1.0. Introduction
A healthy, young person can usually hear pure tones up to around 20 kHz, yet clinical audiometry, the gold-standard for detecting hearing loss, typically only measures tone sensitivity to 8 kHz. In this collection of research reviews and summaries, based on a symposium at the Annual Mid-Winter Meeting of the Association for Research in Otolaryngology in February 2019, we present arguments and evidence pointing to the role of extended high frequency (EHF) hearing, above 8 kHz, in human auditory perception. We conclude by suggesting that, while a causal connection between EHF hearing loss and impaired speech perception is not yet certain, there is an overwhelming body of evidence consistent with that hypothesis. Of huge clinical significance is the evidence that EHF hearing loss is easily measured and may be a very early warning sign of age-related hearing impairment.
Brian Monson opens this compilation by pointing to the role of EHF in speech perception and localization, and Lord Raleigh’s observation more than a century ago that the location of a talker facing away from a listener is degraded. Bringing that story right up to date, Monson and colleagues have recently shown that EHF hearing supports speech-in-noise (SiN) performance when background talkers’ head rotations are varied, because EHF energy radiates primarily toward the front of a talker. David Moore and Lina Motlagh Zadeh studied the correlation between EHFs speech reception thresholds, emphasizing self-reported SiN difficulty as a strong predictor of EHF hearing loss. Their approach included digits-in-noise (DIN) performance where the masker had more or less energy only in the EHF range, showing that listeners do better with less masking of EHFs. Standard and EHF thresholds were highly correlated, however, with a small elevation (~3 dB) in standard frequencies in the presence of a larger EHF hearing loss. What is the effect of such slightly elevated standard thresholds in relation to EHF hearing? Beverly Wright takes up this question, continuing the theme of SiN hearing. She reviews a number of studies, showing that both standard and EHF hearing loss have been widely found to associate with difficulty understanding SiN. She posits four intriguing hypotheses that provide fertile ground for new research and could explain the common phenomenon of reported difficulty in SiN hearing, despite “normal” standard frequency pure tone hearing.
EHF hearing is known to be highly age-dependent, but also highly variable. A high-precision approach by Sumitrajit Dhar, Samantha Stiepan and Jonathan Siegel compares distortion product otoacoustic emissions (DPOAEs) with behavioral thresholds across the lifespan, targeting EHF as the primary spectral region of change. Using these combined techniques, age-related decline in the EHF region first becomes “clinically significant” in 36-45-year-olds and is across a broader and lower frequency range for OAE than for EHF thresholds. Reduced DPOAEs precede standard hearing threshold elevation, providing an earlier warning sign. Lisa Hunter, Chelsea Blankenship and David Moore discuss the importance of sensitive thresholds in young children during the crucial time of intensive language learning, to allow discrimination between consonants that are distinguished by EHF energy. Using pure tone audiometry and SiN in older children assessed for listening difficulties or dosed with aminoglycoside antibiotics, EHF hearing is highly susceptible to middle ear disease and ototoxicity. Frequent surveillance of EHF hearing through childhood for these risk categories is therefore advocated. Finally, clinical rationale and methods to deliver EHF surveillance are the themes taken up by Kevin Munro. Several myths exist around testing EHFs, including standing waves, instrumentation availability, measurement calibration, and reliability, but these are systematically reviewed and shown to be relatively feasible to deal with in clinical settings. Based on these contributions, we suggest that professional bodies and research funders alike should now rethink EHFs with an eye towards further research and early clinical implementation, especially for at-risk individuals.
2.0. Ecological Relevance of EHF Hearing
Brian B. Monson
A fundamental principle of biology is that each species’ and individual’s sensory systems are tailored to meet the demands placed upon them by their environments and experiences. Accordingly, auditory systems across different species exhibit distinctive upper and lower limits for the frequency range of hearing (Fay, 1988; Heffner and Heffner, 2007; Heffner, 2004; Masterton et al., 1969). The audible frequency range for humans spans approximately 20 Hz to 20 kHz. Frequencies beyond 8 kHz have been termed extended high frequencies (EHFs).
At first glance, EHF hearing for humans may seem somewhat puzzling: what is its ecological utility? Audiological assessment of EHF sensitivity is not part of standard clinical routine at present, the implicit assumption being that it is not critical for daily functioning. Perhaps this is because, relative to midrange frequencies, humans display poorer sensitivity to EHF tones, which require higher sound pressure levels to achieve audibility (ISO 226). Furthermore, in typical forms of hearing loss, EHF hair cells, located at the base of cochlea, are the most vulnerable to damage. Indeed, common age-related hearing loss at EHFs begins as early as young adulthood, with substantial losses observed across the EHF range by age 50 for the typically aging population (Green et al., 1987; Stelmachowicz et al., 1989). How, then, are EHFs relevant to the environmental demands placed upon the human auditory system?
2.1. Localization and EHFs.
The dominant view is that sensitivity to EHFs is ecologically relevant because it facilitates sound localization (Heffner and Heffner, 2008). Several studies have demonstrated that low-pass filtering of broadband sounds at 8 kHz increases front/back confusions and elevation judgment errors, although lateral-angle judgments remain relatively stable (Brungart and Simpson, 2009; Carlile et al., 1999; King and Oldfield, 1997; Langendijk and Bronkhorst, 1999). Elevation-dependent and front/back-dependent spectral changes observed at EHFs in head-related transfer functions are thought to explain these findings (Langendijk and Bronkhorst, 1999).
It bears mentioning that most demonstrations of EHF utility for sound localization use synthetic stimuli (clicks and noise bursts) that exhibit artificially high energy levels at EHFs, precluding inferences regarding EHF utility for localization of natural sounds. One notable exception is a study by Best et al., (2005), who used broadband recordings of speech. Their study revealed that audible speech energy at EHFs provides cues to resolve front/back confusions for speech localization. This finding is reminiscent of Lord Rayleigh’s observation more than a century ago that front/back confusions can be introduced by rotating a talker’s head such that the talker faces away from the listener (Rayleigh, 1908). In this scenario, the talker’s speech is effectively low pass filtered at the ear of the listener due to the frequency dependence of speech radiation directivity. That is, radiation patterns for speech are more omnidirectional for lower frequency components and increasingly directional (toward the front of a talker) for higher frequency components (Chu and Warnock, 2002; Halkosaari et al., 2005; Monson et al., 2012a), with EHFs being most directional (Kocon and Monson, 2018; Monson et al., 2012a).
2.2. Speech perception and EHFs
Despite the report of Best et al. (2005), it has been widely believed that EHFs play little to no role in speech perception. One reason for this view is that seminal studies in speech perception demonstrated that energy below approximately 7 kHz is sufficient to reproduce intelligible speech for transmission over communication systems (Crandall and MacKenzie, 1922; Fletcher and Galt, 1950; Fletcher and Steinberg, 1930; Monson et al., 2014). This work resulted in the widespread conception of a restricted information-bearing “speech bandwidth” that did not include EHFs. Perpetuating this view of EHFs are the reports and widely held belief that little acoustic energy exists in the speech signal beyond 8 kHz. Consequently, study of the audibility and utility of speech spectral energy beyond 8 kHz has been lacking.
Is EHF energy in speech negligible? One reason that little energy is thought to exist in this region may be because of the oft-used long-term average spectral (LTAS) representation of speech (Figure 1A). Energy beyond 8 kHz is approximately 30 dB below peak energy at lower frequencies. However, the typical LTAS representation suffers from two drawbacks. First, spectral energy is often calculated using a linear frequency resolution with the fast Fourier transform (FFT). For example, a 2048-point FFT with sampling rate 44.1 kHz results in 21.5-Hz resolution. Binning acoustic energy into 21.5-Hz-wide bands across all frequencies is not a useful way to represent auditory processing. Using a more physiologically or perceptually relevant binning approach with third-octave bands or equivalent rectangular bandwidths (ERB; Glasberg and Moore, 1990) reveals that averaged speech energy beyond 8 kHz may be only 10-20 dB lower in level than energy at lower frequencies (Figure 1A; Levy et al., 2015; Monson et al., 2012b; Moore et al., 2008). Second, because the LTAS averages energy across time, temporal dynamics of EHF energy bursts, such as those for voiceless fricatives, are lost. A spectrographic representation of speech provides better insight into the nature and potential utility of EHF energy (Figure 1B). With these considerations, we sought to determine the upper limit to EHF audibility in speech. Accordingly, we demonstrated that the “maximum audible low-pass filter cutoff frequency” for speech was approximately 13 kHz for the average young normal-hearing listener, but was correlated with pure tone threshold at 16 kHz (Monson and Caravello, 2019). That listeners can, on average, detect the loss of speech spectral energy beyond 13 kHz may be surprising to many researchers and clinicians.
Figure 1.
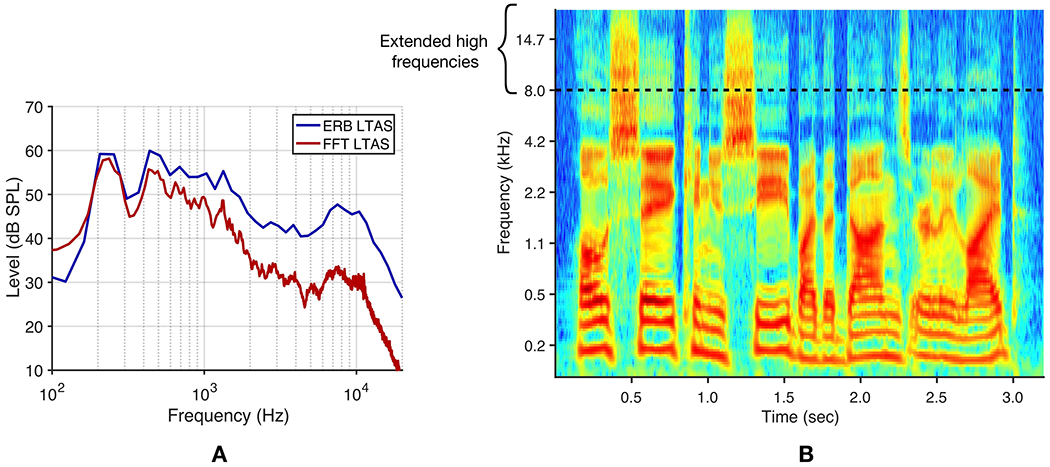
(Color). EHF spectral energy in speech. A. Long-term average spectra for a 60-s two-female-talker babble stimulus using a 2048-point FFT, resulting in 21.5-Hz-wide bands across all frequencies (FFT LTAS) and using equivalent rectangular bandwidths (ERBs), with 1-ERB-wide filter bands and 50% overlap (ERB LTAS). B. Cochleagram for one male talker uttering the phrase, “Oh say, can you see by the dawn’s early light,” using 1-ERB-wide filter bands with 50% overlap (McDermott and Simoncelli, 2011).
It has been proposed that human speech may exert influence on the development and retention of basic auditory system features (Manley, 2017; Theunissen and Elie, 2014), presumably due to the biological relevance of conspecific vocalizations. This proposition provides an alternative to the traditional view of EHFs and speech perception: human auditory system features, including the upper limit of hearing, have developed because of their ecological utility for detection and processing of speech. From this vantage point, the presence of EHF hearing in humans takes on new meaning. The challenge becomes to identify how and the conditions under which EHF hearing supports speech perception. For example, we recently demonstrated that EHF hearing improves the discrimination of a talker’s head rotation (Monson et al., 2019). This is because EHF radiation patterns are highly directional, with EHF energy radiating from the mouth primarily toward the front of a talker (Figure 2A; Chu and Warnock, 2002; Halkosaari et al., 2005; Kocon and Monson, 2018; Monson et al., 2014, 2012b). Thus EHF energy serves as a salient cue to determine the direction a talker is facing, an ability proposed to be important for ascertaining whether one is the intended recipient of an utterance (Neuhoff, 2003).
Figure 2.
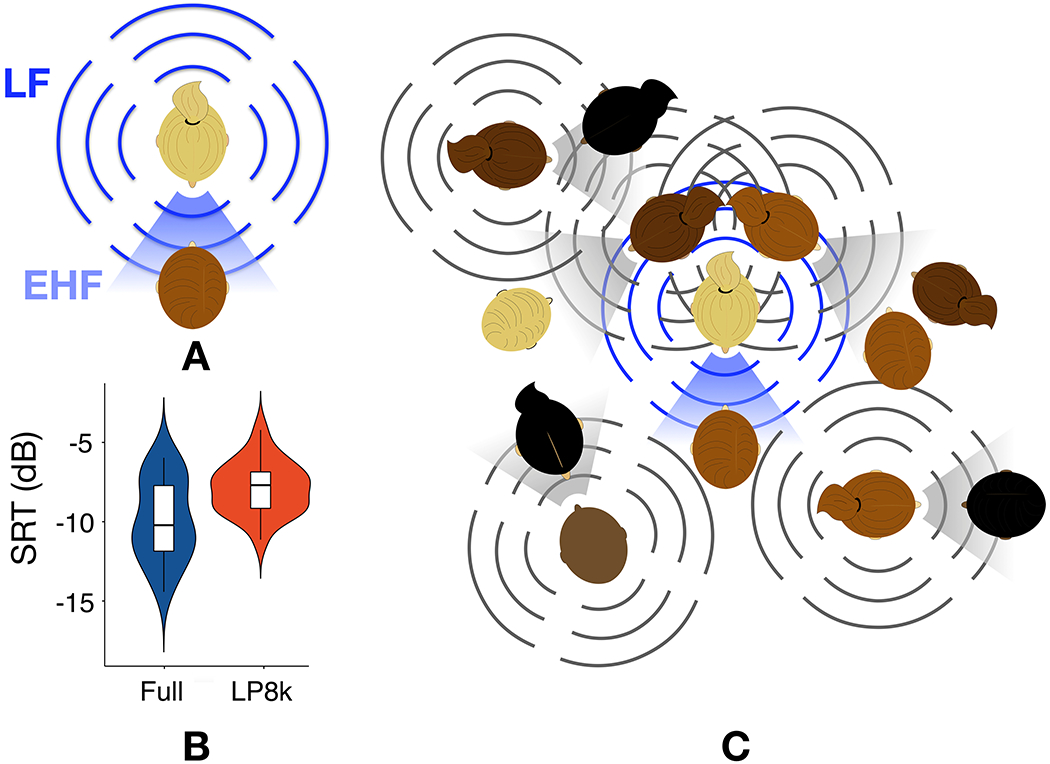
(color). Hearing loss and hearing difficulty. A. Whereas low-frequency (LF) energy in speech radiates nearly omnidirectionally around a talker (bars), EHF energy in speech radiates primarily toward the front of the talker (shading). B. Speech reception thresholds (SRT) for speech-in-speech listening when the target talker is facing the listener but two co-located background talkers’ heads are rotated by 60°. Filtering conditions were full band (Full) or all stimuli low-pass filtered at 8 kHz (LP8k) C. In the ecological cocktail party, the listener primarily receives substantial EHF energy from only the target talker (blue), whereas there would be substantial LF energy interference from other talkers.
2.3. EHF hearing and speech recognition
It has been demonstrated that EHFs in speech provide some phonetic information, useful for vowel and consonant identification when lower-frequency energy is absent or degraded (Lippmann, 1996; Vitela et al., 2015) or when extended bandwidth hearing aids restore EHF audibility (Seeto and Searchfield, 2018). This might explain how some individuals with profound hearing loss below 8 kHz, but residual hearing above 8 kHz, learn to speak with surprisingly good articulation (Berlin et al., 1978). Taking into consideration the directionality of EHFs in speech, we demonstrated that losing access to EHFs via low-pass filtering of speech stimuli at 8 kHz produced a deficit in speech-in-speech recognition for normal hearing listeners when the target talker was facing the listener, but co-located maskers’ heads were rotated away from the listener (Monson et al., 2019). Under these listening conditions, speech reception thresholds for normal hearing listeners were significantly elevated in the 8-kHz low-pass condition (Figure 2B). Whereas the traditional speech-in-speech experiment simulates a listening scenario where both the target talker and maskers are facing a listener (owing to speech materials being recorded with a microphone directly in front of the talker), our approach reflects a more ecologically relevant “cocktail party” listening scenario (Figure 2C), where it would be unusual to have multiple talkers all facing a listener and talking at the same time. Thus, at the ecological cocktail party, a listener receives EHF spectral energy primarily from the target talker, whereas masking energy from background talkers is primarily in the lower frequencies. This directionality effect makes EHFs a salient cue for detection and segregation of the talker of interest from background talkers, in addition to increasing accessibility of phonetic information at EHFs. Consequently, individuals with hearing loss at EHFs may incur deficits in recognizing speech in complex listening environments.
From a theoretical standpoint, EHF hearing is essential for humans. Empirical evidence suggests it has ecological utility for speech perception, particularly in complex listening environments. Adoption of speech materials that incorporate the effects of frequency-dependent speech directionality could improve the ecological relevance of research studies and clinical assessments. The next two sections address other studies on speech perception in noise related to EHF hearing, and self-perception of hearing difficulty in normal and impaired listeners.
3.0. Benefits of EHF hearing for speech perception in noise
David R. Moore, Lina Motlagh Zadeh
Extended high frequency hearing loss may be considered a form of “hidden hearing loss” (Kujawa and Liberman, 2019; Schaette and McAlpine, 2011) in that it can occur in conjunction with normal pure-tone audiometry in the standard frequency range ≤ 8 kHz; (Badri et al., 2011; Motlagh Zadeh et al., 2019). In a study of middle-aged, otherwise normal hearing adults, Yeend and colleagues (2019) showed significant correlations between poorer EHF hearing thresholds and reduced scores on self-report (Speech, Spatial and Qualities of Hearing, SSQ; Gatehouse and Noble, 2004) and speech intelligibility measures (Listening in Spatialized Noise Sentences; LiSN-S, high-cue condition; Cameron and Dillon, 2007). Others have challenged this view, suggesting that sound energy in the EHF range is not functionally useful for listening to speech in challenging environments. For example, after adjusting for age, Mepani et al., (2019) did not find a significant correlation between EHF pure-tone thresholds and word recognition scores.
Digits-in-noise (DIN) is a quick, reliable and automated speech-in-noise measure that both correlates highly with audiometric pure tone average and simulates aspects of hearing that listeners frequently report difficulty with (Jansen et al., 2013; Smits et al., 2013). The typical broadband, BB-DIN presents digit triplets against an adaptively varied level of speech-shaped noise, yielding a speech reception threshold (SRT). To increase DIN sensitivity to high frequency hearing loss, Vlaming and colleagues (2014) substituted a 1.5 kHz low-pass filtered noise, increasing dependency for hearing the digits on the higher frequency end of hearing.
We recently reported higher frequency filtering effects on DIN in 60 adults (18-62 y/o, median=24.5 y/o) with clinically normal hearing (Motlagh Zadeh et al., 2019) in which we substituted an 8 kHz low-pass filtered noise (8 kHz-DIN), increasing dependency for hearing the digits only on EHF. In addition, we used standard and EHF audiometry, and a single question about ease of listening in noise (Moore et al., 2014) to examine whether EHF hearing loss contributes to the difficulty some adults experience in challenging listening conditions. Of these mostly young adults, 34/60 (57%) had an EHF hearing loss relative to adult criteria for normal hearing (> 20 dB HL re: (ISO, 2006) in at least one ear and at one EHF (Fig. 3a). Listeners who self-reported difficulty hearing in noise (16/60; 27%) had higher mean EHF thresholds than those reporting no difficulty (p < 0.0001; Fig. 3b). Of 13 listeners with self-reported difficulty and elevated EHF thresholds, 12 (92%) had bilateral EHF hearing loss (2 to 4 elevated hearing thresholds > 20 dB HL in each ear). Both mean EHF threshold and prevalence of self-reported difficulty increased with age (p < 0.0001). However, when the sample was limited to those 18-30 years old (40/60), the proportion with EHF hearing loss in at least one ear remained high at 19/40 (48%). It is possible that the discrepancy reported here with the results of (Mepani et al., 2019) is due in part to the higher prevalence of younger adults with EHF hearing loss in the current sample, based on a more liberal criterion for EHF hearing loss.
Figure 3.
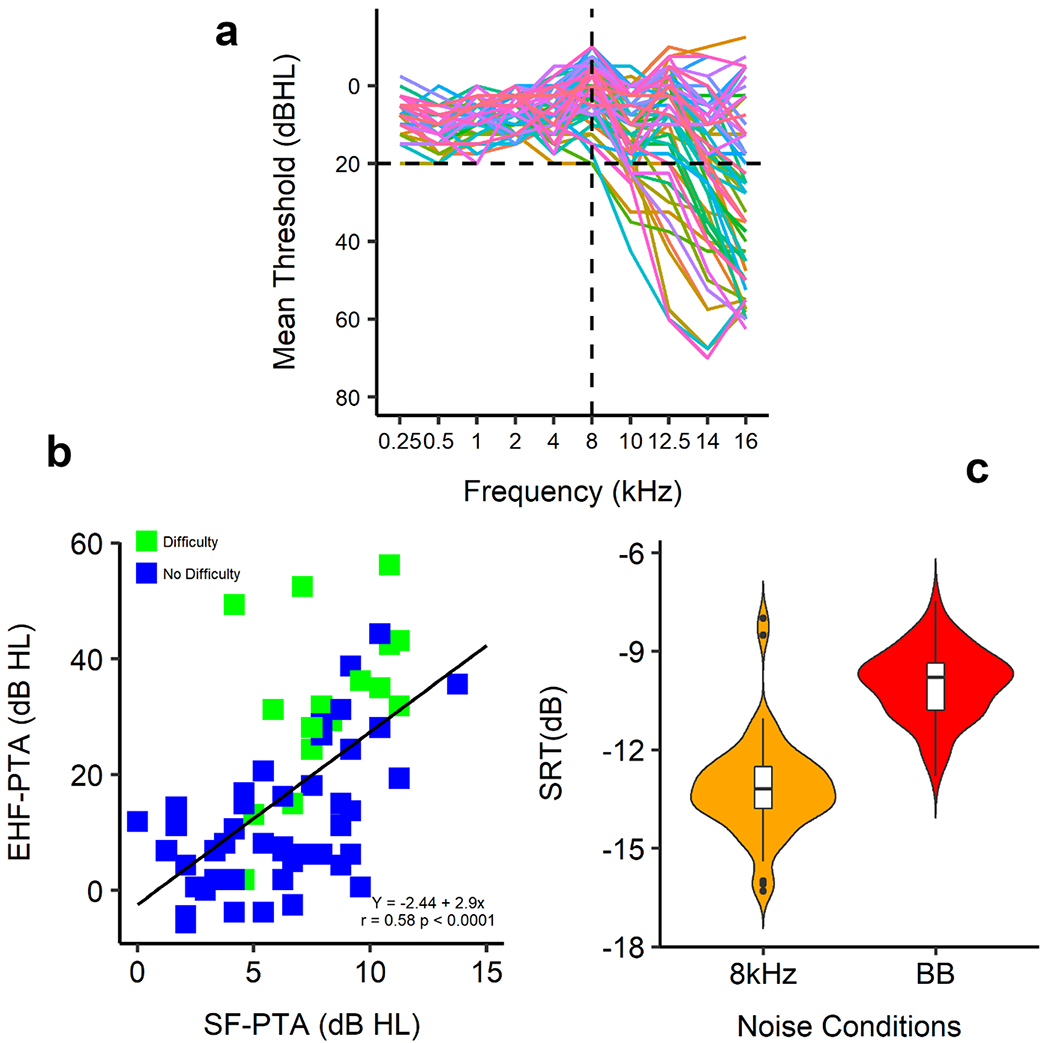
Hearing loss and hearing difficulty. A. Mean hearing thresholds of both ears for standard frequency and extended high-frequency ranges. Black horizontal line shows the level of normal hearing sensitivity (≤ 20 dB HL). Black vertical line indicates standard range of the audiogram (0.25-8 kHz). B. Correlation between pure tone average of standard frequency (PTA-SF) and pure tone average of extended high frequency (PTA-EHF) thresholds for individual listeners with and without self-reported difficulty listening in noise. C. Speech reception threshold (SRT) as a function of broadband (BB) and 8 kHz low-pass filtered noise. Violin plots show kernel probability density of thresholds, boxes are interquartile range (with median), and whiskers are 1.5 times the interquartile range.
Because of correlations between hearing thresholds across frequency, it is possible that listeners with EHF hearing loss may be affected by standard frequency thresholds that, although “normal”, are still elevated relative to those of listeners with normal EHF hearing. In fact, we found a significant correlation between mean standard frequency thresholds and mean EHF thresholds (r = 0.58, p < 0.0001). Those with EHF hearing loss had a mean elevation at standard frequencies of 3.7 dB. Elevated standard frequency thresholds could thus account for some of the differing self-reports of these groups. However, only EHF hearing loss was a significant predictor of self-reported difficulty hearing in noise (F1,58 = 24, p < 0.0001), whereas standard frequency hearing was not a significant predictor (F1,58 = 3.3, p = 0.07). However, only EHF hearing loss correlated with self-reported difficulty (p < 0.0001), whereas standard frequency hearing was not correlated with self-reported difficulty (p = 0.07). Those with EHF hearing loss had a mean elevation of standard frequencies of 3.7 dB. Elevated standard frequency thresholds could thus account for some of the differing self-reports of these groups. However, only EHF hearing loss was a significant predictor of self-reported difficulty hearing in noise (F1, 58 = 24, p < 0.0001), whereas standard frequency hearing was not a significant predictor (F1, 58 = 3.3, p = 0.07). In addition, mean 8 kHz-DIN SRT was significantly better than mean BB-DIN SRT by 3.2 dB (p < 0.0001; Fig. 3c), showing the contribution of EHF hearing to the intelligibility of the digits.
We found the extent of specific EHF hearing loss is related to the number of individuals self-reporting difficulty hearing and to speech perception in noise. EHF hearing loss (Rodriguez Valiente et al., 2014a) and self-reported difficulty (Motlagh Zadeh et al., 2019) are already widespread among younger adults (< 30 y/o) suggesting that, even at this young age, speech perception in challenging conditions is reduced. Extending routine hearing testing to the EHF range thus appears to provide a clinically desirable supplement to conventional audiometry for evaluation of a person’s functional hearing and speech perception capacity. This could in turn lead to more timely and effective prevention of and intervention for further acquired hearing loss later in life.
4.0. EHF Hearing and Difficulty Understanding Speech in Noise
Beverly Wright
As discussed in the previous section, many people report difficulty understanding speech in background noise despite having clinically normal audiograms. This difficulty has been referred to by a variety of different names, including obscure auditory dysfunction (Saunders and Haggard, 1989), auditory disability with normal hearing (King and Stephens, 1992) and King-Kopetzky syndrome (Hinchcliffe, 1992). We will refer to it as difficulty understanding speech in noise despite normal hearing (D-SPiN-NH). Here we review evidence that people with D-SPiN-NH have hearing loss in the EHF range, and discuss possible relationships between that hearing loss and poor SPiN performance.
There is considerable evidence that people with D-SPiN-NH have higher absolute thresholds than controls for frequencies in the EHF range. EHF hearing in people with D-SPiN-NH has been evaluated in five investigations. In all of those cases, thresholds for the D-SPiN-NH group were consistently higher (worse) than for the control group by approximately 6 to 23 dB for at least one frequency in the EHF range (King and Stephens, 1992; Shaw et al., 1996; Badri et al., 2011; Guest et al., 2018; Yeend et al., 2019). In another investigation, thresholds were measured across the standard-audiometric and EHF ranges in groups deemed to be at high risk versus low risk for ear damage based on noise-exposure history and consistency of use of hearing protection (Liberman et al., 2016). Thresholds did not differ between the groups in the standard audiometric range, but were 10-20 dB higher in the EHF range for the high-risk than the low-risk group. Those groups also happened to differ in SPiN perception, with the high-risk group showing the poorer SPiN performance.
The prevalence of hearing loss in the EHF range among people with D-SPiN-NH suggests that the measurement of EHF hearing would be a useful addition to audiological assessments. At a minimum, documentation of an EHF hearing loss in individuals with D-SPiN-NH could confirm their personal assessments that something is wrong with their hearing (also see, e.g., Yeend et al., 2019). However, additional work is needed to determine the relationship between EHF hearing loss and poor SPiN performance. Below we consider four hypotheses: EHF-audibility, low frequencies (LF)-through-EHF, LF-influenced-by-EHF, and EHF-bellwether.
The EHF-audibility hypothesis and the LF-through-EHF hypothesis posit that neurons with characteristic frequencies (CFs) tuned to EHFs provide information that is helpful for speech recognition in noise by encoding either EHF or lower frequency (LF) information in the speech signal. According to the EHF-audibility hypothesis, hearing loss in the EHF range reduces the audibility of speech information in that range, resulting in impaired SPiN performance. This idea essentially reflects a basic assumption of the articulation index--an influential model of speech recognition--that the contribution of any given spectral region to speech recognition is determined entirely by the audibility within that region (French and Steinberg, 1947) According to the LF-through-EHF hypothesis, hearing loss in the EHF range reduces the transmission of speech information at lower frequencies through the tails of tuning curves tuned to EHFs, resulting in impaired SPiN performance. The CF of an auditory neuron is determined by the frequency at the tip of the tuning curve, but auditory neurons also respond to non-CF frequencies, particularly those that are lower than the CF, when the stimulus is of sufficient intensity. Stimulation through these tuning-curve tails therefore encodes information from frequencies other than the CF (e.g., (Kiang and Moxon, 1974). High-CF fibers can even encode low-frequency information more accurately than low-CF fibers in some cases (e.g., Joris et al., 1994). For a mini-review, see Horwitz et al., 2002). Thus, neurons with high (or possibly EHF) CFs may aid in the encoding of speech information at lower frequencies (e.g., Kiang and Moxon, 1974; Greenberg, 1988).
The LF-influenced-by-EHF hypothesis and the EHF-bellwether hypothesis focus instead on neurons with CFs tuned to lower frequencies, positing that those neurons either are, or are not, influenced by EHF processing. According to the LF-influenced-by-EHF hypothesis, hearing loss in the EHF range reduces input that is necessary for the proper functioning of mechanisms acting at lower frequencies, resulting in impaired SPiN performance. This explanation differs from the first two in that it holds that mechanisms in the EHF region aid the encoding of stimuli in the LF range through neurons tuned to frequencies in the LF, rather than the EHF, range. This idea gains some support from evidence that, for example, stimulus evoked otoacoustic emissions evoked by lower-frequency tones arise, at least in part, from more basal regions along the basilar membrane that encode frequencies 2-3 octaves higher than the emission frequency (Charaziak and Siegel, 2015). Alternatively, according to the EHF-bellwether hypothesis, hearing loss in the EHF range is an indicator of hearing damage at lower frequencies, but EHF hearing loss does not contribute directly to difficulties understanding speech in noise. The idea is that the circumstances that induce hearing loss in the EHF range likely also affect LF hearing, for example, by increasing absolute threshold (e.g., Strickland et al., 2004), or by degrading other aspects of hearing even when absolute thresholds are normal (e.g., Badri et al., 2011). A related idea is that, even in the EHF range, the primary contributor to poor SPiN performance, may not be the hearing loss itself, but rather other aspects associated with that loss, such as poorer spectral resolution or reduced dynamic range.
Discriminating among these hypotheses is challenging because they are not all mutually exclusive and several make similar predictions. Nevertheless, points of distinction can be identified. First, only the EHF-audibility hypothesis requires that there be audible, useful, speech information in the EHF range, both of which appear to be the case (Lippmann, 1996; Monson et al., 2019; Motlagh Zadeh et al., 2019; Vitela et al., 2015).
Second, all but the EHF-bellwether hypothesis, predict that reducing the contribution of processing in the EHF range, say through the addition of a high-pass noise, should reduce SPiN performance. That is because the high-pass noise should disrupt the direct encoding of useful EHF information (EHF-audibility), the encoding of LF information (LF-through-EHF), and the proper interaction between the EHF region and the LF neurons (LF-influenced-by-EHF). In this regard, adding high-pass noise with a cutoff frequency between 3 to 4 kHz (well below the EHF range) appears to have had little influence on SPiN performance in participants with normal hearing aside from decrements that could be attributed to downward spread of masking (Strickland, 1994; Strickland et al., 2004). However, SPiN performance with low-pass-filtered speech (cutoff of 1.79 kHz) was poorer in participants with high-frequency hearing loss extending into the EHF range (66-79 years) than in controls (aged 21-35 years), despite similar speech audibility for both groups (Horwitz et al., 2002). This result was interpreted as evidence that intact high-frequency hearing was beneficial even when no speech information was available in that region (Horwitz et al., 2002, but see Strickland, 2004). Equivalent evaluations have yet to be conducted with high-pass noise with cutoff frequencies in the EHF range. If EHF-cutoff high-pass noise diminishes SPiN performance, as predicted, the next step will be to design evaluations that can distinguish among the three hypotheses that make the prediction.
Third, only the LF-influenced-by-EHF and EHF-bellwether hypotheses predict that there should be indications of hearing deficits in LF regions in the D-SPiN-NH population. A number of such deficits have been documented at frequencies < 8 kHz. For example, compared to controls, D-SPiN-NH groups have been reported to have: (1) higher thresholds, typically by 2-6 dB (Saunders and Haggard, 1989; King and Stephens, 1992; Zhao and Stephens, 2000; Yeend et al., 2019); (2) atypical OAEs (e.g., (Stephens et al., 2003; Zhao and Stephens, 2006); (3) poorer frequency selectivity (e.g., Saunders and Haggard, 1989), particularly on the high-frequency side (Badri et al., 2011); and (4) poorer temporal resolution (e.g., King and Stephens, 1992). Therefore, the next step is to determine whether these LF deficits are a consequence of EHF hearing loss. One approach would be to determine if there are cases in which there are deficits at lower frequencies in the absence of EHF loss in this population. A modest example is a report that, among a small group of participants with D-SPiN-NH, 58% had EHF hearing loss, but 77% had wider auditory filter shapes than normal at 2 kHz (Badri et al., 2011), suggesting that the deficits at lower frequencies can occur independently of EHF loss. Of course, just as for hearing loss in the EHF region, determining the relationship between any LF deficits and SPiN performance in people with D-SPiN-NH also requires further evaluation.
Up to this point, we assert that EHF plays a role in speech perception, especially in noise. Elevation in EHF hearing thresholds may occur due to noise, ototoxins, and genetics that together are subsumed under the general construct of “aging”. Testing the hypotheses proposed here needs precise measurement of hearing across the entire audible spectrum in the same individuals. While it may not be possible in a given individual to tease out these contributors to EHF hearing differences, the extent to which pure tone audiometry relates to otoacoustic emissions in an otherwise typical population can shed light on the use of EHF hearing as a marker for aging in the auditory system. In the following section, methods and some results pertaining to such methods are provided.
5.0. EHF Hearing and Distortion Product Otoacoustic Emissions (DPOAE): Growing Older Together
Sumitrajit Dhar, Samantha M. Stiepan, Jonathan H Siegel
The aging auditory system in humans has commonly been characterized by the gradual decline in behavioral hearing thresholds (Gordon-Salant, 2005). In addition to the magnitude of threshold decline, the configuration of hearing loss has also been used to identify the pathologies underlying age-related hearing loss, ARHL (Dubno et al., 2013). In humans, most identified phenotypes of ARHL involve a compromise of the active cochlear processes. Consequently, otoacoustic emissions (OAEs) are an effective marker for aging, especially in the human auditory system. Indeed, the approximate patterns of threshold loss associated with various age-related pathologies are also observed in transient-evoked (TE)OAEs (Vaden et al., 2018). However, whether age-related changes in OAEs can be observed in the absence of, or independent of, threshold changes are debated in the literature.
5.1. Approaches to studying hearing thresholds and OAEs in aging
Although several studies have investigated the relationship between aging, hearing thresholds, and OAEs, their results are neither unequivocal nor easy to interpret. One approach has been to report OAE levels from individuals across a wide age range, while requiring all participants to have hearing thresholds adjusted for their ages (Bonfils et al., 1988). Another approach has been to include individuals across a similarly wide age range but require that all participants have equivalent hearing thresholds over a very narrow frequency range (Collet et al., 1990). In the second approach, where thresholds are equivalent over a narrow range, the influence of aging on thresholds at higher frequencies is ignored. These designs do not allow the segregation of the impact of age and hearing thresholds on OAE levels. Dorn et al. (1998) did find distortion product (DP)OAE levels to vary with age, independently from hearing thresholds; however, others have reported inconsistent or no effects of age on OAEs (Stover and Norton, 1993; Strouse et al., 1996). Further complications in understanding the relationship between aging, hearing thresholds, and OAEs have surfaced more recently with the observation of divergent behavior of different OAE types with increasing age (Abdala et al., 2018). Finally, the possibility of age-related synaptopathy in the cochlea could complicate the relationship between hearing thresholds and OAE levels by altering the efferent control of OAEs but, at least initially, not influencing hearing thresholds (Kujawa and Liberman, 2015).
5.2. Age related trends for EHF hearing and OAEs in Northwestern University studies
One profitable approach in exploring the influence of age on OAEs and hearing thresholds could be to examine their behavior at the EHF where age-related changes are detectable first and are most pronounced thereafter (Lee et al., 2012). Here we compare trends in hearing thresholds (Lee et al., 2012) and DPOAE levels (Poling et al., 2014) at EHF in human participants between 10 and 65 years old while controlling for hearing thresholds through 4 kHz.
Participant characteristics and methods have been reported in detail previously for both the hearing thresholds (Lee et al., 2012) and DPOAEs (Poling et al., 2014). In brief, participants were between 10 and 65 years old with hearing thresholds ≤ 20 dB HL up to 4 kHz, normal middle ear function, and no history of significant noise or ototoxic drug exposure, family history of hearing loss, or history of frequent ear infections or ear surgery. Data are reported from one, randomly chosen, ear of 313 individuals (195 female). Stimulus levels for both threshold and OAE measurements were calibrated using forward pressure level (FPL) to account for the inserted position of the measurement probe in the ear canal, eliminating the influence of ear canal resonances on the stimulus pressure delivered to the ear drum (Souza et al., 2014). Hearing thresholds between 0.125 and 20 kHz were measured using a modified Bekesy tracking method and DPOAEs were recorded by sweeping two stimulus tones (f1 and f2, f2/f1 = 1.22) for f2 frequencies between 0.75 and 20 kHz at equivalent levels of 65- and 55-dB SPL.
As has been observed before, both DPOAE levels and hearing thresholds systematically decline with age, with the most extreme decline in the highest frequencies (Figure 4). Changes in hearing thresholds with age are usually plotted in dB Hearing Level (HL) depicting change compared to a clinical normative value. On occasion hearing thresholds for individuals of various ages are displayed in dB SPL (Stelmachowicz et al., 1989). Here we take an approach similar to that of plotting in dB HL but use the average thresholds obtained from 10- to 21-year-olds in the current cohort as the reference. This is partly necessitated by the use of novel calibration methods and partly motivated to avoid any artificial underestimation of age-related changes due to an existing age bias in the clinical norms. Changes in DPOAE levels are also represented using the same baseline. Additionally, change in thresholds in five age groups is presented after dividing the absolute value by three to allow visualization of DPOAE and threshold changes across the same ordinate range.
Figure 4.
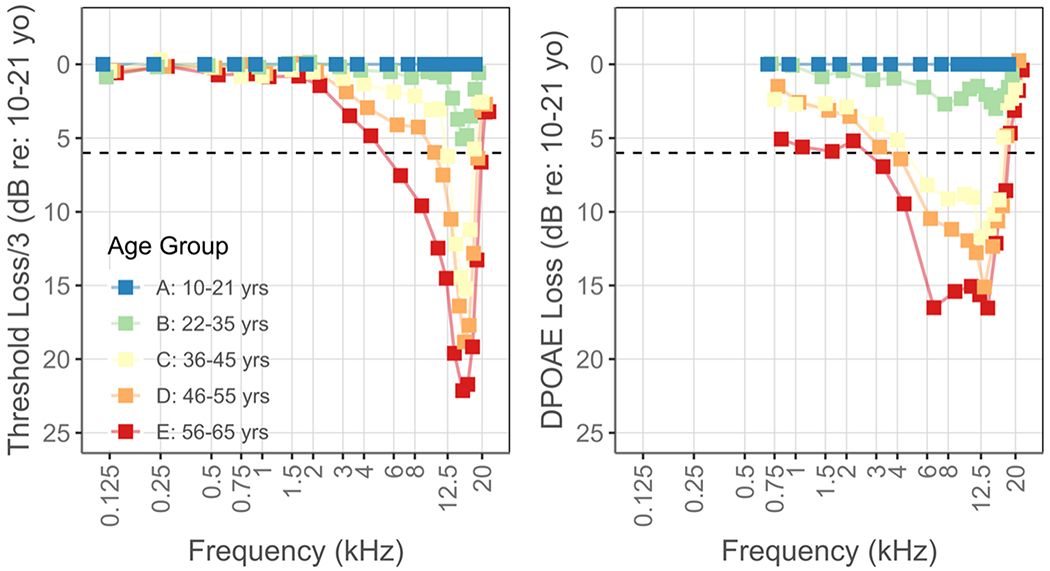
Threshold (left) and DPOAE level (right) loss in older age groups compared to those between 10 and 21 years old. Hearing thresholds are presented after dividing the absolute loss by three to enable visualization using the same range as the DPOAE loss data. The horizontal dashed line at 6 dB approximately marks a clinically significant loss.
Both DPOAE levels and hearing thresholds demonstrate clinically significant loss in the 36 – 45-year-old age group, with the loss being most prominent at 12.5 and 16 kHz for DPOAE levels and thresholds, respectively. Both measures continue to decline while the range of affected frequencies widens with increasing age. Overall, the age-related decline is evident over a wider frequency range in DPOAE levels and the change in DPOAE levels between the 22 – 35 year and 36 – 45-year age groups is more dramatic. An analysis of variance showed that both age and hearing thresholds had statistically significant effects on DPOAE levels (p < 0.001) with a significant interaction between age and hearing thresholds (p < 0.01). The relationship between DPOAE levels and hearing thresholds at six representative frequencies is displayed in Figure 5. These six frequencies were chosen as they demonstrate the gradual transition in the relationship between these variables. In the lowest frequencies, hearing thresholds display a considerably smaller range compared to the DPOAE levels. Consequently, the data cloud displays a vertical drop, depicting a decrease in DPOAE levels with age with comparatively limited change in hearing thresholds. However, with increasing frequencies, the data cloud starts to expand horizontally as the range of measured hearing thresholds increases. Finally, at the highest frequencies, the data cloud displays a horizontal tail formed by data from the older age groups. The horizontal tail is driven in part by DPOAE levels approaching the measurement noise floor and partly by a saturation in DPOAE level change.
Figure 5.
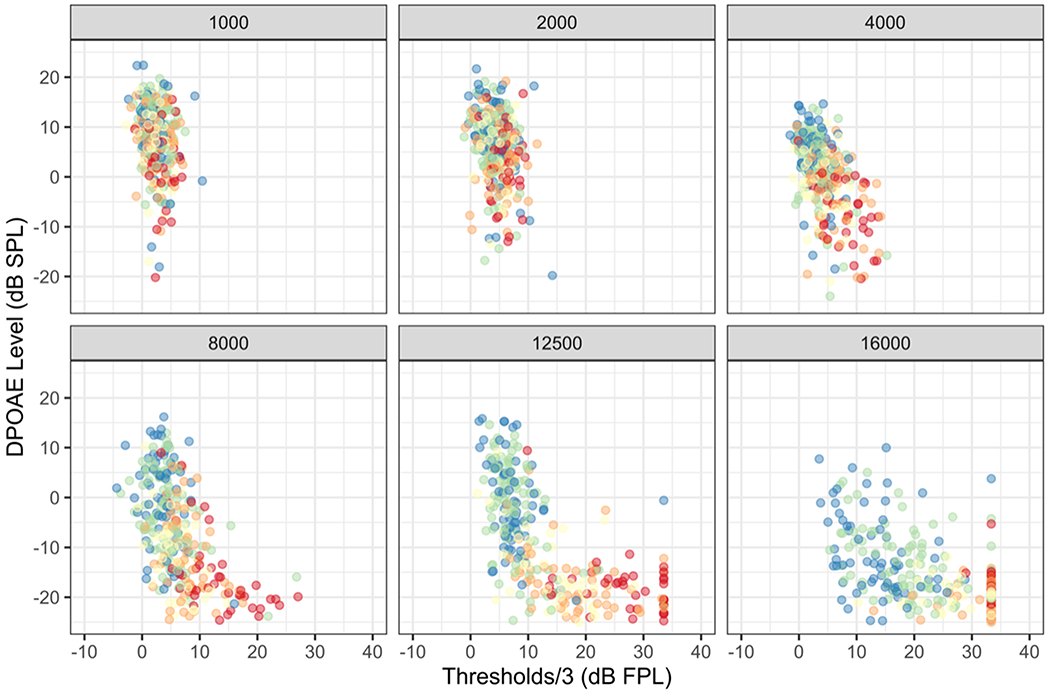
(color). DPOAE levels plotted against hearing thresholds calibrated using forward pressure level (FPL) for each subject at six representative frequencies. The colors representing different age groups are identical to those used in Figure 5.
Historically, the study of auditory aging in humans has typically started in the sixth or seventh decades of life. However, the data presented here provide clear evidence of age-related changes in the auditory periphery in the fourth decade of life, if not earlier. These early changes are observable at the highest audible frequencies of human hearing. Previously, stimulus delivery and calibration at these frequencies in human ears has been problematic (Siegel and Hirohata, 1994; Stelmachowicz et al., 1989). However, reliable data, such as those presented here, are obtainable today with the aid of improved calibration methods. Data such as these help to illuminate initial age-related changes in the auditory system and will allow timely delivery of treatment, whatever form that may take in the future.
The change in hearing thresholds are presented after dividing the absolute value by three following previously demonstrated relationships between different measures of change in cochlear function. Each dB of change in DPOAE thresholds has been shown to be accompanied by approximately 2 dB of change in the auditory brainstem response (ABR) threshold in Mongolian Gerbil chronically exposed to furosemide to mimic metabolic presbycusis (Mills and Schmiedt, 2004). Behavioral and ABR thresholds in humans show a more complicated relationship (eg., McCreery et al., 2015) but a 0.6 dB change in ABR thresholds for each dB of change in behavioral thresholds appears to be a good approximation. Taken together, presenting change in hearing thresholds after dividing by three exposes the greater frequency-range of decline in DPOAE levels with age even when the DPOAE and threshold changes are examined over the same absolute range.
The results shown in Figure 5 suggest that the changes in DPOAE levels are not entirely tied to, nor driven by, changes in hearing thresholds. This is most evident at the lowest frequencies in Figure 5 where a significant drop in DPOAE levels with age is observed with minimal change in hearing thresholds. This argument is bolstered by the reduction in DPOAE levels observed at the lowest frequencies in the right panel of Figure 4 for the older age groups, while hearing thresholds remain near those of the youngest age groups in the left panel. DPOAE levels are altered by age-related changes in the cochlea prior to their manifestation in hearing threshold change.
With this large population study, broad age range and measurement of both pure tone thresholds and DPOAEs, we turn our attention next to factors that can result in premature loss of EHF hearing, and the impact this may have on functional hearing for speech perception in children and teens.
6.0. EHF hearing and causes of premature loss in children
Lisa L. Hunter, Chelsea M. Blankenship and David R. Moore
Children and teens have exceptionally sensitive hearing in the EHF region in comparison to adults, but EHF sensitivity progressively decreases from early in adulthood (Rodriguez Valiente et al., 2014b; Schechter et al., 1986). By the second decade of life, significant decreases in sensitivity have occurred, and continue to progress in a basal to apical pattern. EHF hearing is highly susceptible to ototoxic drugs (Fausti et al., 1993, 1984; Garinis et al., 2018) otitis media with effusion (Cordeiro et al., 2018; Hunter et al., 1996) and impulse noise due to firearm use (Fausti et al., 1981; Laffoon et al., 2019). However, variability of the impact to EHF hearing is large among all these risk factors, presumably due to genetic variants. Because healthy children and young adults can perceive pure tones up to at least 20 kHz (Schechter et al., 1986) and the frequencies above 8 kHz most sensitively reveal the impact of insults, inclusion of this frequency range in the audiologic test battery is warranted.
Because infants are learning speech phonemes to build the units of language, good audibility is critical across the frequency range of hearing. The pristine hearing sensitivity of young children extended to the frequencies above 8 kHz theoretically enhances perception of differences in phonemes such as stop consonants, sonorants and fricatives that carry linguistic meaning. The importance of higher frequency audibility for these phonemes is evident in the short-term spectra measured at the ear for /s/ and /sh/ in the 10 kHz region, with distinct differences for speech tokens spoken by male and female adults compared to children (Pittman et al., 2003). Further evidence regarding differences in phoneme acquisition at 14 to 16 months of age for normal hearing children compared to those with congenital hearing loss was provided by Stelmachowicz et al. (2004). Fricatives are acquired later than are vowels and other consonant classes, both in normal hearing children and those with early-identified congenital hearing loss. Evidence for differential energy for these speech sounds from 3 to above 10 kHz was reported for the phonemes /ch, f, j, s, sh, th, v, and zZ (Alexander et al., 2014). Thus, substantial spectral energy is available in the frequencies above 8 kHz for linguistically important speech sounds, and hearing loss negatively affects acquisition of these speech sounds in young children.
6.1. Impact of otitis media on EHF hearing and relation to listening difficulties
Extended high-frequency hearing loss has been consistently linked to histories of OME in childhood, related to severity or number of pressure equalization (PE) tube surgeries (Hunter et al., 1996; Laitila et al., 1997). In ears that have fully recovered from the mechanical effects of OME (as assessed with wideband reflectance), the EHF hearing loss is permanent and of sensory origin (Margolis et al., 2000). The mechanism for such cochlear damage has been attributed to transmission of bacterial endotoxins through the round window membrane, resulting in basal outer hair cell damage, as demonstrated in animal studies (Engel et al., 1998; Lundman et al., 1992). High frequency hearing loss in children (aged 6-15 years) with histories of chronic OME essentially accelerates the aging process, in that hearing sensitivity for frequencies above 8 kHz is like an average 40 to 50-year-old (Hunter et al., 1996). We do not know what impact such losses have for speech perception in these children, especially in noisy or reverberant environments, and this is an important area to study.
Recent studies point to EHF hearing assessment as a highly sensitive method to study speech perception deficits in children and teenagers. One such example is in otherwise unexplained Listening Difficulties (LiD) in children, presenting as significant difficulty understanding speech in noisy environments, such as in classrooms. We have recently studied peripheral hearing function in 60 children aged 6-14 years (Hunter et al., n.d.) who have LiD reported by their parents on questionnaires using the ECLiPS validated questionnaire published by Barry et al. (2015). These children were compared to age-matched children without LiD or other developmental problems. Assessment of EHF hearing revealed that children with listening difficulties combined with histories of OME and PE tubes had significantly poorer EHF hearing, but this was also the case in an age-matched control group, some of who also had histories of OME and PE tubes. Thus, EHF hearing was more associated with histories of OME and PE tubes than to LiD per se. Presence of EHF hearing loss was related to poorer speech in noise performance on the Listening in Spatialized Noise test (LiSN-S), as shown in Figure 6. We measured the spectral energy using circum-aural earphones for the LiSN-S test and found that sufficient speech energy was present in the 10-16 kHz region to allow audibility. Frequencies below 10 kHz were not significantly related to the test condition in which sentences and competing sentences are co-located in a simulated binaural condition under earphones (low-cue). At the higher frequency bands (10-12 and 14-16 kHz) however, significant relationships were found for the low-cue condition (Fig. 6). Relationships were also found for the high cue condition (spatially separated target and a different competing talker), as well the condition in which the target and the same competing speech were separated. The relationships appeared to be driven primarily by the children with listening difficulties, shown by the yellow circles in the scatterplot. They tended to have the poorest performance on the LiSN-S, coupled with poorer hearing thresholds.
Figure 6.
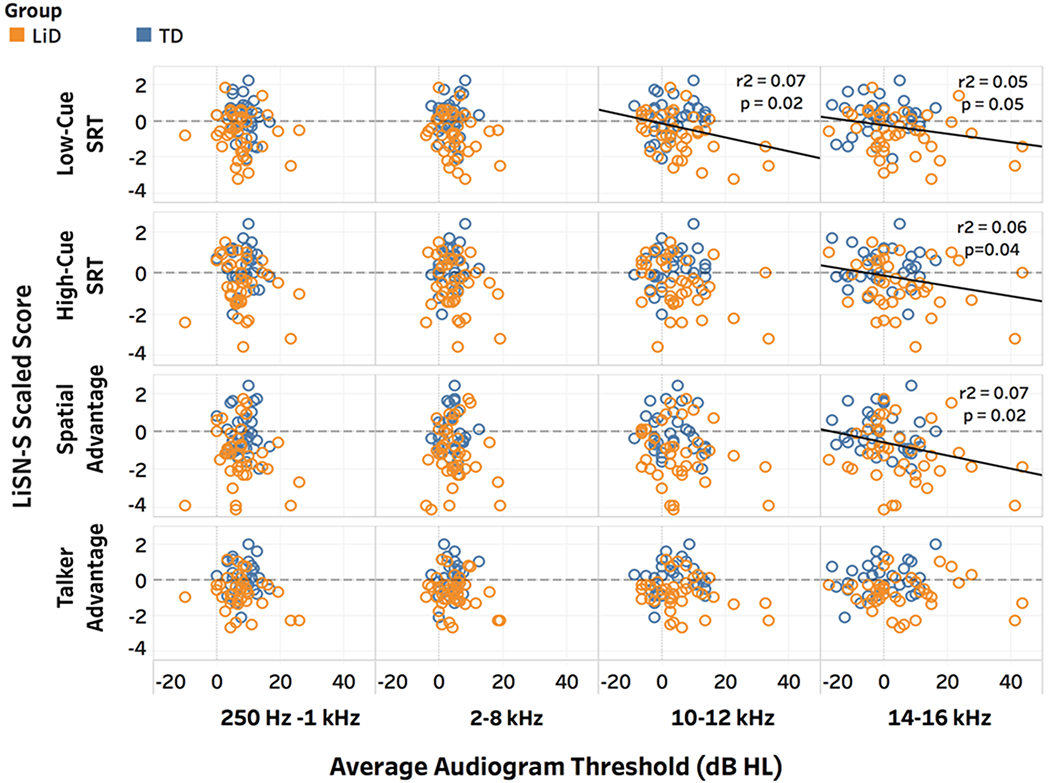
(color). Scatterplots of individual results for children with listening difficulties (gold circles), compared to typically developing children (blue circles) for four different conditions of the LiSN-S standardized test of speech perception in competing noise. The y-axis plots the LiSN-S scaled score, in which negative numbers are worse, with 0 (dotted line) showing average scores. The x-axis plots average pure tone hearing thresholds in dB HL for lower to higher frequency bands.
6.2. Impact of ototoxicity on EHF hearing and relation to listening difficulties
A second line of evidence for EHF hearing contribution to speech perception in children is ototoxicity. Due to the known, direct relation between ototoxic drugs and EHF hearing, regular monitoring is recommended (Konrad-Martin et al., 2017;Campbell and Le Prell, 2018). Aminoglycoside (AG) drugs (e.g., tobramycin, amikacin) are frequently used to treat drug-resistant chronic lung infections in patients such as those with cystic fibrosis (CF). Unfortunately, AGs cause hearing loss due to active hair cell uptake of the drugs, causing oxidative stress that leads to cell death starting first in the basal cochlea. We studied hearing in 91 patients with CF aged 15 yrs. to 63 yrs. (mean age = 27) receiving IV tobramycin, amikacin and/or vancomycin to prevent or treat bacterial lung infections (Garinis et al., 2017). Results were compared to 37 normally hearing young adults without a history of CF or similar drug treatments. Audiometry showed that 81 of 157 ears (52%) had EHF hearing loss (> 25 dB HL) for at least one tested frequency. Because the majority of ears had pre-existing hearing loss in the EHFs, prediction of onset was not possible, yet the cumulative burden of ototoxicity on hearing was clear. The same cohort was then categorized by cumulative IV-AG and/or vancomycin cumulative doses, after adjusting for gender and age. Patients with the highest cumulative AG doses were 4.5 times more likely to have permanent EHF hearing loss than patients with the lowest cumulative doses (Garinis et al., 2017).
A second cohort of 58 children and teenagers with CF aged 7-19 (mean=15.5) admitted for lung infections with IV-AG treatment were tested with standard and EHF audiometry, clinical DPOAEs, and experimental chirp stimulus TEOAEs (Hunter et al., 2018). This group was compared to 50 age-matched controls using EHF pure tone threshold testing and speech perception in noise (BKB-SIN test). For the CF group, hearing loss (>15 dB HL) was found in 52% of ears for the EHF (10-16 kHz) range, and 19% in the conventional clinical frequency range. This result highlights the much greater sensitivity of EHF audiometry to ototoxicity. Not previously reported in such studies is that self-perceived hearing difficulties were reported by 28% of the patients treated with AGs, and persistent tinnitus was reported by 53% (Hunter et al., 2018). Significantly poorer SIN scores for the Bamford-Kowal-Bench speech in noise test (BKB-SIN) were found for cases treated with AGs compared to control ears (p < .001), and for ears with hearing loss compared to those with normal hearing (p=.045).. Mild to moderate SIN deficits were found in 40% of CF cases (Hunter et al., 2018), but the correlation with EHF hearing thresholds was low (R2 = .0422), while correlation with conventional frequencies was higher (R2 = .1225), as shown in Figure 7. This may be because the speech stimuli used were highly contextual sentences, or that damage important for speech perception in noise occurs secondarily in the EHF or alternatively, that damage is due to neural or synaptic loss in addition to hair cell loss. The functional impact of SIN difficulty as shown in these causes of EHF hearing loss in children and teens is highly relevant for the exceptionally noisy and reverberant environments that they must listen and learn in, such as in the classroom, sports, work and social settings. Prospective research to study relationships between EHF hearing, shifts in hearing with ototoxic drugs and speech sensitized to EHF is needed.
Figure 7.
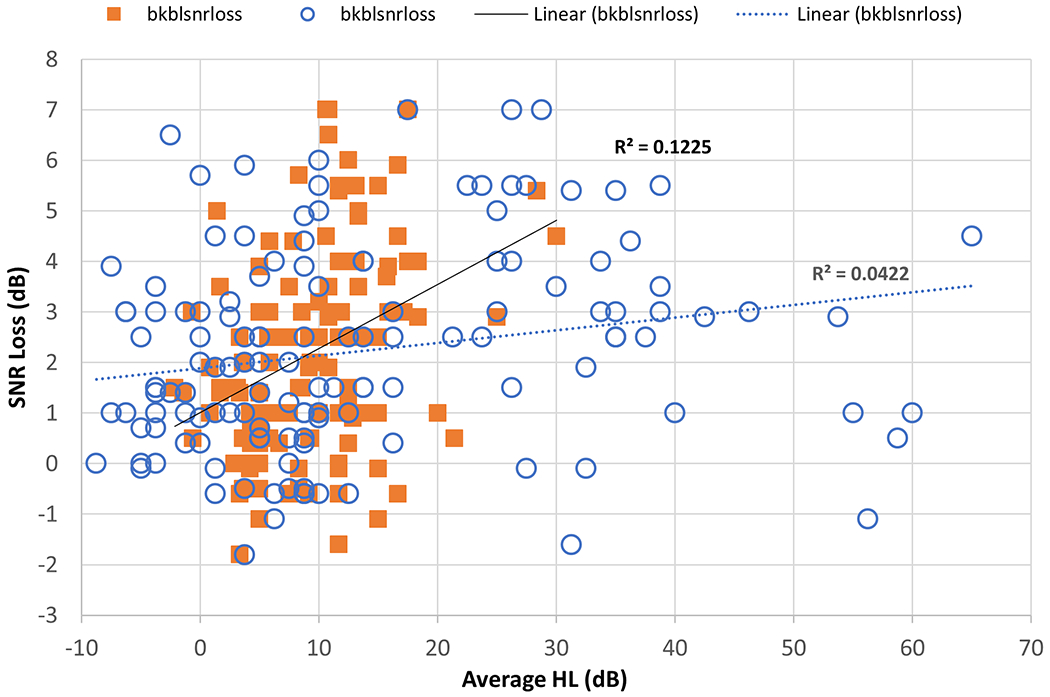
Scatterplot of BKB-SIN scores (y-axis) for AG-treated patients with cystic fibrosis compared to average conventional (.25-8 kHz, filled squares) and EHF (10-16 kHz, open circles) pure tone thresholds on the x-axis.
In order to facilitate both clinical and research applications use of EHF hearing assessment, attention to measurement issues is important. The final section reviews studies and standards relevant to obtain accurate EHF measures.
7.0. Measurement of EHF audiometry
Kevin Munro
Pure tone audiometry, the detection of quiet tones of varying frequency, has assumed the role of ‘gold standard’ test of hearing for more than 70 years (Johnson, 1970). Recommended procedures exist for performing clinical pure-tone air- and bone-conduction threshold audiometry (eg., BSA, 2018). These clinical procedures generally assume that the upper frequency for air-conduction testing will not exceed 8 kHz. However, clinical practice may be changing, and audiometer calibration services are reporting an increase in the number of requests for calibration of EHFs (personal communication with Jonathan Proffitt).
There is a move away from the traditional stand-alone clinical audiometers to software suites that integrate clinical tests including tympanometry, otoacoustic emissions, hearing instrument tests and probe microphone measurements. These software suites have an optional ‘add-on’ module, consisting of a license, which allows access to test frequencies up to around 20 kHz, and earphones with an appropriate EHF response. An informal survey of UK audiologists on the British Academy of Audiology Facebook page (January 2019) revealed that current clinical applications of EHF audiometry include: (1) early warning and detection of hearing loss e.g., ototoxicity or noise monitoring; (2) understanding unexplained hearing difficulties including tinnitus; and (3) guiding the fitting of hearing aids with an EHF response e.g., Earlens. Rodriguez Valiente et al., (2016) present several case studies that illustrate the potential usefulness of EHF audiometry.
There are three classes of earphones: supra-aural, insert and circum-aural. Historically, the standard clinical air-conduction transducer was a supra-aural earphone (e.g., Telephonics TDH39 earphone with an MX-41-AR cushion) that sits on the pinna. In many countries, there has been a move away from the supra-aural earphone to the insert earphone (e.g., Etymotic Research ER3A), placed into the external ear canal. The insert earphone is reported to have a number of advantages over the supra-aural earphone including increased patient comfort, reduced occurrence of ear canal collapsing and increased inter-aural attenuation (for example, see Munro and Agnew, 1999). The ER-3A was designed to have the same frequency response as the TDH-39 earphone and neither are suitable for EHF audiometry. Circum-aural earphones, full earphones that surround the pinna, are usually used for EHF audiometry, but research insert earphones are also available.
In order to provide uniformity in the use of hearing threshold levels throughout the world, standards exist that provide reference equivalent SPL for different earphones. For example, International Organization for Standardization (ISO) 389 is a multi-part standard, with reference values for the calibration of audiometric equipment. Parts One and Two of ISO 389 provide reference values for the supra-aural and insert earphone, respectively. Part Eight of ISO 389 provides reference values for the frequency range 8 to 16 kHz (ISO 389-8, 2004); however, these are based on a small number of studies and the KOSS HV/IA and Sennheisser HDA 200 (Wedemark, Germany) earphones cited in the standard are now out of production. A new earphone, the RadioEar DD450 (New Eagle, PA), has been designed to simulate the characteristics of the HDA 200 (Smull et al., 2019). Although reference values for the HDA 200 earphone have yet to be incorporated into ISO 389, these have already been incorporated into audiometer specifications contained within ANSI S3.6-2018 (American National Standards Institute, 2018). The Sennheiser HDA 280 earphone may serve as a low-cost alternative to the HDA 200 (Folkeard et al., 2019) but reference data for EHFs have not yet been incorporated into the relevant national and international standards.
Audiometer maintenance and calibration can be separated into biological checks and physical measurements (ISO 8253-1). Routine biological checks require that the listener has good EHF hearing sensitivity. As detailed in section 4, early age-related changes in EHF sensitivity mean that even relatively young listeners may not have normal thresholds of 0-20 dB HL in the EHF range. Thus, it may be inappropriate to use adult reference values when testing younger age groups as this may miss or under-estimate the severity of hearing loss at EHFs. Unless the purpose is to simply identify changes in hearing threshold between two time points, correction factors could be applied to adjust for the better EHF tone detection in children.
Thresholds at EHF have higher inter-subject variability than at frequencies below 8 kHz. The reason for this is not completely understood. There has been concern that this is an artifact due to standing waves in the ear canal creating spatially non-uniform sound pressures. However, circum-aural earphones do not necessarily lead to greater intra-subject variability, perhaps due to their impedance characteristics mitigating the generation of standing waves in the ear canal. Also, studies that have compensated for variations in ear canal sound pressure level have also demonstrated high inter-subject variability at EHFs (for example, see Figure 6 in Lee et al., 2012).
While test-retest differences are likely to be smaller with methods that reference the sound pressure level at the ear drum, clinically acceptable test-retest reliability can be obtained if care is taken to ensure there is good placement of the earphone centered around the pinna so that the centre of the circum-aural earphone and the opening of the external canal is consistent between tests (Fausti et al., 1990). Frank (2001) used the HDA 200 circum-aural earphone to obtain EHF hearing thresholds (8 to 16 kHz) in 100 adults on four separate occasions. The results show that 98% of hearing thresholds (8 to 16 kHz) were within 10 on retest. Flamme et al. (2015) compared the Sennheisser HDA 200 circum-aural and the TDH39 supra-aural earphone. Test-retest reliability with the circum-aural earphone was reported to be at least as good, and possibly better, than the supra-aural earphone. Studies in children have also demonstrated acceptable intrasubject reliability for audiometric use (Beahan et al., 2012; Hunter et al., 1996). Because children have shorter ear canals, the impact of standing waves is theoretically less than that for adults, although there is a developmental effect, as children younger than 6 years tend to have slightly higher test-retest variability than older children (Beahan et al., 2012).
A practical issue for the tester is whether they should continue to use the supra-aural or insert earphones for ‘standard’ audiometry frequencies and swap to circum-aural when measuring EHF. The alternative is to abandon supra-aural and insert earphones and use the circum-aural earphone for all test frequencies. For organizations that use ANSI for calibration purposes, reference equivalent threshold sound pressure level values (RETSPL) values are available for pure tones from 0.125 kHz through to 16 kHz. For organizations that rely on ISO for calibration purposes, it is unlikely they will abandon supra-aural or insert transducers until RETSPL values for the circum-aural earphone are published across the entire audiometric frequency range. The inconvenience of swapping earphones is a potential barrier to widespread implementation, as may be the case if there is no financial reimbursement for testing at EHFs.
EHF stimuli may not always be perceived as tonal with a distinct single pitch and the upper frequency of hearing is sometimes described by audiologists as having a noise or hiss-like quality. Careful client instruction is required to avoid confusion. Standing waves can be an issue when using high-frequency signals. A simple cross-check is to remove, then reposition the earphone before making verification measures.
Measuring hearing sensitivity at EHFs will add test time but could be minimized by judicious selection of test frequencies e.g., the highest frequency required for ototoxicity monitoring is likely to be different from the highest frequency required for fitting an extended bandwidth hearing aid. In any case, the selection of pure tone frequencies to be included may vary from one person to another and is contingent on many factors. There is some evidence that testers apply a test battery approach without any contingent decision making. By way of example, audiologists have slavishly measured bone-conduction hearing thresholds at 4 kHz despite the occurrence of unexpected 15-20 dB air-bone gaps (likely due to an error in the reference force level; , and have then generally ignored the test finding (Margolis et al., 2013). An important consideration in audiometric testing is inter-aural attenuation (IA) i.e. drop of intensity of an acoustic signal from the test ear audiometric transducer to the non-test ear cochlea. For clinical purposes, minimum values of IA determine the need for masking of the non-test ear in order to prevent cross hearing. Brannstrom and Lantz (2010) evaluated IA for pure tones (0.125 kHz to 16 kHz) using HDA 200 and the TDH39 earphones. They followed the same procedure as Munro and Agnew (1999) and Munro and Contractor (2010) and tested adults with unilateral deafness. The findings revealed that the HDA 200 earphone provided greater IA than the TDH39, reducing the need for masking. IA data have yet to be established for the RadioEar DD450.
Unlike many research developments, implementation of EHF audiometry into clinical practice is relatively easy. Inclusion of EHF audiometry in clinical practice guidelines by the relevant professional bodies will help accelerate implementation into clinical practice.
8.0. Conclusions
The present compilation of contributions provides clarity regarding several issues related to EHF hearing. Namely, it is clear that EHF hearing has ecological relevance and utility, particularly in challenging listening conditions. Independent studies concur that EHFs contribute to speech-innoise listening. Loss of EHFs per se (via masking or low-pass filtering) leads to decrements in speech-in-noise and speech-in-speech performance for normal-hearing listeners. Additionally, EHFs are the most vulnerable to damage or loss incurred through ototoxicity, otitis media, noise exposure, and aging. EHF hearing can be reliably assessed in the clinic and the laboratory, and such assessment reveals that individuals with clinically normal hearing at standard audiometric frequencies show substantial variability in thresholds at EHFs.
At the same time, questions remain regarding EHF hearing and the utility of EHF assessment in the clinic. It is not yet certain whether elevated EHF thresholds per se are causal to speech-in-noise difficulties observed for individuals with EHF hearing loss. It is possible that elevated EHF thresholds are a marker for other mechanisms that lead to the observed listening difficulties, such as elevated thresholds in the standard audiometric range, broadened auditory filters, or synaptopathy. Early EHF loss might serve as a harbinger of premature debilitating hearing loss or other auditory dysfunction, and this possibility warrants further investigation. That is, it may be that screening for EHF loss in the clinic could assist in identifying populations at risk for listening difficulties or other dysfunction incident to ototoxicity, otitis media, noise exposure, or aging. While technology and inner ear therapies to remediate or prevent EHF hearing loss are not available in today’s audiology repertoire, a better understanding of the functional impacts would motivate efforts to improve hearing conservation and rehabilitation through targeted research for future clinical applications.
Acknowledgements
This work was supported by a Place Award and Decibel Therapeutics (LLH, CB); NIH grants R01DC014078 and R21DC016241 (DRM, LLH, LMZ, CB); NIH grant DC008420 and Northwestern University (SD); NIHR Manchester Biomedical Research Centre (KJM, DRM).
REFERENCES
- Abdala C, Ortmann AJ, Shera CA, 2018. Reflection- and Distortion-Source Otoacoustic Emissions: Evidence for Increased Irregularity in the Human Cochlea During Aging. J. Assoc. Res. Otolaryngol 19, 493–510. 10.1007/s10162-018-0680-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JM, Kopun JG, Stelmachowicz PG, 2014. Effects of Frequency Compression and Frequency Transposition on Fricative and Affricate Perception in Listeners With Normal Hearing and Mild to Moderate Hearing Loss. Ear Hear. 35, 519–532. 10.1097/AUD.0000000000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American National Standards Institute, 2018. ANSI/ASA S3.6-2018 Specifications for Audiometers. [Google Scholar]
- Badri R, Siegel JH, Wright BA, 2011. Auditory filter shapes and high-frequency hearing in adults who have impaired speech in noise performance despite clinically normal audiograms. J. Acoust. Soc. Am 129, 852–863. 10.1121/1.3523476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry JG, Tomlin D, Moore DR, Dillon H, 2015. Use of Questionnaire-Based Measures in the Assessment of Listening Difficulties in School-Aged Children. Ear Hear. 36, e300–e313. 10.1097/AUD.0000000000000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beahan N, Kei J, Driscoll C, Charles B, Khan A, 2012. High-Frequency Pure-Tone Audiometry in Children: A Test-Retest Reliability Study Relative to Ototoxic Criteria. Ear Hear. 33, 104–111. 10.1097/AUD.0b013e318228a77d [DOI] [PubMed] [Google Scholar]
- Berlin CI, Wexler KF, Jerger JF, Halperin HR, Smith S, 1978. Superior ultra-audiometric hearing: a new type of hearing loss which correlates highly with unusually good speech in the “profoundly deaf”. Otolaryngology 86, 111–116. [DOI] [PubMed] [Google Scholar]
- Best V, Carlile S, Jin C, van Schaik A, 2005. The role of high frequencies in speech localization. J. Acoust. Soc. Am 118, 353–363. 10.1121/1.1926107 [DOI] [PubMed] [Google Scholar]
- Bonfils P, Bertrand Y, Uziel A, 1988. Evoked Otoacoustic Emissions: Normative Data and Presbycusis. Int. J. Audiol 27, 27–35. 10.3109/00206098809081571 [DOI] [PubMed] [Google Scholar]
- Brannstrom KJ, Lantz J, 2010. Interaural attenuation for Sennheiser HDA 200 circumaural earphones. Int. J. Audiol 49, 467–471. 10.3109/14992021003663111 [DOI] [PubMed] [Google Scholar]
- Brungart DS, Simpson BD, 2009. Effects of bandwidth on auditory localization with a noise masker. J. Acoust. Soc. Am 126, 3199–3208. 10.1121/L3243309 [DOI] [PubMed] [Google Scholar]
- BSA, 2018. Recommended procedure. Pure-tone air-conduction and Bone-conduction threshold audiometry with and without masking. Br. Soc. Audiol August, 1–39. [Google Scholar]
- Cameron S, Dillon H, 2007. Development of the Listening in Spatialized Noise-Sentences Test (LISN-S). Ear Hear. 28, 196–211. 10.1097/AUD.0b013e318031267f [DOI] [PubMed] [Google Scholar]
- Campbell KC, Le Prell CG, 2018. Drug-Induced Ototoxicity: Diagnosis and Monitoring. Drug Saf. 41, 451–464. [DOI] [PubMed] [Google Scholar]
- Carlile S, Delaney S, Corderoy A, 1999. The localisation of spectrally restricted sounds by human listeners. Hear. Res 128, 175–189. 10.1016/S0378-5955(98)00205-6 [DOI] [PubMed] [Google Scholar]
- Charaziak KK, Siegel JH, 2015. Tuning of SFOAEs Evoked by Low-Frequency Tones Is Not Compatible with Localized Emission Generation. J. Assoc. Res. Otolaryngol 16, 317–329. 10.1007/s10162-015-0513-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W, Warnock A, 2002. Detailed directivity of sound fields around human talkers. Inst. Res. Constr. Natl. Res. Counc. Canada, Tech. Rep December [Google Scholar]
- Collet L, Moulin A, Gartner M, Morgon A, 1990. Age-Related Changes in Evoked Otoacoustic Emissions. Ann. Otol. Rhinol. Laryngol 99, 993–997. 10.1177/000348949009901212 [DOI] [PubMed] [Google Scholar]
- Cordeiro FP, da Costa Monsanto R, Kasemodel ALP, de Almeida Gondra L, de Oliveira Penido N, 2018. Extended high-frequency hearing loss following the first episode of otitis media. Laryngoscope 128, 2879–2884. 10.1002/lary.27309 [DOI] [PubMed] [Google Scholar]
- Crandall IB, MacKenzie D, 1922. Analysis of the Energy Distribution in Speech 1. Bell Syst. Tech. J 1, 116–128. 10.1002/j.1538-7305.1922.tb00383.x [DOI] [Google Scholar]
- Dorn PA, Piskorski P, Keefe DH, Neely ST, Gorga MP, 1998. On the existence of an age/threshold/frequency interaction in distortion product otoacoustic emissions. J. Acoust. Soc. Am 104, 964–971. 10.1121/L423339 [DOI] [PubMed] [Google Scholar]
- Dubno JR, Eckert MA, Lee F-S, Matthews LJ, Schmiedt RA, 2013. Classifying Human Audiometric Phenotypes of Age-Related Hearing Loss from Animal Models. J. Assoc. Res. Otolaryngol 14, 687–701. 10.1007/s10162-013-0396-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel F, Blatz R, Schliebs R, Palmer M, Bhakdi S, 1998. Bacterial cytolysin perturbs round window membrane permeability barrier in vivo: Possible cause of sensorineural hearing loss in acute otitis media. Infect. Immun 66, 343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausti SA, Erickson DA, Frey RH, Rappaport BZ, 1981. The Effects of Impulsive Noise Upon Human Hearing Sensitivity (8 to 20 kHz). Scand. Audiol 10, 21–29. 10.3109/01050398109076158 [DOI] [PubMed] [Google Scholar]
- Fausti SA, Frey RH, Henry JA, Knutsen JL, Olson DJ, 1990. Reliability and validity of high-frequency (8–20 kHz) thresholds obtained on a computer-based audiometer as compared to a documented laboratory system. J. Am. Acad. Audiol 1, 162–171. [PubMed] [Google Scholar]
- Fausti SA, Henry JA, Schaffer HI, Olson DJ, Frey RH, Bagby GC, 1993. High-Frequency Monitoring for Early Detection of Cisplatin Ototoxicity. Arch. Otolaryngol. - Head Neck Surg 119, 661–666. 10.1001/archotol.1993.01880180081015 [DOI] [PubMed] [Google Scholar]
- Fausti SA, Rappaport BZ, Schechter MA, Frey RH, Ward TT, Brummett RE, 1984. Detection of aminoglycoside ototoxicity by high-frequency auditory evaluation: Selected case studies. Am. J. Otolaryngol 5, 177–182. 10.1016/S0196-0709(84)80009-5 [DOI] [PubMed] [Google Scholar]
- Fay RR, 1988. Comparative psychoacoustics. Hear. Res 34, 295–305. 10.1016/0378-5955(88)90009-3 [DOI] [PubMed] [Google Scholar]
- Flamme GA, Geda K, McGregor KD, Wyllys K, Deiters KK, Murphy WJ, Stephenson MR, 2015. Stimulus and transducer effects on threshold. Int. J. Audiol 54, S19–S29. 10.3109/14992027.2014.979300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher H, Galt RH, 1950. The Perception of Speech and Its Relation to Telephony. J. Acoust. Soc. Am 22, 89–151. 10.1121/1.1906605 [DOI] [Google Scholar]
- Fletcher H, Steinberg JC, 1930. Articulation Testing Methods. J. Acoust. Soc. Am 1, 17–21. 10.1121/1.1915183 [DOI] [Google Scholar]
- Folkeard P, Hawkins M, Scollie S, Sheikh B, Parsa V, 2019. An evaluation of the Sennheiser HDA 280-CL circumaural headphone for use in audiometric testing. Int. J. Audiol 58, 427–433. 10.1080/14992027.2019.1594415 [DOI] [PubMed] [Google Scholar]
- Frank T, 2001. High-Frequency (8 to 16 kHz) Reference Thresholds and Intrasubject Threshold Variability Relative to Ototoxicity Criteria Using a Sennheiser HDA 200 Earphone. Ear Hear. 22, 161–168. 10.1097/00003446-200104000-00009 [DOI] [PubMed] [Google Scholar]
- French NR, Steinberg JC, 1947. Factors Governing the Intelligibility of Speech Sounds. J. Acoust. Soc. Am 19, 90–119. 10.1121/1.1916407 [DOI] [Google Scholar]
- Garinis AC, Cross CP, Srikanth P, Carroll K, Feeney MP, Keefe DH, Hunter LL, Putterman DB, Cohen DM, Gold JA, Steyger PS, 2017. The cumulative effects of intravenous antibiotic treatments on hearing in patients with cystic fibrosis. J. Cyst. Fibros 16 10.1016/jjcf.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garinis AC, Keefe DH, Hunter LL, Fitzpatrick DF, Putterman DB, McMillan GP, Gold JA, Feeney MP, 2018. Chirp-Evoked Otoacoustic Emissions and Middle Ear Absorbance for Monitoring Ototoxicity in Cystic Fibrosis Patients. Ear Hear. 39, 69–84. 10.1097/AUD.0000000000000464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatehouse S, Noble W, 2004. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int. J. Audiol 43, 85–99. 10.1080/14992020400050014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasberg BR, Moore BCJ, 1990. Derivation of auditory filter shapes from notched-noise data. Hear. Res 47, 103–138. 10.1016/0378-5955(90)90170-T [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, 2005. Hearing loss and aging: New research findings and clinical implications. J. Rehabil. Res. Dev 42, 9 10.1682/JRRD.2005.01.0006 [DOI] [PubMed] [Google Scholar]
- Green DM, Kidd G, Stevens KN, 1987. High-frequency audiometric assessment of a young adult population. J. Acoust. Soc. Am 81, 485–494. 10.1121/L394914 [DOI] [PubMed] [Google Scholar]
- Greenberg S, 1988. The ear as a speech analyzer. J. Phonol 16, 139–149. [Google Scholar]
- Guest H, Munro KJ, Prendergast G, Millman RE, Plack CJ, 2018. Impaired speech perception in noise with a normal audiogram: No evidence for cochlear synaptopathy and no relation to lifetime noise exposure. Hear. Res 364, 142–151. 10.1016/j.heares.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkosaari T, Vaalgamaa M, Karjalainen M, 2005. Directivity of Artificial and human speech. AES J. Audio Eng. Soc 53, 620–631. [Google Scholar]
- Heffner HE, Heffner RS, 2008. High-frequency hearing, in: Dallos P, Oertel D, Hoy R (Ed.), Handbook of the Senses: Audition. Elsevier, New York, pp. 55–60. [Google Scholar]
- Heffner HE, Heffner RS, 2007. Hearing ranges of laboratory animals. J. Am. Assoc. Lab. Anim. Sci 41, 2959–2965. 10.1080/003979n.20n.596109 [DOI] [PubMed] [Google Scholar]
- Heffner RS, 2004. Primate hearing from a mammalian perspective. Anat. Rec 281A, 1111–1122. 10.1002/ar.a.20117 [DOI] [PubMed] [Google Scholar]
- Hinchcliffe R, 1992. King-Kopetzky syndrome: An auditory stress disorder? J. Audiol. Med 1, 89–98. [Google Scholar]
- Horwitz AR, Dubno JR, Ahlstrom JB, 2002. Recognition of low-pass-filtered consonants in noise with normal and impaired high-frequency hearing. J. Acoust. Soc. Am 111, 409–416. 10.1121/L1427357 [DOI] [PubMed] [Google Scholar]
- Hunter L, Garinis AC, Dong M, Macdonald K, Burg G, Blankenship C, Feeney P, Clancy JP, 2018. Hearing Loss in Cystic Fibrosis Treated with Aminoglycosides. Pediatr. Pulmonol 53, S148–S456. 10.1002/ppul.24152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter LL, Blankenship CM, Sloat NT, Perdew A, Stewart H, Moore DR, n.d. Peripheral Auditory Involvement in Childhood Listening Difficulty. Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter LL, Margolis RH, Rykken JR, Le CT, Daly KA, Giebink GS, 1996. High Frequency Hearing Loss Associated with Otitis Media. Ear Hear. 17, 1–11. 10.1097/00003446-199602000-00001 [DOI] [PubMed] [Google Scholar]
- ISO, 2006. Acoustics — Reference zero for the calibration of audiometric equipment — Part 5: Reference equivalent threshold sound pressure levels for pure tones in the frequency range 8 kHz to 16 kHz. [Google Scholar]
- ISO 389-8, 2004. Acoustics — Reference zero for the calibration of audiometric equipment --Part 8: Reference equivalent threshold sound pressure levels for pure tones and circumaural earphones, Natural Language Engineering. [Google Scholar]
- Jansen S, Luts H, Dejonckere P, van Wieringen A, Wouters J, 2013. Efficient Hearing Screening in Noise-Exposed Listeners Using the Digit Triplet Test. Ear Hear. 34, 773–778. 10.1097/AUD.0b013e318297920b [DOI] [PubMed] [Google Scholar]
- Johnson EW, 1970. Tuning Forks to Audiometers and Back Again. Laryngoscope 80, 49–68. 10.1288/00005537-197001000-00005 [DOI] [PubMed] [Google Scholar]
- Joris PX, Smith PH, Yin TCT, 1994. Enhancement of neural synchronization in the anteroventral cochlear nucleus. II. Responses in the tuning curve tail. J. Neurophysiol 71, 1037–1051. 10.1152/jn.1994.71.3.1037 [DOI] [PubMed] [Google Scholar]
- Kiang NYS, Moxon EC, 1974. Tails of tuning curves of auditory-nerve fibers. J. Acoust. Soc. Am 55, 620–630. 10.1121/1.1914572 [DOI] [PubMed] [Google Scholar]
- King K, Stephens D, 1992. Auditory and Psychological Factors in ‘Auditory Disability with Normal Hearing’. Scand. Audiol 21, 109–114. 10.3109/01050399209045990 [DOI] [PubMed] [Google Scholar]
- King RB, Oldfield SR, 1997. The Impact of Signal Bandwidth on Auditory Localization: Implications for the Design of Three-Dimensional Audio Displays. Hum. Factors J. Hum. Factors Ergon. Soc 39, 287–295. 10.1518/001872097778543895 [DOI] [Google Scholar]
- Kocon P, Monson BB, 2018. Horizontal directivity patterns differ between vowels extracted from running speech. J. Acoust. Soc. Am 144, EL7–EL12. 10.1121/1.5044508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad-Martin D, Poling GL, Garinis AC, Ortiz CE, Hopper J, O’Connell Bennett K, Dille MF, 2017. Applying U.S. national guidelines for ototoxicity monitoring in adult patients: perspectives on patient populations, service gaps, barriers and solutions. Int. J. Audiol 0, 1–16. 10.1080/14992027.2017.1398421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC, 2019. Translating animal models to human therapeutics in noise-induced and age-related hearing loss. Hear. Res 377, 44–52. 10.1016/j.heares.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC, 2015. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear. Res 330, 191–199. 10.1016/j.heares.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffoon SM, Stewart M, Zheng Y, Meinke DK, 2019. Conventional audiometry, extended high-frequency audiometry, and DPOAEs in youth recreational firearm users. Int. J. Audiol 58, S40–S48. 10.1080/14992027.2018.1536833 [DOI] [PubMed] [Google Scholar]
- Laitila P, Karma P, Sipila M, Manninen M, Rahko T, 1997. Extended high frequency hearing and history of acute otitis media in 14-year-old children in Finland, in: Acta Oto-Laryngologica, Supplement. pp. 27–29. [DOI] [PubMed] [Google Scholar]
- Langendijk EH, Bronkhorst AW, 1999. The contribution of spectral cues to human sound localization. J. Acoust. Soc. Am 105, 1036–1036. 10.1121/1.424945 [DOI] [PubMed] [Google Scholar]
- Lee J, Dhar S, Abel R, Banakis R, Grolley E, Lee Jungwha, Zecker S, Siegel J, 2012. Behavioral Hearing Thresholds Between 0.125 and 20 kHz Using Depth-Compensated Ear Simulator Calibration. Ear Hear. 33, 315–329. 10.1097/AUD.0b013e31823d7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy SC, Freed DJ, Nilsson M, Moore BCJ, Puria S, 2015. Extended High-Frequency Bandwidth Improves Speech Reception in the Presence of Spatially Separated Masking Speech. Ear Hear. 36, e214–e224. 10.1097/AUD.0000000000000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Epstein MJ, Cleveland SS, Wang H, Maison SF, 2016. Toward a differential diagnosis of hidden hearing loss in humans. PLoS One 11, 1–15. 10.1371/journal.pone.0162726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann RP, 1996. Accurate consonant perception without mid-frequency speech energy. IEEE Trans. Speech Audio Process 4, 66 10.1109/TSA.1996.481454 [DOI] [Google Scholar]
- Lundman L, Bagger-Sjoback D, Juhn SK, Morizono T, 1992. Pseudomonas aeurignosa exotoxin A and Haemophilus influenzae type b endotoxin. Effect on the inner ear and passage through the round window membrane of the chinchilla, in: Acta Oto-Laryngologica, Supplement. pp. 101:437–444. [PubMed] [Google Scholar]
- Manley GA, 2017. Comparative Auditory Neuroscience: Understanding the Evolution and Function of Ears. J. Assoc. Res. Otolaryngol 18, 1–24. 10.1007/s10162-016-0579-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis RH, Eikelboom RH, Johnson C, Ginter SM, Swanepoel DW, Moore BCJ, 2013. False air-bone gaps at 4 kHz in listeners with normal hearing and sensorineural hearing loss. Int. J. Audiol 10.3109/14992027.2013.792437 [DOI] [PubMed] [Google Scholar]
- Margolis RH, Saly GL, Hunter LL, 2000. High-frequency hearing loss and wideband middle ear impedance in children with otitis media histories. Ear Hear. 21, 206–211. 10.1097/00003446-200006000-00003 [DOI] [PubMed] [Google Scholar]
- Masterton B, Heffner H, Ravizza R, 1969. The Evolution of Human Hearing. J. Acoust. Soc. Am 45, 966–985. 10.1121/1.1911574 [DOI] [PubMed] [Google Scholar]
- McCreery RW, Kaminski J, Beauchaine K, Lenzen N, Simms K, Gorga MP, 2015. The Impact of Degree of Hearing Loss on Auditory Brainstem Response Predictions of Behavioral Thresholds. Ear Hear. 36, 309–319. 10.1097/AUD.0000000000000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mepani AM, Kirk SA, Hancock KE, Bennett K, de Gruttola V, Liberman MC, Maison SF, 2019. Middle Ear Muscle Reflex and Word Recognition in “Normal-Hearing” Adults. Ear Hear 1 10.1097/AUD.0000000000000804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills DM, Schmiedt RA, 2004. Metabolic Presbycusis: Differential Changes in Auditory Brainstem and Otoacoustic Emission Responses with Chronic Furosemide Application in the Gerbil. JARO - J. Assoc. Res. Otolaryngol 5, 1–10. 10.1007/s10162-003-4004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson BB, Caravello J, n.d. The maximum audible low-pass cutoff frequency for speech. J Acoust Soc Am. [DOI] [PubMed] [Google Scholar]
- Monson BB, Hunter EJ, Story BH, 2012a. Horizontal directivity of low- and high-frequency energy in speech and singing. J. Acoust. Soc. Am 132, 433–441. https://doi.org/10.1121A.4725963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson BB, Lotto AJ, Story BH, 2014. Gender and vocal production mode discrimination using the high frequencies for speech and singing. Front. Psychol 5, 1–7. 10.3389/fpsyg.2014.01239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson BB, Lotto AJ, Story BH, 2012b. Analysis of high-frequency energy in long-term average spectra of singing, speech, and voiceless fricatives. J. Acoust. Soc. Am 132, 1754–1764. https://doi.org/10.1121A.4742724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson BB, Rock J, Schulz A, Hoffman E, Buss E, 2019. Ecological cocktail party listening reveals the utility of extended high-frequency hearing. Hear. Res 381, 107773 10.1016/j.heares.2019.107773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BCJ, Stone MA, Fullgrabe C, Glasberg BR, Puria S, 2008. Spectro-Temporal Characteristics of Speech at High Frequencies, and the Potential for Restoration of Audibility to People with Mild-to-Moderate Hearing Loss. Ear Hear. 29, 907–922. 10.1097/AUD.0b013e31818246f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Edmondson-Jones M, Dawes P, Fortnum H, McCormack A, Pierzycki RH, Munro KJ, 2014. Relation between Speech-in-Noise Threshold, Hearing Loss and Cognition from 40–69 Years of Age. PLoS One 9, e107720 10.1371/journal.pone.0107720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motlagh Zadeh L, Silbert HN, Sternasty K, Swanepoel DW, Hunter LL, Moore RD, 2019. Extended high frequency hearing enhances speech perception in noise. Proc. Natl. Acad. Sci 116, 23753–23759. 10.1073/pnas.1903315116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro KJ, Agnew N, 1999. A comparison of inter-aural attenuation with the Etymotic ER-3A insert earphone and the Telephonies TDH-39 supra-aural earphone. Br. J. Audiol 33, 259–262. 10.3109/03005369909090106 [DOI] [PubMed] [Google Scholar]
- Munro KJ, Contractor A, 2010. Inter-aural attenuation with insert earphones. Int. J. Audiol 49, 799–801. 10.3109/14992027.2010.497940 [DOI] [PubMed] [Google Scholar]
- Neuhoff JG, 2003. Twist and Shout: Audible Facing Angles and Dynamic Rotation. Ecol. Psychol 15, 335–351. 10.1207/s15326969eco1504_7 [DOI] [Google Scholar]
- Pittman AL, Stelmachowicz PG, Lewis DE, Hoover BM, 2003. Spectral Characteristics of Speech at the Ear. J. Speech, Lang. Hear. Res 46, 649–657. 10.1044/1092-4388C2003/051) [DOI] [PubMed] [Google Scholar]
- Poling GL, Siegel JH, Jungmee Lee, Jungwha Lee, Dhar S, 2014. Characteristics of the 2 f 1 - f 2 distortion product otoacoustic emission in a normal hearing population. J. Acoust. Soc. Am 135, 287–299. 10.1121/1.4845415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayleigh Lord, 1908. XVIII. Acoustical notes .—VIII. London, Edinburgh, Dublin Philos. Mag. J. Sci 16, 235–246. 10.1080/14786440808636505 [DOI] [Google Scholar]
- Rodriguez Valiente A, Garcia Berrocal JR, Roldan Fidalgo A, Trinidad A, Ramirez Camacho R, 2014a. Earphones in extended high-frequency audiometry and ISO 389–5. Int. J. Audiol 53, 595–603. 10.3109/14992027.2014.903339 [DOI] [PubMed] [Google Scholar]
- Rodriguez Valiente A, Roldan Fidalgo A, Villarreal IM, Garcia Berrocal JR, 2016. Extended High-frequency Audiometry (9000–20000 Hz). Usefulness in Audiological Diagnosis. Acta Otorrinolaringol. English Ed 67, 40–44. 10.1016/j.otoeng.2015.02.001 [DOI] [PubMed] [Google Scholar]
- Rodriguez Valiente A, Trinidad A, Garcia Berrocal JR, Gorriz C, Ramirez Camacho R, 2014b. Extended high-frequency (9–20 kHz) audiometry reference thresholds in 645 healthy subjects. Int. J. Audiol 53, 531–545. 10.3109/14992027.2014.893375 [DOI] [PubMed] [Google Scholar]
- Saunders GH, Haggard MP, 1989. The Clinical Assessment of Obscure Auditory Dysfunction— 1. Auditory and Psychological Factors. Ear Hear. 10, 200–208. 10.1097/00003446-198906000-00011 [DOI] [PubMed] [Google Scholar]
- Schaette R, McAlpine D, 2011. Tinnitus with a Normal Audiogram: Physiological Evidence for Hidden Hearing Loss and Computational Model. J. Neurosci 31, 13452–13457. 10.1523/JNEUROSa.2156-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter MA, Fausti SA, Rappaport BZ, Frey RH, 1986. Age categorization of high-frequency auditory threshold data. J. Acoust. Soc. Am 79, 767–771. 10.1121/1.393466 [DOI] [PubMed] [Google Scholar]
- Seeto A and Searchfield GD (2018). Investigation of Extended Bandwidth Hearing Aid Amplification on Speech Intelligibility and Sound Quality in Adults with Mild-to-Moderate Hearing Loss. J. Am. Acad. Aud 29:243–254. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Jardine CA, Fridjhon P, 1996. A pilot investigation of high-frequency audiometry in obscure auditory dysfunction (OAD) patients. Br. J. Audiol 30, 233–237. 10.3109/03005369609076770 [DOI] [PubMed] [Google Scholar]
- Siegel JH, Hirohata ET, 1994. Sound calibration and distortion product otoacoustic emissions at high frequencies. Hear. Res 80, 146–152. 10.1016/0378-5955(94)90106-6 [DOI] [PubMed] [Google Scholar]
- Smits C, Theo Goverts S, Festen JM, 2013. The digits-in-noise test: Assessing auditory speech recognition abilities in noise. J. Acoust. Soc. Am 133, 1693–1706. 10.1121/1.4789933 [DOI] [PubMed] [Google Scholar]
- Smull CC, Madsen B, Margolis RH, 2019. Evaluation of Two Circumaural Earphones for Audiometry. Ear Hear. 40, 177–183. 10.1097/AUD.0000000000000585 [DOI] [PubMed] [Google Scholar]
- Souza NN, Dhar S, Neely ST, Siegel JH, 2014. Comparison of nine methods to estimate ear-canal stimulus levels. J. Acoust. Soc. Am 136, 1768–1787. 10.1121/1.4894787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmachowicz PG, Beauchaine KA, Kalberer A, Kelly WJ, Jesteadt W, 1989. High-frequency audiometry: Test reliability and procedural considerations. J. Acoust. Soc. Am 85, 879–887. 10.1121/1.397559 [DOI] [PubMed] [Google Scholar]
- Stelmachowicz PG, Pittman AL, Hoover BM, Lewis DE, Moeller MP, 2004. The Importance of High-Frequency Audibility in the Speech and Language Development of Children With Hearing Loss. Arch. Otolaryngol. Neck Surg 130, 556 10.1001/archotol.130.5.556 [DOI] [PubMed] [Google Scholar]
- Stephens D, Zhao F, Kennedy V, 2003. Is there an association between noise exposure and King Kopetzky Syndrome? Noise Heal. 5, 55–62. [PubMed] [Google Scholar]
- Stover L, Norton SJ, 1993. The effects of aging on otoacoustic emissions. J. Acoust. Soc. Am 94, 2670–2681. 10.1121/1.407351 [DOI] [PubMed] [Google Scholar]
- Strickland EA, Viemeister NF, Van Tasell DJ, Preminger JE, 2004. The role of high-CF fibers in speech perception: Comments on Horwitz et al. (2002) (L). J. Acoust. Soc. Am 116, 49–50. 10.1121/1.1756614 [DOI] [PubMed] [Google Scholar]
- Strickland EA, Viemeister NF, Van Tasell DJ, Preminger JE, 1994. Is useful speech information carried by fibers with high characteristic frequencies? J. Acoust. Soc. Am 95, 497–501. 10.1121/1.408343 [DOI] [PubMed] [Google Scholar]
- Strouse AL, Ochs MT, Hall JW, 1996. Evidence against the influence of aging on distortion-product otoacoustic emissions. J. Am. Acad. Audiol 7, 339–345. [PubMed] [Google Scholar]
- Theunissen FE, Elie JE, 2014. Neural processing of natural sounds. Nat. Rev. Neurosci 15, 355–366. 10.1038/nrn3731 [DOI] [PubMed] [Google Scholar]
- Vaden KI, Matthews LJ, Dubno JR, 2018. Transient-Evoked Otoacoustic Emissions Reflect Audiometric Patterns of Age-Related Hearing Loss. Trends Hear. 22, 233121651879784 10.1177/2331216518797848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitela AD, Monson BB, Lotto AJ, 2015. Phoneme categorization relying solely on high-frequency energy. J. Acoust. Soc. Am 137, EL65–EL70. 10.1121/1.4903917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaming MSMG, MacKinnon RC, Jansen M, Moore DR, 2014. Automated Screening for High-Frequency Hearing Loss. Ear Hear. 35, 667–679. 10.1097/AUD.0000000000000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeend I, Beach EF, Sharma M, 2019. Working Memory and Extended High-Frequency Hearing in Adults. Ear Hear. 40, 458–467. 10.1097/AUD.0000000000000640 [DOI] [PubMed] [Google Scholar]
- Zhao F, Stephens D, 2006. Distortion product otoacoustic emissions in patients with King-Kopetzky syndrome. Int. J. Audiol 45, 34–39. 10.1080/02640410500243939 [DOI] [PubMed] [Google Scholar]
- Zhao F, Stephens D, 2000. Subcategories of Patients with King-Kopetzky Syndrome. Br. J. Audiol 34, 241–256. 10.3109/03005364000000134 [DOI] [PubMed] [Google Scholar]


