Alternatively, the FSI-mediated feedforward circuit may be potentiated equally to all MSNs, but the selective potentiation of inputs encoding cocaine-related information to MSNs allows MSNs to be activated by cocaine-related stimuli, but not other, non-specific stimuli, which also refines cocaine-encoding MSN ensembles. This scenario may also contribute to the encoding of other, relatively weak incentive signals in the NAc or anhedonia associated with cocaine withdrawal110,129,135. In addition, cocaine-induced adaptations in NAc FSIs may also compromise behavioral flexibility and promote continued drug use despite changes in outcome, such that the incoming information that would otherwise shift the NAc output to change behavior fails to activate related MSNs. Regardless, enhanced FSI-mediated inhibition may improve the signal-to-noise ratio of the NAc output, with MSNs becoming more responsive to cocaine-related inputs than non-cocaine-related inputs, thus improving behavioral performance. In addition, given the critical role of FSIs in synchronizing neural activity22,23, an enhancement of FSI-mediated feedforward inhibition may also improve the temporal output of the NAc for behavioral refinement. Furthermore, through inhibitory signaling-mediated regulation of synaptic plasticity136, FSI inputs to MSNs may influence the induction of plasticity at excitatory synapses onto MSNs, which may either facilitate the formation of MSN ensembles encoding cocaine associated information or exclude MSNs that encode extraneous information from the drug ensembles66. Overall, these proposed scenarios may help depict excitatory synaptic input to FSIs as a key means for cocaine experience to shape and orchestrate NAc MSN ensembles that contribute to cocaine memories and drive drug seeking behaviors.
Concluding remarks
Increasing evidence suggests that the local FSI circuit embedded in the NAc is a unique neural target through which cocaine experience reshapes the functional output of NAc and, ultimately, motivated behaviors. Results thus far depict that excitatory synaptic inputs that drive this circuit are likely the locations hosting cocaine-induced adaptive changes, while the backbone FSI-to-MSN connection appears to be conservatively inert. Furthermore, cocaine-induced adaptations at excitatory synapses on NAc FSIs versus MSNs are differentially implicated in different aspects/phases of cocaine-induced behaviors. These findings set up future studies to explore the molecular and cellular underpinnings of these cocaine-induced changes, and how adaptations in FSIs influences the in vivo dynamics of NAc MSNs during drug taking and seeking. In addition, the unveiled role of NAc FSIs in cocaine seeking encourages future studies to further explore whether FSIs are also implicated in increased drug motivation, compulsive drug taking and seeking, and other “late-stage” symptoms after prolonged drug exposure. We propose that FSIs orchestrate the output of the NAc by gating the activities of different functional MSNs ensembles, and cocaine-induced adaptations in FSIs may refine this process to enhance the encoding of cocaine-induced behaviors. Determining how drugs of abuse alter FSI-mediated feedforward inhibition in future studies will provide a more complete understanding of how drug-associated information (e.g., cues) are integrated within the local circuits of the NAc to promote drug seeking and other addiction-related behaviors. Furthermore, given their low numbers and clear molecular features, NAc FSIs can be selectively manipulated in vivo, making them attractive targets for potential clinical manipulations.
Acknowledgements:
The authors’ work was partially supported by NIH grants DA043940 (WJW), DA023206 (YD), DA047861 (YD), DA040620 (YD), DA044538 (YD).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Wolf ME Synaptic mechanisms underlying persistent cocaine craving. Nature Reviews Neuroscience 17, 351–365, doi: 10.1038/nrn.2016.39 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sesack SR & Grace AA Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology 35, 27–47, doi: 10.1038/npp.2009.93 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Floresco SB The nucleus accumbens: an interface between cognition, emotion, and action. Annual review of psychology 66, 25–52, doi: 10.1146/annurev-psych-010213-115159 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Dong Y & Nestler EJ The neural rejuvenation hypothesis of cocaine addiction. Trends in pharmacological sciences 35, 374–383, doi: 10.1016/j.tips.2014.05.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley AE Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44, 161–179, doi: 10.1016/j.neuron.2004.09.016 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Huang YH, Schlüter OM & Dong Y Cocaine-induced homeostatic regulation and dysregulation of nucleus accumbens neurons. Behavioural brain research 216, 9–18, doi: 10.1016/j.bbr.2010.07.039 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tepper JM, Tecuapetla F, Koos T & Ibanez-Sandoval O Heterogeneity and diversity of striatal GABAergic interneurons. Frontiers in neuroanatomy 4, 150, doi: 10.3389/fnana.2010.00150 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright WJ, Schlüter OM & Dong Y A Feedforward Inhibitory Circuit Mediated by CB1-Expressing Fast-Spiking Interneurons in the Nucleus Accumbens. Neuropsychopharmacology 42, 1146–1156, doi: 10.1038/npp.2016.275 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J et al. Nucleus accumbens feedforward inhibition circuit promotes cocaine self-administration. Proceedings of the National Academy of Sciences of the United States of America 56, 201707822, doi: 10.1073/pnas.1707822114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scudder SL, Baimel C, Macdonald EE & Carter AG Hippocampal-Evoked Feedforward Inhibition in the Nucleus Accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience 38, 9091–9104, doi: 10.1523/JNEUROSCI.1971-18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trouche S et al. A Hippocampus-Accumbens Tripartite Neuronal Motif Guides Appetitive Memory in Space. Cell, 1–31, doi: 10.1016/j.cell.2018.12.037 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi J et al. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nature Neuroscience 19, 725–733, doi: 10.1038/nn.4281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu H, Gan J & Jonas P Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345, 1255263–1255263, doi: 10.1126/science.1255263 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Xue M, Atallah BV & Scanziani M Equalizing excitation-inhibition ratios across visual cortical neurons. Nature 511, 596–600, doi: 10.1038/nature13321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winters BD et al. Cannabinoid receptor 1-expressing neurons in the nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America 109, E2717–2725, doi: 10.1073/pnas.1206303109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hefft S & Jonas P Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nature Neuroscience 8, 1319–1328, doi: 10.1038/nn1542 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi Y Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. The Journal of neuroscience : the official journal of the Society for Neuroscience 13, 4908–4923 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bracci E, Centonze D, Bernardi G & Calabresi P Voltage-dependent membrane potential oscillations of rat striatal fast-spiking interneurons. The Journal of physiology 549, 121–130, doi: 10.1113/jphysiol.2003.040857 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brumberg JC, Nowak LG & McCormick DA Ionic mechanisms underlying repetitive high-frequency burst firing in supragranular cortical neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 20, 4829–4843, doi: 10.1523/JNEUROSCI.20-13-04829.2000 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golomb D et al. Mechanisms of Firing Patterns in Fast-Spiking Cortical Interneurons. PLOS Computational Biology 3, e156–115, doi: 10.1371/journal.pcbi.0030156 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau T, Gage GJ, Berke JD & Zochowski M Local dynamics of gap-junction-coupled interneuron networks. Physical biology 7, 16015, doi: 10.1088/1478-3975/7/1/016015 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cobb SR, Buhl EH, Halasy K, Paulsen O & Somogyi P Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378, 75–78, doi: 10.1038/378075a0 (1995). [DOI] [PubMed] [Google Scholar]
- 23.Bonifazi P et al. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science 326, 1419–1424, doi: 10.1126/science.1175509 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Gan J, Weng S.-m., Pernía-Andrade AJ, Csicsvari J & Jonas P Phase-Locked Inhibition, but Not Excitation, Underlies Hippocampal Ripple Oscillations in Awake Mice In Vivo. Neuron 93, 308–314, doi: 10.1016/j.neuron.2016.12.018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taverna S, Canciani B & Pennartz CMA Membrane properties and synaptic connectivity of fast-spiking interneurons in rat ventral striatum. Brain Research 1152, 49–56, doi: 10.1016/j.brainres.2007.03.053 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Araya R, Eisenthal KB & Yuste R Dendritic spines linearize the summation of excitatory potentials. Proceedings of the National Academy of Sciences 103, 18799–18804, doi: 10.1073/pnas.0609225103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon T, Sakamoto M, Peterka DS & Yuste R Attenuation of Synaptic Potentials in Dendritic Spines. Cell reports 20, 1100–1110, doi: 10.1016/j.celrep.2017.07.012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svoboda K, Tank DW & Denk W Direct measurement of coupling between dendritic spines and shafts. Science 272, 716–719, doi: 10.1126/science.272.5262.716 (1996). [DOI] [PubMed] [Google Scholar]
- 29.Murakoshi H, Wang H & Yasuda R Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature 472, 100–104, doi: 10.1038/nature09823 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S-JR, Escobedo-Lozoya Y, Szatmari EM & Yasuda R Activation of CaMKII in single dendritic spines during long-term potentiation. Nature 458, 299–304, doi: 10.1038/nature07842 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meitzen J, Pflepsen KR, Stern CM, Meisel RL & Mermelstein PG Measurements of neuron soma size and density in rat dorsal striatum, nucleus accumbens core and nucleus accumbens shell: Differences between striatal region and brain hemisphere, but not sex. Neuroscience Letters 487, 177–181, doi: 10.1016/j.neulet.2010.10.017 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramanathan S, Hanley JJ, Deniau JM & Bolam JP Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience 22, 8158–8169 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng T & Wilson CJ Corticostriatal combinatorics: the implications of corticostriatal axonal arborizations. Journal of neurophysiology 87, 1007–1017, doi: 10.1152/jn.00519.2001 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Everitt BJ & Robbins TW Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience 8, 1481–1489, doi: 10.1038/nn1579 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Kalivas P, Volkow N & Seamans J Unmanageable Motivation in Addiction: A Pathology in Prefrontal-Accumbens Glutamate Transmission. Neuron 45, 647–650, doi: 10.1016/j.neuron.2005.02.005 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Wolf ME Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci, doi: 10.1038/nrn.2016.39 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheeler RA & Carelli RM Dissecting motivational circuitry to understand substance abuse. Neuropharmacology 56 Suppl 1, 149–159, doi: 10.1016/j.neuropharm.2008.06.028 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berke JD Uncoordinated firing rate changes of striatal fast-spiking interneurons during behavioral task performance. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 10075–10080, doi: 10.1523/JNEUROSCI.2192-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lansink CS, Goltstein PM, Lankelma JV & Pennartz CMA Fast-spiking interneurons of the rat ventral striatum: temporal coordination of activity with principal cells and responsiveness to reward. The European journal of neuroscience 32, 494–508, doi: 10.1111/j.1460-9568.2010.07293.x (2010). [DOI] [PubMed] [Google Scholar]
- 40.Gerfen CR et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432, doi: 10.1126/science.2147780 (1990). [DOI] [PubMed] [Google Scholar]
- 41.Kupchik YM et al. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nature Neuroscience 18, 1230–1232, doi: 10.1038/nn.4068 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lobo MK et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330, 385–390, doi: 10.1126/science.1188472 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Connor EC et al. Accumbal D1R Neurons Projecting to Lateral Hypothalamus Authorize Feeding. Neuron 88, 553–564, doi: 10.1016/j.neuron.2015.09.038 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Gibson GD et al. Distinct Accumbens Shell Output Pathways Promote versus Prevent Relapse to Alcohol Seeking. Neuron, 1–16, doi: 10.1016/j.neuron.2018.03.033 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Al-Hasani R et al. Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron 87, 1063–1077, doi: 10.1016/j.neuron.2015.08.019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pennartz CM, Groenewegen HJ & Lopes da Silva FH The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Progress in neurobiology 42, 719–761, doi: 10.1016/0301-0082(94)90025-6 (1994). [DOI] [PubMed] [Google Scholar]
- 47.Roitman MF, Wheeler RA & Carelli RM Nucleus Accumbens Neurons Are Innately Tuned for Rewarding and Aversive Taste Stimuli, Encode Their Predictors, and Are Linked to Motor Output. Neuron 45, 587–597, doi: 10.1016/j.neuron.2004.12.055 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Carelli RM, Ijames SG & Crumling AJ Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. The Journal of neuroscience : the official journal of the Society for Neuroscience 20, 4255–4266 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carelli RM Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. ‘natural’ reinforcement. Physiology & Behavior 76, 379–387, doi: 10.1016/s0031-9384(02)00760-6 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Barbera G et al. Spatially Compact Neural Clusters in the Dorsal Striatum Encode Locomotion Relevant Information. Neuron, 1–13, doi: 10.1016/j.neuron.2016.08.037 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klaus A et al. The Spatiotemporal Organization of the Striatum Encodes Action Space. Neuron 95, 1171–1180.e1177, doi: 10.1016/j.neuron.2017.08.015 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parker JG et al. Diametric neural ensemble dynamics in parkinsonian and dyskinetic states. Nature, 1–32, doi: 10.1038/s41586-018-0090-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taha SA & Fields HL Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience 25, 1193–1202, doi: 10.1523/JNEUROSCI.3975-04.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taha SA & Fields HL Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 217–222, doi: 10.1523/JNEUROSCI.3227-05.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krause M, German PW, Taha SA & Fields HL A Pause in Nucleus Accumbens Neuron Firing Is Required to Initiate and Maintain Feeding. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 4746–4756, doi: 10.1523/JNEUROSCI.0197-10.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ambroggi F, Ghazizadeh A, Nicola SM & Fields HL Roles of Nucleus Accumbens Core and Shell in Incentive-Cue Responding and Behavioral Inhibition. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 6820–6830, doi: 10.1523/JNEUROSCI.6491-10.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghazizadeh A, Ambroggi F, Odean N & Fields HL Prefrontal Cortex Mediates Extinction of Responding by Two Distinct Neural Mechanisms in Accumbens Shell. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 726–737, doi: 10.1523/JNEUROSCI.3891-11.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicola SM, Yun IA, Wakabayashi KT & Fields HL Cue-Evoked Firing of Nucleus Accumbens Neurons Encodes Motivational Significance During a Discriminative Stimulus Task. Journal of neurophysiology 91, 1840–1865, doi: 10.1152/jn.00657.2003 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Peoples LL & West MO Phasic firing of single neurons in the rat nucleus accumbens correlated with the timing of intravenous cocaine self-administration. The Journal of neuroscience : the official journal of the Society for Neuroscience 16, 3459–3473 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang JY, Sawyer SF, Lee RS & Woodward DJ Electrophysiological and pharmacological evidence for the role of the nucleus accumbens in cocaine self-administration in freely moving rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 14, 1224–1244 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koya E et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nature Neuroscience 12, 1069–1073, doi: 10.1038/nn.2364 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cruz FC et al. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 7437–7446, doi: 10.1523/JNEUROSCI.0238-14.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gritton HJ et al. Unique contributions of parvalbumin and cholinergic interneurons in organizing striatal networks during movement. Nature Neuroscience, 1–20, doi: 10.1038/s41593-019-0341-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Owen SF, Berke JD & Kreitzer AC Fast-Spiking Interneurons Supply Feedforward Control of Bursting, Calcium, and Plasticity for Efficient Learning. Cell 172, 683–695.e615, doi: 10.1016/j.cell.2018.01.005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agetsuma M, Hamm JP, Tao K, Fujisawa S & Yuste R Parvalbumin-Positive Interneurons Regulate Neuronal Ensembles in Visual Cortex. Cerebral Cortex 32, 1–15, doi: 10.1093/cercor/bhx169 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morrison DJ et al. Parvalbumin interneurons constrain the size of the lateral amygdala engram. Neurobiology of Learning and Memory 135, 91–99, doi: 10.1016/j.nlm.2016.07.007 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Roberts BM, White MG, Patton MH, Chen R & Mathur BN Ensemble encoding of action speed by striatal fast-spiking interneurons. Brain structure & function, 1–10, doi: 10.1007/s00429-019-01908-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gage GJ, Stoetzner CR, Wiltschko AB & Berke JD Selective activation of striatal fast-spiking interneurons during choice execution. Neuron 67, 466–479, doi: 10.1016/j.neuron.2010.06.034 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pisansky MT et al. Nucleus Accumbens Fast-Spiking Interneurons Constrain Impulsive Action. Biological Psychiatry, doi: 10.1016/j.biopsych.2019.07.002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Hare JK et al. Striatal fast-spiking interneurons selectively modulate circuit output and are required for habitual behavior. eLife 6, 32, doi: 10.7554/eLife.26231 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kravitz AV et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626, doi: 10.1038/nature09159 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui G et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238–242, doi: 10.1038/nature11846 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meng C et al. Spectrally Resolved Fiber Photometry for Multi- component Analysis of Brain Circuits. Neuron, 1–16, doi: 10.1016/j.neuron.2018.04.012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsutsui-Kimura I et al. Distinct Roles of Ventromedial versus Ventrolateral Striatal Medium Spiny Neurons in Reward-Oriented Behavior. Current Biology 27, 3042–3048.e3044, doi: 10.1016/j.cub.2017.08.061 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Natsubori A et al. Ventrolateral Striatal Medium Spiny Neurons Positively Regulate Food-Incentive, Goal-Directed Behavior Independently of D1 and D2 Selectivity. The Journal of neuroscience : the official journal of the Society for Neuroscience 37, 2723–2733, doi: 10.1523/JNEUROSCI.3377-16.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Calipari ES et al. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proceedings of the National Academy of Sciences of the United States of America 113, 2726–2731, doi: 10.1073/pnas.1521238113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Owesson-White C et al. Cue-Evoked Dopamine Release Rapidly Modulates D2 Neurons in the Nucleus Accumbens During Motivated Behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 6011–6021, doi: 10.1523/JNEUROSCI.0393-16.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Freund TF Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends in neurosciences 26, 489–495, doi: 10.1016/S0166-2236(03)00227-3 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Buzsáki G & Chrobak JJ Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Current Opinion in Neurobiology 5, 504–510 (1995). [DOI] [PubMed] [Google Scholar]
- 80.Düzel E, Penny WD & Burgess N Brain oscillations and memory. Current Opinion in Neurobiology 20, 143–149, doi: 10.1016/j.conb.2010.01.004 (2010). [DOI] [PubMed] [Google Scholar]
- 81.van der Meer M Integrating early results on ventral striatal gamma oscillations in the rat. Frontiers in neuroscience, 1–12, doi: 10.3389/fnins.2010.00300 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Freund TF & Katona I Perisomatic inhibition. Neuron 56, 33–42, doi: 10.1016/j.neuron.2007.09.012 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Sohal VS, Zhang F, Yizhar O & Deisseroth K Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702, doi: 10.1038/nature07991 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van der Meer MAA & Redish AD Low and High Gamma Oscillations in Rat Ventral Striatum have Distinct Relationships to Behavior, Reward, and Spiking Activity on a Learned Spatial Decision Task. Frontiers in integrative neuroscience 3, 9, doi: 10.3389/neuro.07.009.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berke JD, Okatan M, Skurski J & Eichenbaum HB Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43, 883–896, doi: 10.1016/j.neuron.2004.08.035 (2004). [DOI] [PubMed] [Google Scholar]
- 86.Sjulson L, Peyrache A, Cumpelik A, Cassataro D & Buzsáki G Cocaine Place Conditioning Strengthens Location- Specific Hippocampal Coupling to the Nucleus Accumbens. Neuron, 1–15, doi: 10.1016/j.neuron.2018.04.015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akam T & Kullmann DM Oscillations and filtering networks support flexible routing of information. Neuron 67, 308–320, doi: 10.1016/j.neuron.2010.06.019 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akam T & Kullmann DM Oscillatory multiplexing of population codes for selective communication in the mammalian brain. Nature Reviews Neuroscience 15, 111–122, doi: 10.1038/nrn3668 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koos T & Tepper JM Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nature Neuroscience 2, 467–472, doi: 10.1038/8138 (1999). [DOI] [PubMed] [Google Scholar]
- 90.Vida I, Bartos M & Jonas P Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron 49, 107–117, doi: 10.1016/j.neuron.2005.11.036 (2006). [DOI] [PubMed] [Google Scholar]
- 91.Otaka M et al. Exposure to cocaine regulates inhibitory synaptic transmission in the nucleus accumbens. J Neurosci 33, 6753–6758, doi: 10.1523/JNEUROSCI.4577-12.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mu P et al. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci 30, 3689–3699, doi: 10.1523/JNEUROSCI.4063-09.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilson CJ & Kawaguchi Y The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 16, 2397–2410 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stern EA, Jaeger D & Wilson CJ Membrane potential synchrony of simultaneously recorded striatal spiny neurons in vivo. Nature 394, 475–478, doi: 10.1038/28848 (1998). [DOI] [PubMed] [Google Scholar]
- 95.Korpi ER et al. Mechanisms of Action and Persistent Neuroplasticity by Drugs of Abuse. Pharmacological Reviews 67, 872–1004, doi: 10.1124/pr.115.010967 (2015). [DOI] [PubMed] [Google Scholar]
- 96.Di Chiara G & Imperato A Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences 85, 5274–5278, doi: 10.1073/pnas.85.14.5274 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Phillips PEM, Stuber GD, Heien MLAV, Wightman RM & Carelli RM Subsecond dopamine release promotes cocaine seeking. Nature 422, 614–618, doi: 10.1038/nature01476 (2003). [DOI] [PubMed] [Google Scholar]
- 98.Volkow ND, Wise RA & Baler R The dopamine motive system: implications for drug and food addiction. Nature Reviews Neuroscience 18, 741–752, doi: 10.1038/nrn.2017.130 (2017). [DOI] [PubMed] [Google Scholar]
- 99.Tritsch NX & Sabatini BL Dopaminergic Modulation of Synaptic Transmission in Cortex and Striatum. Neuron 76, 33–50, doi: 10.1016/j.neuron.2012.09.023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bracci E, Centonze D, Bernardi G & Calabresi P Dopamine excites fast-spiking interneurons in the striatum. Journal of neurophysiology 87, 2190–2194, doi: 10.1152/jn.00754.2001 (2002). [DOI] [PubMed] [Google Scholar]
- 101.Wiltschko AB, Pettibone JR & Berke JD Opposite effects of stimulant and antipsychotic drugs on striatal fast-spiking interneurons. Neuropsychopharmacology 35, 1261–1270, doi: 10.1038/npp.2009.226 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koob GF & Volkow ND Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238, doi: 10.1038/npp.2009.110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Everitt BJ & Robbins TW Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annual review of psychology 67, 23–50, doi: 10.1146/annurev-psych-122414-033457 (2016). [DOI] [PubMed] [Google Scholar]
- 104.Hearing M, Graziane N, Dong Y & Thomas MJ Opioid and Psychostimulant Plasticity: Targeting Overlap in Nucleus Accumbens Glutamate Signaling. Trends in pharmacological sciences, 1–19, doi: 10.1016/j.tips.2017.12.004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mu P et al. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 3689–3699, doi: 10.1523/JNEUROSCI.4063-09.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang J et al. Cascades of Homeostatic Dysregulation Promote Incubation of Cocaine Craving. The Journal of neuroscience : the official journal of the Society for Neuroscience 38, 4316–4328, doi: 10.1523/JNEUROSCI.3291-17.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Otaka M et al. Exposure to Cocaine Regulates Inhibitory Synaptic Transmission in the Nucleus Accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 6753–6758, doi: 10.1523/JNEUROSCI.4577-12.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hollander JA & Carelli RM Abstinence from cocaine self-administration heightens neural encoding of goal-directed behaviors in the accumbens. Neuropsychopharmacology 30, 1464–1474, doi: 10.1038/sj.npp.1300748 (2005). [DOI] [PubMed] [Google Scholar]
- 109.Hollander JA & Carelli RM Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 3535–3539, doi: 10.1523/JNEUROSCI.3667-06.2007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guillem K, Ahmed SH & Peoples LL Escalation of cocaine intake and incubation of cocaine seeking are correlated with dissociable neuronal processes in different accumbens subregions. Biological Psychiatry 76, 31–39, doi: 10.1016/j.biopsych.2013.08.032 (2014). [DOI] [PubMed] [Google Scholar]
- 111.Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP & West MO Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 7239–7245 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Augustin SM & Lovinger DM Functional Relevance of Endocannabinoid-Dependent Synaptic Plasticity in the Central Nervous System. ACS Chemical Neuroscience 9, 2146–2161, doi: 10.1021/acschemneuro.7b00508 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kullmann DM, Moreau AW, Bakiri Y & Nicholson E Plasticity of inhibition. Neuron 75, 951–962, doi: 10.1016/j.neuron.2012.07.030 (2012). [DOI] [PubMed] [Google Scholar]
- 114.Mathur BN, Tanahira C, Tamamaki N & Lovinger DM Voltage drives diverse endocannabinoid signals to mediate striatal microcircuit-specific plasticity. Nature Neuroscience 16, 1275–1283, doi: 10.1038/nn.3478 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grueter BA, Brasnjo G & Malenka RC Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nature Neuroscience 13, 1519–1525, doi: 10.1038/nn.2685 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Purgianto A, Loweth JA, Miao JJ, Milovanovic M & Wolf ME Surface expression of GABAA receptors in the rat nucleus accumbens is increased in early but not late withdrawal from extended-access cocaine self-administration. Brain Research 1642, 336–343, doi: 10.1016/j.brainres.2016.04.014 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ribeiro EA et al. Transcriptional and physiological adaptations in nucleus accumbens somatostatin interneurons that regulate behavioral responses to cocaine. Nature Communications, 1–10, doi: 10.1038/s41467-018-05657-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dobbs LK et al. Dopamine Regulation of Lateral Inhibition between Striatal Neurons Gates the Stimulant Actions of Cocaine. Neuron 90, 1100–1113, doi: 10.1016/j.neuron.2016.04.031 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suska A, Lee BR, Huang YH, Dong Y & Schlüter OM Selective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaine. Proceedings of the National Academy of Sciences of the United States of America 110, 713–718, doi: 10.1073/pnas.1206287110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Neumann PA et al. Cocaine-Induced Synaptic Alterations in Thalamus to Nucleus Accumbens Projection. Neuropsychopharmacology 41, 2399–2410, doi: 10.1038/npp.2016.52 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boudreau AC, Reimers JM, Milovanovic M & Wolf ME Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 10621–10635, doi: 10.1523/JNEUROSCI.2163-07.2007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Conrad KL et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454, 118–121, doi: 10.1038/nature06995 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pascoli V et al. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509, 459–464, doi: 10.1038/nature13257 (2014). [DOI] [PubMed] [Google Scholar]
- 124.Dong Y et al. CREB modulates excitability of nucleus accumbens neurons. Nature Neuroscience 9, 475–477, doi: 10.1038/nn1661 (2006). [DOI] [PubMed] [Google Scholar]
- 125.Wright WJ et al. Silent Synapses Dictate Cocaine Memory Destabilization and Reconsolidation. Nature Neuroscience In Press (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang YH et al. In vivo cocaine experience generates silent synapses. Neuron 63, 40–47, doi: 10.1016/j.neuron.2009.06.007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee BR et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nature Neuroscience 16, 1644–1651, doi: 10.1038/nn.3533 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma Y-Y et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83, 1453–1467, doi: 10.1016/j.neuron.2014.08.023 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Burton AC et al. Previous cocaine self-administration disrupts reward expectancy encoding in ventral striatum. Neuropsychopharmacology, 1–11, doi: 10.1038/s41386-018-0058-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.MacAskill AF, Cassel JM & Carter AG Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens. Nature Neuroscience 17, 1198–1207, doi: 10.1038/nn.3783 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Neumann PA et al. Cocaine-Induced Synaptic Alterations in Thalamus to Nucleus Accumbens Projection. Neuropsychopharmacology, doi: 10.1038/npp.2016.52 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Purgianto A, Weinfeld ME & Wolf ME Prolonged withdrawal from cocaine self-administration affects prefrontal cortex- and basolateral amygdala-nucleus accumbens core circuits but not accumbens GABAergic local interneurons. Addiction Biology, 1–13, doi: 10.1111/adb.12430 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grewe BF et al. Neural ensemble dynamics underlying a long-term associative memory. Nature, 1–23, doi: 10.1038/nature21682 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peters AJ, Chen SX & Komiyama T Emergence of reproducible spatiotemporal activity during motor learning. Nature 510, 263–267, doi: 10.1038/nature13235 (2014). [DOI] [PubMed] [Google Scholar]
- 135.Gawin FH & Kleber HD Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Archives of general psychiatry 43, 107–113 (1986). [DOI] [PubMed] [Google Scholar]
- 136.Hayama T et al. GABA promotes the competitive selection of dendritic spines by controlling local Ca2+ signaling. Nature Neuroscience 16, 1409–1416, doi: 10.1038/nn.3496 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


