Abstract
Before the background of increasingly frequent outbreaks and cases of mosquito-borne diseases in various European countries, Germany recently realised the necessity of updating decade-old data on the occurrence and spatiotemporal distribution of culicid species. Starting in 2011, a mosquito monitoring programme was therefore launched with adult and immature mosquito stages being collected at numerous sites all over Germany both actively by trapping, netting, aspirating and dipping, and passively by the citizen science project ‘Mueckenatlas’. Until the end of 2019, about 516,000 mosquito specimens were analysed, with 52 (probably 53) species belonging to seven genera found, including several species not reported for decades due to being extremely rare (Aedes refiki, Anopheles algeriensis, Culex martinii) or local (Culiseta alaskaensis, Cs. glaphyroptera, Cs. ochroptera). In addition to 43 (probably 44 including Cs. subochrea) out of 46 species previously described for Germany, nine species were collected that had never been documented before. These consisted of five species recently established (Ae. albopictus, Ae. japonicus, Ae. koreicus, An. petragnani, Cs. longiareolata), three species probably introduced on one single occasion only and not established (Ae. aegypti, Ae. berlandi, Ae. pulcritarsis), and a newly described cryptic species of the Anopheles maculipennis complex (An. daciae) that had probably always been present but not been differentiated from its siblings. Two species formerly listed for Germany could not be documented (Ae. cyprius, Ae. nigrinus), while presence is likely for another species (Cs. subochrea), which could not be demonstrated in the monitoring programme as it can neither morphologically nor genetically be reliably distinguished from a closely related species (Cs. annulata) in the female sex. While Cs. annulata males were collected in the present programme, this was not the case with Cs. subochrea. In summary, although some species regarded endemic could not be found during the last 9 years, the number of culicid species that must be considered firmly established in Germany has increased to 51 (assuming Cs. subochrea and Ae. nigrinus are still present) due to several newly emerged ones but also to one species (Ae. cyprius) that must be considered extinct after almost a century without documentation. Most likely, introduction and establishment of the new species are a consequence of globalisation and climate warming, as three of them are native to Asia (Ae. albopictus, Ae. japonicus, Ae. koreicus) and three (Ae. albopictus, An. petragnani, Cs. longiareolata) are relatively thermophilic. Another thermophilic species, Uranotaenia unguiculata, which had been described for southwestern Germany in 1994 and had since been found only at the very site of its first detection, was recently documented at additional localities in the northeastern part of the country. As several mosquito species found in Germany are serious pests or potential vectors of disease agents and should be kept under permanent observation or even be controlled immediately on emergence, the German mosquito monitoring programme has recently been institutionalised and perpetuated.
Keywords: Culicidae, Germany, Inventory, Mosquito monitoring, Invasive species, Globalisation, Climate warming
Introduction
Similar to other countries, the list of German culicids was subject to tremendous changes until the second half of the twentieth century regarding scientific names and numbers of species. This was mainly due to taxonomic revisions, including dissolutions of synonymities, restructuring of the generic system and reassignments of species to genera, as well as to descriptions of previously unrecognised, closely related species, including sibling species.
Consequently, Martini (1915), for example, noted 20 mosquito species for Germany, Eckstein (1920) 21, Vogel (1929) 35, Peus (1932) 38, Peus (1937a, 1950) 40, Britz (1955) 42 and Mohrig (1969) 44. In 1968, Peus (1970) added a 45th species. The increase in species numbers is the more impressing as it is in contrast to the reduction in German territory as a consequence of the two world wars (e.g. Eastern Prussia, Eastern Pomerania, Silesia, Alsace-Lorraine). Since all species described for Germany until 1968 had been, and —with the exception of one species (see below)— still are, considered endemic, the preceding recognition of new species was merely a result of scientific progress, i.e. increased knowledge and improved approaches and taxonomic methodologies.
After the disappearance of malaria in the middle of the twentieth century from most of Europe (Bruce-Chwatt and de Zulueta 1980), mosquito research experienced a sharp decline in Germany. Mosquitoes were not considered dangerous vectors anymore and faded from the view of researchers and funders. Data on the mosquito fauna were subsequently collected only locally or, at maximum, regionally, mainly due to being nuisance pests and linked to control activities (e.g. Becker and Ludwig 1983). Thus, Mohrig’s compilation of the ‘Culicidae of Germany’ (Mohrig 1969), which is based both on the literature and the author’s own collections, remained a standard textbook for German mosquito researchers and was referred to as a guide to the biology, occurrence and distribution of German mosquitoes for decades. According to the valid species list of mosquito taxonomic names as of 16 January 2020 (Harbach 2020), it listed 44 species ever reported for Germany and was only supplemented by Aedes geminus, described in 1970 as a sister species to Ae. cinereus (Peus 1970), and Uranotaenia unguiculata, a newly established species detected in 1994 that had invaded from the south (Becker and Kaiser 1995). While additional species were not recognised for almost two decades and those 46 species were listed in a checklist of German dipterans published in 1999 (Dahl et al. 1999), no information was available on the fate of several rare and less common species not encountered for many years.
In 2008, another invasive culicid, Ae. japonicus, was found and soon demonstrated to have established (Schaffner et al. 2009; Becker et al. 2011), although already in 2007, eggs of Ae. albopictus had been detected in southwestern Germany in an oviposition trap, initially remaining without evidence of establishment (Pluskota et al. 2008).
Triggered by these findings of invasive mosquito species in Germany, which represented potential vectors of disease agents, and the continuing demonstrated spread of Ae. albopictus in southern Europe (Scholte and Schaffner 2007; Medlock et al. 2015) as well as increasing numbers of culicid-borne West Nile fever incidents (Hubálek and Halouzka 1999; Bellini et al. 2014) and the emergence of chikungunya and dengue in Europe (Rezza 2014), scientific, political and public interest in mosquitoes experienced a renaissance in Germany and finally resulted in a monitoring programme launched in 2011.
We here present benchmark results of the German monitoring programme, targeting the occurrence and spatiotemporal distribution of both native and invasive species, together with an updated inventory of the mosquito species of Germany. Information on the specific distributions of the species found, including distribution maps and their phenology, will follow separately.
Materials and methods
Mosquito collection
Mosquitoes were collected actively and passively. Active collections of adult specimens were carried out by various kinds of traps during the vegetative seasons each year (April to October). At a total of 109 collection sites throughout Germany, BG Sentinel traps (Biogents, Regensburg, Germany) equipped with a CO2 source (gas tank releasing 500 g CO2/24 h) and baited with BG Lure® (Biogents) as attractants were operated for 24 h per week from April to October during a first monitoring phase (2011–2014). Thirteen of the sites were sampled for 4 years, 41 sites for 3 years, 15 sites for 2 years and 40 sites for 1 year. In addition to the BG Sentinels, CO2-baited EVS (encephalitis virus surveillance) traps (BioQuip Products, Compton, CA, USA) (with the equivalent concentration of released gas) were used in the same rhythm (i.e. once per week for 24 h from April to October) at 52 sites, with 15 sites sampled for 2 years and 37 sites sampled for 1 year. In a second project phase (2015–2017), 64 BG Sentinels were annually operated for 24 h once per month in April and October and twice per month from May to September at different sites per year in the eastern half of Germany. For organisational reasons, no systematic trapping took place in 2018, but ten EVS traps each were operated for 2 to 3 weeks on four limited areas in eastern Germany following West Nile virus (WNV) emergence (Kampen et al. 2020). In 2019, systematic trapping covering the complete vegetative season was resumed with 31 BG Sentinels, run at more or less evenly distributed sites throughout Germany which promised to offer a high species diversity and a high abundance of mosquitoes, and an additional 20 EVS traps at a WNV hotspot in the Wildlife Park Berlin in September (Kampen et al. 2020).
Furthermore, adult mosquitoes were caught throughout Germany by aspirating females from resting places in animal (predominantly sheep, goat, cattle and horse) shelters and zoo settings (305 collections, 58 stables, barns, etc.) (Heym et al. 2018; Werner and Kampen, unpublished) and hibernating females in winter shelters, such as caves, dungeons and cellars (137 objects, with one object sampled twice), as well as by netting from bushy vegetation (e.g. in forests) and from both animal and human baits (Werner and Kampen, unpublished).
Finally, mosquito larvae and pupae were actively collected throughout Germany by dipping and sieving in natural and artificial breeding places (ca. 3000 collections at 1500 sites).
To enlarge the data pool and support data collection in terms of quantity (number of mosquitoes) and quality (species spectrum), mosquitoes were also collected passively by a citizen science project, the ‘Mueckenatlas’, which had been launched in April 2012 (Kampen et al. 2015; Walther and Kampen 2017). In that project, citizens are asked to capture mosquitoes in their private surroundings and submit them for scientific analysis.
Mosquito identification
For practical reasons, morphological determination of mosquitoes was generally performed on adult specimens using the identification keys by Mohrig (1969), Schaffner et al. (2001) and Becker et al. (2010). Collected immature stages were therefore kept in jars and beakers containing water taken from their breeding sites until adult emergence. Adult mosquito specimens were killed by freezing for at least 1 h at − 20 °C.
Individuals belonging to the Culex pipiens and Anopheles maculipennis complexes were genetically identified by species-specific PCR assays (Proft et al. 1999; Rudolf et al. 2013; Kronefeld et al. 2014). Other closely related species or damaged specimens that could not be identified based on morphological characters were subjected to CO1 (cytochrome c oxidase subunit 1) PCR and sequencing (Folmer et al. 1994; Hébert et al. 2003).
Data management
All collection data were fed into the German mosquito database CULBASE which, for the purpose of the present contribution, was filtered for mosquito species and developmental stage, number of collection sites and mode of sampling.
Results and discussion
Within the scope of the present monitoring project, a total of more than 516,000 mosquito specimens collected from 2011 to 2019 throughout Germany were analysed, including some 300,000 trapped specimens, ca. 62,000 adult hand catches (aspirated, netted), ca. 137,000 mosquitoes submitted to the ‘Mueckenatlas’ scheme and at least 17,000 immature stages collected from their breeding sites. The number of collected immature stages was actually much higher than 17,000 as often only one larva per species, site and collection event was entered into the database, although more specimens had been collected and identified. This discrepancy is due to non-standardised collection efforts and time.
The mosquitoes belonged to an assured 52 species out of seven culicid genera and were collected at roughly 22,600 sites (Table 1, Fig. 1). Probably, specimens of a 53rd species (Cs. subochrea) were among the collections, but this species could not be reliably distinguished from a closely related species (Cs. annulata) in the life stages available. Forty-three (possibly 44, assuming the presence of Cs. subochrea) of the collected species were included in the 46 thought to occur in Germany prior to the onset of the monitoring programme (Dahl et al. 1999). In addition, several invasive species were captured, five of which are now considered established: Culiseta longiareolata, An. petragnani, Ae. albopictus, Ae. japonicus and Ae. koreicus.
Table 1.
Mosquito species listed for Germany by Dahl et al. (1999) and found within the scope of the presented monitoring programme (non-native species with single detections excluded)
| Species | First documented on the territory of present-day Germany1 | Found last in Germany (documented last2) |
Found in this monitoring project | Considered established | ||
|---|---|---|---|---|---|---|
| Adults | Immature stages | |||||
| Trap | Mueckenatlas | |||||
|
Ae. (Stegomyia) albopictus Skuse, 1895 |
2007 (Pluskota et al. 2008) (as Stegomyia albopicta) |
2019 (this study) | + | + | + | + |
|
Ae. (Ochlerotatus) annulipes Meigen, 1830 |
1898 (Mohrig 2000) (as Cx. annulipes) |
2019 (this study) | + | + | + | + |
|
Ae. (Ochlerotatus) cantans Meigen, 1818 |
1906 (Eysell 1907) (as Cx. cantans) |
2019 (this study) | + | + | + | + |
|
Ae. (Ochlerotatus) caspius Pallas, 1771 |
1928 (Peus 1929) | 2019 (this study) | + | + | + | + |
|
Ae. (Ochlerotatus) cataphylla Dyar, 1916 |
1920 (Martini 1920b) (as Ae. rostochiensis) |
2019 (this study) | + | + | + | + |
|
Ae. (Aedes) cinereus Meigen, 1818 |
1897 (Mohrig 2000) | 2019 (this study) | + | + | + | + |
|
Ae. (Ochlerotatus) communis de Geer, 1776 |
1896 (Mohrig 2000) | 2018 (this study) | + | + | + | + |
|
Ae. (Ochlerotatus) cyprius Ludlow, 1920 |
1900 (Edwards 1921) (as Ae. freyi) |
1925 (Peus 1937b) | − | − | − | −3 |
|
Ae. (Ochlerotatus) detritus Haliday, 1833 |
1919 (Martini 1920b) (as Ae. salinus) |
2019 (this study) | + | + | + | + |
|
Ae. (Ochlerotatus) diantaeus Howard, Dyar & Knab, 1913 |
1893 (Mohrig 2000) | 2017 (this study) | + | + | − | + |
|
Ae. (Ochlerotatus) dorsalis Meigen, 1830 |
1914 (Martini 1920b) | 2019 (this study) | + | + | + | + |
|
Ae. (Ochlerotatus) excrucians Walker, 1856 |
1898 (Mohrig 2000) | 2019 (this study) | + | + | + | + |
|
Ae. (Ochlerotatus) flavescens Müller, 1764 |
1895 (Mohrig 2000) | 2019 (this study) | + | + | + | + |
|
Ae. (Aedes) geminus Peus 1970 |
1968 (Peus 1970) | 2019 (this study) | + | + | + | + |
|
Ae. (Dahliana) geniculatus Olivier, 1791 |
1898 (Mohrig 2000) (as Cx. ornatus) |
2019 (this study) | + | + | + | + |
|
Ae. (Ochlerotatus) intrudens Dyar, 1919 |
1928 (Peus 1929) | 2018 (this study) | + | + | + | + |
|
Ae. (Hulecyteomyia) japonicus Theobald, 1901 |
2008 (Schaffner et al. 2009) | 2019 (Kampen et al. 2020) | + | + | + | + |
|
Ae. (Hulecyteomyia) koreicus Edwards, 1917 |
2015 (Werner et al. 2016) | 2019 (this study) | − | + | + | + |
|
Ae. (Ochlerotatus) leucomelas Meigen, 1804 |
1896 (Mohrig 2000) | 2019 (this study) | + | + | + | + |
|
Ae. (Ochlerotatus) nigrinus Eckstein, 1918 |
1932 (Peus 1933) | 1993 (Becker and Kaiser 1995) | − | − | − | ? |
|
Ae. (Ochlerotatus) pullatus Coquillett, 1904 |
1916 (Kühlhorn 1954) | 2016 (this study) | + | + | + | + |
|
Ae. (Ochlerotatus) punctor Kirby, 1837 |
1913 (Kühlhorn 1954) | 2019 (this study) | + | + | + | + |
|
Ae. (Rusticoidus) refiki Medschid, 1928 |
1897 (Vogel 1931) | 2017 (Kuhlisch et al. 2017) | + | + | + | + |
|
Ae. (Ochlerotatus) riparius Dyar & Knab, 1907 |
1919 (Martini 1920a) (as Ae. semicantans) |
2019 (this study) | + | + | + | + |
|
Ae. (Aedes) rossicus Dolbeskin, Gorickaja & Mitrofanova, 1930 |
1963 (Müller 1965) | 2019 (this study) | + | + | + | + |
|
Ae. (Ochlerotatus) rusticus Rossi, 1790 |
1914 (Martini 1920a) (as Ae. diversus) |
2019 (this study) | + | + | + | + |
|
Ae. (Ochlerotatus) sticticus Meigen, 1838 |
1901 (Mohrig 2000) (as Cx. sticticus) |
2019 (this study) | + | + | + | + |
|
Ae. (Aedimorphus) vexans Meigen, 1830 |
1900 (Mohrig 2000) (as Cx. vexans) |
2019 (this study) | + | + | + | + |
|
An. (Anopheles) algeriensis Theobald, 1903 |
1931 (Martini 1931) | 2019 (this study) | + | + | − | + |
|
An. (Anopheles) atroparvus van Thiel, 1927 |
1931 (Martini et al. 1931) | 2016 (Kampen et al. 2016b) | + | + | + | + |
|
An. (Anopheles) claviger Meigen, 1804 |
1911/12 (Schneider 1913) (as An. bifurcatus) |
2019 (this study) | + | + | + | + |
|
An. (Anopheles) daciae Linton, Nicolescu & Harbach, 2004 |
2007 (Weitzel et al. 2012) | 2019 (this study) | + | + | + | + |
|
An. (Anopheles) maculipennis Meigen, 1818 |
1931 (Martini et al. 1931) | 2019 (this study) | + | + | + | + |
|
An. (Anopheles) messeae Falleroni, 1926 |
1931 (Martini et al. 1931) | 2019 (this study) | + | + | + | + |
|
An. (Anopheles) petragnani del Vecchio, 1939 |
2015 (Becker et al. 2016, Kampen et al. 2017) | 2019 (this study) | + | + | + | + |
|
An. (Anopheles) plumbeus Stephens, 1828 |
1911 (Schneider 1913) (as An. nigripes) |
2019 (this study) | + | + | + | + |
|
Cq. (Coquillettidia) richiardii Ficalbi, 1889 |
1896 (Mohrig 2000) | 2019 (this study) | + | + | + | + |
|
Cx. (Culex) hortensis Ficalbi, 1889 |
1927 (Peus 1929) | 2019 (this study) | + | + | + | + |
|
Cx. (Culex) martinii Medschid, 1930 |
1935 (Peus 1950) | 2017 (Kuhlisch et al. 2018b) | + | − | − | + |
|
Cx. (Culex) modestus Ficalbi, 1890 |
1960/61 (Mohrig 1963) | 2019 (Kampen et al. 2020) | + | + | + | + |
|
Cx. (Culex) pipiens Linnaeus, 1758 |
1900 (Mohrig 2000) | 2019 (Kampen et al. 2020) | + | + | + | + |
|
Cx. (Culex) territans Walker, 1856 |
1911 (Schneider 1913) | 2019 (this study) | + | + | + | + |
|
Cx. (Culex) torrentium Martini, 1925 |
1924 (Martini 1924b) | 2019 (Kampen et al. 2020) | + | + | + | + |
|
Cs. (Culiseta) alaskaensis Ludlow, 1906 |
1897 (Mohrig 2000) (as Cx. annulatus) |
2017 (this study) | + | + | + | + |
|
Cs. (Culiseta) annulata Schrank, 1776 |
1896 (Mohrig 2000) (as Cx. annulatus) |
2019 (this study) | + | + | + | + |
|
Cs. (Culicella) fumipennis Stephens, 1825 |
1930 (Peus 1950) | 2019 (this study) | + | ? | ? | + |
|
Cs. (Culiseta) glaphyroptera Schiner, 1864 |
1923 (Martini 1924b) | 2018 (this study) | + | + | + | + |
|
Cs. (Allotheobaldia) longiareolata Macquart, 1838 |
2011 (Becker and Hoffmann 2011, Werner et al. 2012) | 2019 (this study) | + | + | + | + |
|
Cs. (Culicella) morsitans Theobald, 1901 |
1911 (Schneider 1913) (as Culicada morsitans) |
2019 (this study) | + | + | + | + |
|
Cs. (Culicella) ochroptera Peus 1935 |
1928 (Peus 1935) (as Theobaldia. glaphyroptera) |
2017 (Kuhlisch et al. 2019) | + | + | + | + |
|
Cs. (Culiseta) subochrea Edwards 1921 |
1914 (Martini 1924a) (as Th. annulata var. ferruginata) |
1991 (Becker and Kaiser 1995) | ? | ? | ? | + |
|
Ur. (Pseudoficalbia) unguiculata Edwards, 1913 |
1994 (Becker and Kaiser 1995) | 2019 (this study) | + | − | + | + |
| Total number of species | 52 | 48 (49?) | 46 (48?) | 45 (47?) | 50 | |
| 49 (50?) | ||||||
+/–: included/not included in the collections; ?: reliable identification not possible due to high morphological similarity and CO1-DNA sequence homology with other species, lack of males and processing of adults only; 1based on literature published from the year 1900 onwards; 2references provided only when most recent documentation occurred before most recent finding in the present monitoring programme; 3should not be considered belonging to the German mosquito fauna anymore
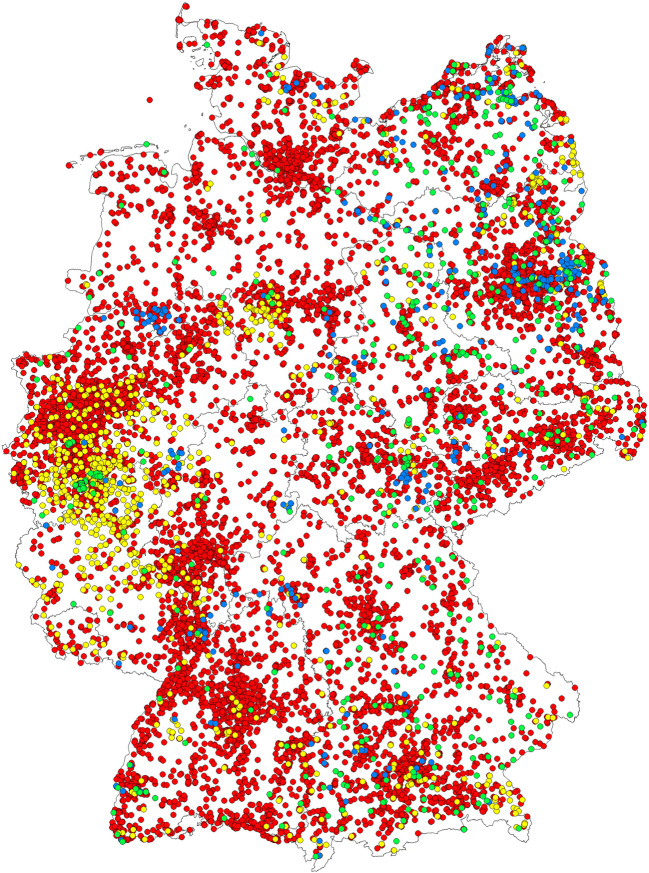
Fig. 1.
Mosquito collections 2011–2019 (green dots—trap collections; red dots—‘Mueckenatlas’ submissions; blue dots—netting, aspirating and baited collections of adults; yellow dots—dipping/sieving of immature developmental stages)
Culiseta longiareolata, a thermophilic species widely distributed in the Mediterranean (Becker et al. 2010), was discovered in southwestern Germany in 2011 (Becker and Hoffmann 2011; Werner et al. 2012; Kampen et al. 2013b). In the following years, it was repeatedly found in more central and northern parts of Germany (Kampen et al. 2017), while in 2018 and 2019, it was for the first time demonstrated at exactly the same places in the West German cities of Worms and Alzey (cemeteries) as in 2017, indicating overwintering and establishment (Kampen and Werner, unpublished data).
Anopheles petragnani is another thermophilic species which predominantly occurs in southwestern Europe (Becker et al. 2010). In Germany, it was first detected in 2015 (Becker et al. 2016) but has since been reported from four sites in the southern half of the country, with annual larval collections at one of these sites (rock pools in the river Murg close to the city of Forbach in the federal state of Baden-Württemberg) from 2015 to 2019, indicating persistent maintenance (Kampen et al. 2017; Kampen and Werner, unpublished data).
The Asian tiger mosquito Ae. albopictus, the most invasive mosquito species of the world within the last 30 years or so (Paupy et al. 2009), has been reported from 28 European countries in 20 of which it succeeded in establishing (Medlock et al. 2015; Robert et al. 2019). By producing diapausing eggs, this thermophilic species has become adapted to more temperate climates, and a strong tendency to spread northwards can be observed (e.g. Armstrong et al. 2017). Aedes albopictus was initially trapped in the southwestern part of Germany only, predominantly on service stations along motorways entering the country from the south (Werner et al. 2012; Becker et al. 2013; Kampen et al. 2013a). These findings were generally attributed to introductions by vehicle transport from southern Europe, such as Italy, where the species is widely distributed (Romi et al. 2008). More recently, Ae. albopictus was increasingly often reported from other parts of southern Germany and remote from motorways, linked to local reproduction over extended periods of time (Werner and Kampen 2015). Repeated overwintering suggests establishment of the species at various localities in Germany, including the northernmost population worldwide (Pluskota et al. 2016; Becker et al. 2017; Walther et al. 2017; Kuhlisch et al. 2018a).
A fourth invasive and established species found, Ae. japonicus, originates from East Asia and is well adapted to temperate climates (Kampen and Werner 2014; Kaufman and Fonseca 2014). The first records within the present monitoring programme were again from southwestern Germany (Werner et al. 2012) where the species had been known to occur since 2009 (Schaffner et al. 2009; Becker et al. 2011). In 2012, 2013 and 2015, specimens collected in western, northern and southeastern Germany were submitted to the ‘Mueckenatlas’, resulting in the detection of three additional, previously unknown populations of the species (Kampen et al. 2012; Werner and Kampen 2013; Zielke et al. 2016). The southwest German population of Ae. japonicus included, which spread to France in the west and to Switzerland in the south, four populations of Ae. japonicus existed, completely or partly, on German territory in 2015 (Kampen and Werner 2014; Zielke et al. 2016). Both ‘Mueckenatlas’ submissions and field collections from 2016 from outside the known population areas suggested that the species kept spreading (Kampen et al. 2017). As of 2017, the various German populations had either merged or were close to merging, with numerous collection sites throughout the southern half of Germany, although much more dense in the western part (Koban et al. 2019). This process continued until 2019 when putative gaps in the distribution map of Ae. japonicus in southern Germany filled (Kampen et al. 2020).
Finally, Ae. koreicus succeeded in establishing in Germany. After the first finding of a specimen in Bavaria in 2015 (Werner et al. 2016), the species emerged in the federal state of Hesse in 2016 where it could be found again in 2017 and 2018 at several places, apparently having built up a population (Pfitzner et al. 2018; Steinbrink et al. 2019).
In addition to the invasive thermophilic species, climate warming obviously also has an impact on thermophilic mosquito species already present in Germany, such as Ur. unguiculata, which is widely distributed in the Mediterranean (Becker et al. 2010). This species was first detected in Germany in 1994 in the northern Upper Rhine valley (Becker and Kaiser 1995) and from then on repeatedly encountered at that very same site, but nowhere else. Only in 2016 (two sites), and again in 2017, 2018 and 2019 (one site each), the species was discovered, both as larvae and as adults, in northeastern Germany (Tippelt et al. 2017; Werner and Kampen, unpublished data).
Single specimens of three further non-indigenous species, Ae. aegypti, Ae. berlandi and Ae. pulcritarsis, were demonstrated on one occasion each in 2016 after submission to the ‘Mueckenatlas’ scheme (Kampen et al. 2016a, 2017; Werner and Kampen, unpublished) and can therefore not be considered belonging to the German mosquito fauna.
However, an eighth species previously not listed although probably present was demonstrated to occur (Kronefeld et al. 2012, 2014): Anopheles daciae, a cryptic species of the An. maculipennis complex which was only separated from its sibling, An. messeae, in 2004 on the basis of fixed genetic differences (Nicolescu et al. 2004). The species does not appear to be particularly rare in Germany, but seems to have major distribution areas in southern and northeastern Germany (Kronefeld et al. 2014; Kampen et al. 2016b; Lühken et al. 2016; Czajka et al. 2020; Kampen and Werner, unpublished data). Findings from outside Germany suggest that An. daciae is much more frequent and widespread in Europe than initially assumed (e.g. Rydzanicz et al. 2017; Blažejová et al. 2018>; Kavran et al. 2018; Culverwell et al. 2020). Coincidently with the detections of An. daciae in Germany, documentations of An. atroparvus, another member of the An. maculipennis complex, have become quite rare.).
Among the 52 (53 including Cs. subochrea) species found, several less frequent and even rare species were registered (Kampen et al. 2014), including three Culiseta species which had not been reported for decades: Cs. alaskaensis, Cs. glaphyroptera and Cs. ochroptera (Kampen et al. 2013b; Kuhlisch et al. 2019). Extremely rare species re-discovered are Ae. refiki, An. algeriensis and Cx. martinii (Krüger and Tannich 2014; Kuhlisch et al. 2017, 2018b; Tippelt et al. 2018).
Two species listed in previous mosquito checklists were not found at all within the scope of the monitoring project: Ae. cyprius and Ae. nigrinus. In the case of Ae. cyprius, it is highly questionable whether it still occurs in Germany. It had been found only in the mid-1920s at very few sites around Berlin (Peus 1937b), and not much later, Peus (1950) already considered it extinct. Aedes nigrinus is another extremely rare species, which—to the best of the authors’ knowledge—was documented four times only for Germany, each time with larval findings: twice in the 1930s from northwestern Baden-Württemberg and Upper Bavaria (Peus 1933, 1950), once in 1980 from Central Germany (Heitkamp et al. 1985), although the species identification in that case must be doubted as the biotopes (forest ponds) were untypical, and once and last in 1993 with one single specimen found in the Upper Rhine valley (Becker and Kaiser 1995).
It is unclear whether Cs. subochrea was or was not found during the presented monitoring programme due to close relatedness and high morphological and genetic similarity to Cs. annulata. While several Cs. annulata males could be unambiguously identified based on characteristics of their genitalia, this was never the case with Cs. subochrea. According to the original literature on German mosquitoes, Cs. subochrea was observed last in 1991 (Becker and Kaiser 1995). However, although the latter species apparently is not very common, it is supposed to be widely distributed in Europe and can be assumed to still occur in Germany.
While 48 (probably 49 including Cs. subochrea) of the registered species had been trapped, 49 species (51 including Cs. subochrea and Cs. fumipennis) had been collected and submitted by citizens to the ‘Mueckenatlas’ (Table 1). Culex martinii was the only species only caught by traps (disregarding Cs. fumipennis and Cs. subochrea of which no pertinent data exist) while Ae. aegypti and Ae. koreicus adults were only obtained via the ‘Mueckenatlas’, but not by trapping. Although both active and passive approaches collected almost the same number of species until the end of 2019, the detection of new introductions or populations of invasive Aedes species could almost always be credited to the ‘Mueckenatlas’ scheme (Werner and Kampen, unpublished).
As opposed to all established species caught as adults, 45 of them (47 including Cs. subochrea and Cs. fumipennis) were collected as aquatic stages (i.e. larvae and pupae). Aedes detritus, Ae. diantaeus and An. algeriensis, which were collected as adults, were not represented among the immature mosquito stages.
Not surprisingly, females of Cx. pipiens s.l., An. maculipennis s.l., Anopheles sp., Aedes sp. and Culiseta sp. were found in winter shelters, but their analysis according to species level remains to be done.
Species identification was difficult or impossible in some groups of closely related species, when morphological characters were missing or ambiguous. Thus, Ae. annulipes/cantans/excrucians/riparius, Ae. cataphylla/leucomelas, Ae. cinereus/geminus/rossicus, Ae. intrudens/diantaeus, Cs. morsitans/fumipennis and, as already mentioned, Cs. annulata/subochrea could often not be differentiated, even by CO1-barcoding. For these groups of species, it would be rather helpful to have reliable species-specific genetic markers at hand.
Conclusion
The German mosquito monitoring programme provided valuable data as to the present occurrence of culicid species in Germany. Apparently, changes in the mosquito fauna have recently occurred, not least caused by the introduction, establishment and spread of invasive species. These changes therefore mainly apply to additional species rather than lost species. It is evident that environmental and ecological changes have an impact on the availability of habitats for some specialised and stenoecious mosquito species (e.g. An. atroparvus, Cs. ochroptera and Cs. glaphyroptera, which have become rare in Germany due to a loss of suitable breeding sites), but these do not seem to have resulted in species extinction yet. The two species listed in previous checklists but neither found during this monitoring programme nor documented by others in Germany for decades, Ae. cyprius and Ae. nigrinus, are breeders of pools in open landscapes such as meadows and floodplains, which are still existent. Thus, their absence, or lack of finding, may have other reasons. In summary, the current number of culicid species established in Germany amounts to 51 (Table 1).
As Ae. albopictus, Ae. japonicus and Ae. koreicus are spreading and, together with various indigenous taxa (e.g. Ae. vexans, An. maculipennis s.l., Cx. modestus, Cx. pipiens s.l.), may pose a risk to human and animal health (Kampen and Walther 2018), the German mosquito monitoring programme has recently been institutionalised and perpetuated with the aim of collecting, assessing and distributing data on spatial occurrence, seasonal population dynamics and abundance of both native and invasive species.
Acknowledgements
This work was financially supported by the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE), grant numbers 2810HS022, 2819104115, 2819104615 and 2818SE001, and by the Robert-Koch-Institute, grant number 1362/1-982. In the time period covering this study, numerous qualification works (B.Sc., M.Sc., Ph.D. theses) were prepared contributing to the monitoring programme. We are grateful to Marco Dittmann, Linus Früh, Susan Jahn, Marcel Koban, Iris Kröger, Jakob Martin, Nadja Pernat, Eva Rogasch, Mandy Schäfer, Dorothee Scheuch and Lisa Tippelt. We also thank Fanny Breski, Brigitte Dannenfeld, Jutta Falland, Juliane Horenk, Martina Pusch, Antje Wehrhan, Fermin Georgio Lorenzen-Schmidt, Oliver Tauchmann, Christin Henke and Maxi Uecker for technical assistance in the laboratory, and to Petra Kranz and colleagues from the FLI Institute for Epidemiology for establishing and maintaining databases.
Funding Information
Open Access funding provided by Projekt DEAL.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Armstrong PM, Andreadis TG, Shepard JJ, Thomas MC. Northern range expansion of the Asian tiger mosquito (Aedes albopictus): analysis of mosquito data from Connecticut, USA. PLoS Negl Trop Dis. 2017;11:e0005623. doi: 10.1371/journal.pntd.0005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker N, Hoffmann D. First record of Culiseta longiareolata (Macquart) for Germany. Eur Mosq Bull. 2011;29:143–150. [Google Scholar]
- Becker N, Kaiser A. Die Culicidenvorkommen in den Rheinauen des Oberrheingebiets mit besonderer Berücksichtigung von Uranotaenia (Culicidae, Diptera)—einer neuen Stechmückengattung für Deutschland. Mitt Dtsch Ges Allg Angew Entomol. 1995;10:407–413. [Google Scholar]
- Becker N, Ludwig HW. Mosquito control in West Germany. Bull Soc Vector Ecol. 1983;8:85–93. [Google Scholar]
- Becker N, Petrić D, Zgomba M, Boase C, Madon M, Dahl C, Kaiser A. Mosquitoes and their control. 2. Heidelberg: Springer; 2010. [Google Scholar]
- Becker N, Huber K, Pluskota B, Kaiser A. Ochlerotatus japonicus japonicus—a newly established neozoan in Germany and a revised list of the German mosquito fauna. Eur Mosq Bull. 2011;28:88–102. [Google Scholar]
- Becker N, Geier M, Balczun C, Bradersen U, Huber K, Kiel E, Krüger A, Lühken R, Orendt C, Plenge-Bönig A, Rose A, Schaub GA, Tannich E. Repeated introduction of Aedes albopictus into Germany, July to October 2012. Parasitol Res. 2013;112:1787–1790. doi: 10.1007/s00436-012-3230-1. [DOI] [PubMed] [Google Scholar]
- Becker N, Pfitzner WP, Czajka C, Kaiser A, Weitzel T (2016) Anopheles (Anopheles) petragnani Del Vecchio 1939—a new mosquito species for Germany. Parasitol Res 115:2671–2677 [DOI] [PMC free article] [PubMed]
- Becker N, Schön S, Klein AM, Ferstl I, Kizgin A, Tannich E, Kuhn C, Pluskota B, Jöst A. First mass development of Aedes albopictus (Diptera: Culicidae)—its surveillance and control in Germany. Parasitol Res. 2017;116:847–858. doi: 10.1007/s00436-016-5356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini R, Zeller H, van Bortel W. A review of the vector management methods to prevent and control outbreaks of West Nile virus infection and the challenge for Europe. Parasit Vectors. 2014;7:e323. doi: 10.1186/1756-3305-7-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blažejová H, Šebesta O, Rettich F, Mendel J, Čabanová V, Miterpáková M, Betášová L, Peško J, Hubálek Z, Kampen H, Rudolf I. Cryptic species Anopheles daciae (Diptera: Culicidae) found in the Czech Republic and Slovakia. Parasitol Res. 2018;117:315–321. doi: 10.1007/s00436-017-5670-0. [DOI] [PubMed] [Google Scholar]
- Britz L. Über die Stechmücken-Fauna des Stadtkreises Leipzig. Z Angew Zool. 1955;42:61–79. [Google Scholar]
- Bruce-Chwatt LJ, de Zulueta J. The rise and fall of malaria in Europe. Oxford: Oxford University Press; 1980. [Google Scholar]
- Culverwell CL, Vapalahti OP, Harbach RE (2020) Anopheles daciae, a new country record for Finland. Med Vet Entomol 34:145–150 [DOI] [PubMed]
- Czajka C, Weitzel T, Kaiser A, Pfitzner WP, Becker N. Species composition, geographical distribution and seasonal abundance of the Anopheles maculipennis complex along the upper Rhine, Germany. Parasitol Res. 2020;119:75–84. doi: 10.1007/s00436-019-06551-z. [DOI] [PubMed] [Google Scholar]
- Dahl CI, Kaiser A, Becker N (1999) Culicidae. In: Schuhmann H, Bährmann R, Stark A (eds), Checkliste der Dipteren Deutschlands. Studia Dipterologica, Suppl 2:51–52
- Eckstein F (1920) Die einheimischen Stechmücken. Verlag Natur und Kultur Dr. Franz Josef Völler, München
- Edwards FW. A revision of the mosquitos of the Palaearctic region. Bull Ent Res. 1921;12:263–351. [Google Scholar]
- Eysell A. Beiträge zur Biologie der Stechmücken. Arch Schiffs- Tropenhyg. 1907;11:197–211. [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- Harbach RE (2020) Mosquito Taxonomic Inventory. http://mosquito-taxonomic-inventory.info, accessed 13 June 2020
- Hébert PD, Ratnasingham S, de Waard JR. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc R Soc Lond B. 2003;270(Suppl):S96–S99. doi: 10.1098/rsbl.2003.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkamp U, Gottwald J, Klapp K. Untersuchungen zur Erstbesiedlung der Fauna in neu angelegten Tümpeln im Vergleich mit restaurierten Gewässern. Mitt Flora Fauna Süd-Nieders. 1985;7:95–130. [Google Scholar]
- Heym EC, Kampen H, Walther D. Mosquito species composition and phenology (Diptera, Culicidae) in two German zoological gardens imply different risks of mosquito-borne pathogen transmission. J Vector Ecol. 2018;43:80–88. doi: 10.1111/jvec.12286. [DOI] [PubMed] [Google Scholar]
- Hubálek Z, Halouzka J. West Nile fever—a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999;5:643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampen H, Walther D. Vector potential of mosquito species (Diptera: Culicidae) occurring in Central Europe. Parasitol Res Monogr. 2018;10:41–68. [Google Scholar]
- Kampen H, Werner D. Out of the bush: the Asian bush mosquito Aedes japonicus japonicus (Theobald, 1901) (Diptera, Culicidae) becomes invasive. Parasit Vectors. 2014;7:e59. doi: 10.1186/1756-3305-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampen H, Zielke D, Werner D. A new focus of Aedes japonicus japonicus (Theobald, 1901) (Diptera, Culicidae) distribution in western Germany: rapid spread or a further introduction event? Parasit Vectors. 2012;5:e284. doi: 10.1186/1756-3305-5-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampen H, Kronefeld M, Zielke D, Werner D. Further specimens of the Asian tiger mosquito Aedes albopictus (Diptera, Culicidae) trapped in Southwest Germany. Parasitol Res. 2013;112:905–907. doi: 10.1007/s00436-012-3128-y. [DOI] [PubMed] [Google Scholar]
- Kampen H, Kronefeld M, Zielke D, Werner D. Three rarely encountered and a new Culiseta species in Germany. J Eur Mosq Control Assoc. 2013;31:36–39. [Google Scholar]
- Kampen H, Kronefeld M, Zielke D, Werner D. Some new, less frequent and rare mosquito species (Diptera, Culicidae) recently collected in Germany. Mitt Dtsch Ges Allg Angew Entomol. 2014;19:123–130. [Google Scholar]
- Kampen H, Medlock JM, Vaux AG, Koenraadt CJ, van Vliet AJ, Bartumeus F, Oltra A, Sousa CA, Chouin S, Werner D. Approaches to passive mosquito surveillance in the EU. Parasit Vectors. 2015;8:e9. doi: 10.1186/s13071-014-0604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampen H, Jansen S, Schmidt-Chanasit J, Walther D (2016a) Indoor development of Aedes aegypti in Germany, 2016. Euro Surveill 21:pii=30407 [DOI] [PMC free article] [PubMed]
- Kampen H, Schäfer M, Zielke DE, Walther D. The Anopheles maculipennis complex (Diptera: Culicidae) in Germany: an update following recent monitoring activities. Parasitol Res. 2016;115:3281–3294. doi: 10.1007/s00436-016-5189-9. [DOI] [PubMed] [Google Scholar]
- Kampen H, Schuhbauer A, Walther D. Emerging mosquito species in Germany—a synopsis after six years of mosquito monitoring (2011–2016) Parasitol Res. 2017;116:3253–3263. doi: 10.1007/s00436-017-5619-3. [DOI] [PubMed] [Google Scholar]
- Kampen H, Holicki CM, Ziegler U, Groschup MH, Tews BA, Werner D. West Nile virus mosquito vectors (Diptera: Culicidae) in Germany. Viruses. 2020;12:e493. doi: 10.3390/v12050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MG, Fonseca DM. Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae) Annu Rev Entomol. 2014;59:31–49. doi: 10.1146/annurev-ento-011613-162012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavran M, Zgomba M, Weitzel T, Petric D, Manz C, Becker N (2018) Distribution of Anopheles daciae and other Anopheles maculipennis complex species in Serbia. Parasitol Res 117:3277–3287 [DOI] [PMC free article] [PubMed]
- Koban MB, Frueh L, Kuhlisch C, Schaub GA, Steidle JLM, Scheuch DE, Janssen N, Kampen H, Werner D. The Asian bush mosquito Aedes japonicus japonicus (Diptera: Culicidae) in Europe, 17 years after its first detection, with a focus on monitoring methods. Parasit Vectors. 2019;12:e109. doi: 10.1186/s13071-019-3349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronefeld M, Dittmann M, Zielke D, Werner D, Kampen H. Molecular confirmation of the occurrence in Germany of Anopheles daciae (Diptera, Culicidae) Parasit Vectors. 2012;5:2e50. doi: 10.1186/1756-3305-5-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronefeld M, Werner D, Kampen H. PCR identification and distribution of Anopheles daciae (Diptera, Culicidae) in Germany. Parasitol Res. 2014;113:2079–2086. doi: 10.1007/s00436-014-3857-1. [DOI] [PubMed] [Google Scholar]
- Krüger A, Tannich E. Rediscovery of Anopheles algeriensis Theob. (Diptera: Culicidae) in Germany after half a century. J Eur Mosq Control Assoc. 2014;31:14–16. [Google Scholar]
- Kühlhorn F. Beitrag zur Verbreitung und Oekologie oberbayerischer Culiciden (Culex, Theobaldia, Aedes/Dipt.) Nachrichtenbl Bayer Entomol. 1954;3(47–50):59–61. [Google Scholar]
- Kuhlisch C, Kampen H, Walther D. Two new distribution records of Aedes (Rusticoidus) refiki Medschid, 1928 (Diptera: Culicidae) from Germany. J Eur Mosq Control Assoc. 2017;35:18–24. [Google Scholar]
- Kuhlisch C, Kampen H, Walther D. The Asian tiger mosquito Aedes albopictus (Diptera: Culicidae) in Central Germany: surveillance in its northernmost distribution area. Acta Trop. 2018;188:78–85. doi: 10.1016/j.actatropica.2018.08.019. [DOI] [PubMed] [Google Scholar]
- Kuhlisch C, Kampen H, Walther D. Rediscovery of Culex (Neoculex) martinii Medschid, 1930 (Diptera, Culicidae) in Germany. Parasitol Res. 2018;17:3351–3354. doi: 10.1007/s00436-018-6056-7. [DOI] [PubMed] [Google Scholar]
- Kuhlisch C, Kampen H, Werner D. On the distribution and ecology of Culiseta (Culicella) ochroptera (Peus) (Diptera: Culicidae) in Germany. Zootaxa. 2019;4576:544–558. doi: 10.11646/zootaxa.4576.3.7. [DOI] [PubMed] [Google Scholar]
- Lühken R, Czajka C, Steinke S, Jöst H, Schmidt-Chanasit J, Pfitzner W, Becker N, Kiel E, Krüger A, Tannich E. Distribution of individual members of the mosquito Anopheles maculipennis complex in Germany identified by newly developed real-time PCR assays. Med Vet Entomol. 2016;30:144–154. doi: 10.1111/mve.12161. [DOI] [PubMed] [Google Scholar]
- Martini E. Über drei weniger bekannte Kuliziden: Aëdes ornatus Meigen; Mansonia richiardii Fic. Und Anopheles (Coelodiazesis) nigripes Stäger. Arch Schiffs- Tropenhyg. 1915;19:585–607. [Google Scholar]
- Martini E. Über Stechmücken, besonders deren europäische Arten, und ihre Bekämpfung. Arch Schiffs- Tropenhyg. 1920;24(Beih 1):1–267. [Google Scholar]
- Martini E. Über mecklenburgische Culiciden. Sitzungsber Abh Naturforsch Ges Rostock. 1920;7:203–209. [Google Scholar]
- Martini E. Culiciden-Beobachtungen 1922, 1923. Z Angew Entomol. 1924;10:436–447. [Google Scholar]
- Martini E. Zwei bemerkenswerte Culiciden von einem eigenartigen biotop. Int Rev Ges Hydrobiol Hydrogeogr. 1924;12:333–337. [Google Scholar]
- Martini E. Über Anopheles bifurcatus und algeriensis. Anz Schädlingsk. 1931;7:109–110. [Google Scholar]
- Martini E, Missiroli A, Hackett LW (1931) Versuche zum Rassenproblem des Anopheles maculipennis. Arch Schiffs- Tropenhyg 35:622–643
- Medlock JM, Hansford KM, Versteirt V, Cull B, Kampen H, Fontenille D, Hendrickx G, Zeller H, van Bortel W, Schaffner F. An entomological review of invasive mosquitoes in Europe. Bull Ent Res. 2015;105:637–663. doi: 10.1017/S0007485315000103. [DOI] [PubMed] [Google Scholar]
- Mohrig W. Erstnachweis von Culex (Barraudius) modestus Ficalbi, 1890 in Deutschland (Diptera, Culicidae) Dtsch Entomol Z. 1963;10:331–334. [Google Scholar]
- Mohrig W. Die Culiciden Deutschlands. Gustav Fischer Verlag, Jena. Parasitol Schriftenr. 1969;18:1–260. [Google Scholar]
- Mohrig W (2000) Culicidae. In: Ziegler J, Menzel F (eds), Die historische Dipteren-Sammlung Carl Friedrich Ketel. Revision einer zwischen 1884 und 1903 angelegten Sammlung von Zweiflüglern (Diptera) aus Mecklenburg-Vorpommern. Nova Suppl Ent Berlin 14:42–43
- Müller P. Beiträge zur Kenntnis der Culicinenfauna in einigen Erholungsgebieten der Bezirke Rostock und Frankfurt/Oder. Angew Parasitol. 1965;5:90–101. [Google Scholar]
- Nicolescu G, Linton YM, Vladimirescu A, Howard TM, Harbach RE. Mosquitoes of the Anopheles maculipennis group (Diptera: Culicidae) in Romania, with the discovery and formal recognition of a new species based on molecular and morphological evidence. Bull Ent Res. 2004;6:525–535. doi: 10.1079/ber2004330. [DOI] [PubMed] [Google Scholar]
- Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microb Infect. 2009;11:1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Peus F. Beiträge zur Faunistik und Ökologie der einheimischen Culiciden. Z Desinfekt. 1929;21(76–81):92–98. [Google Scholar]
- Peus F. Die Stechmückenplage im Spreewald und die Möglichkeiten ihrer Bekämpfung. Z Gesundheitstech Städtehyg. 1932;3(4):133–142. [Google Scholar]
- Peus F. Zur Kenntnis der Aedes-Arten des deutschen Faunengebietes (Dipt., Culicid.). Die Weibchen der Aedes communis-Gruppe. Konowia. 1933;12:145–159. [Google Scholar]
- Peus F. Theobaldia (subg. Cullicella) ochroptera sp. n., eine bisher unbekannte Stechmücke. Märk Tierwelt. 1935;1:113–121. [Google Scholar]
- Peus F. Wieviele Stechmückenarten gibt es in Deutschland? Z Hyg Zool Schädlingsbek. 1937;29:117–119. [Google Scholar]
- Peus F. Aedes cyprius Ludlow (=A. freyi Edwards) und seine Larve (Dipt.: Culicidae) Arch Hydrobiol. 1937;31:242–252. [Google Scholar]
- Peus F. Stechmücken. Die Neue Brehm-Bücherei. 1950;20:1–80. [Google Scholar]
- Peus F (1970) Bemerkenswerte Mücken am Tegeler Fließ. Berl Naturschutzbl, Special Issue May:18–26
- Pfitzner WP, Lehner A, Hoffmann D, Czajka C, Becker N. First record and morphological characterization of an established population of Aedes (Hulecoeteomyia) koreicus (Diptera: Culicidae) in Germany. Parasit Vectors. 2018;11:e662. doi: 10.1186/s13071-018-3199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluskota B, Storch V, Braunbeck T, Beck M, Becker N. First record of Stegomyia albopicta (Skuse) (Diptera: Culicidae) in Germany. Eur Mosq Bull. 2008;26:1–5. [Google Scholar]
- Pluskota B, Jöst A, Augsten X, Stelzner L, Ferstl I, Becker N. Successful overwintering of Aedes albopictus in Germany. Parasitol Res. 2016;115:3245–3247. doi: 10.1007/s00436-016-5078-2. [DOI] [PubMed] [Google Scholar]
- Proft J, Maier WA, Kampen H. Identification of six sibling species of the Anopheles maculipennis complex (Diptera: Culicidae) by a polymerase chain reaction assay. Parasitol Res. 1999;85:837–843. doi: 10.1007/s004360050642. [DOI] [PubMed] [Google Scholar]
- Rezza G. Dengue and chikungunya: long-distance spread and outbreaks in naive areas. Pathog Glob Health. 2014;108:349–355. doi: 10.1179/2047773214Y.0000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V, Günay F, Le Goff G, Boussès P, Sulesco T, Khalin A, Medlock J, Kampen H, Petrić D, Schaffner F. Distribution chart for Euro-Mediterranean mosquitoes (western Palaearctic region) J Eur Mosq Control Assoc. 2019;37:1–28. [Google Scholar]
- Romi R, Toma L, Severini F, Di Luca M. Twenty years of the presence of Aedes albopictus in Italy—from the annoying pest mosquito to the real disease vector. Eur Infect Dis. 2008;2:98–101. [Google Scholar]
- Rudolf M, Czajka C, Börstler J, Melaun C, Jöst H, von Thien H, Badusche M, Becker N, Schmidt-Chanasit J, Krüger A, Tannich E, Becker S. First nationwide surveillance of Culex pipiens complex and Culex torrentium mosquitoes demonstrated the presence of Culex pipiens biotype pipiens/molestus hybrids in Germany. PLoS One. 2013;8:e71832. doi: 10.1371/journal.pone.0071832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydzanicz K, Czułowska A, Manz C, Jawień P. First record of Anopheles daciae (Linton, Nicolescu & Harbach, 2004) in Poland. J Vector Ecol. 2017;42:196–199. doi: 10.1111/jvec.12257. [DOI] [PubMed] [Google Scholar]
- Schaffner F, Angel G, Geoffroy B, Hervy JP, Rhaiem A, Brunhes J (2001) The mosquitoes of Europe. An identification and training programme (CD-ROM). IRD Éditions & EID Méditerrannée, Montpellier, France
- Schaffner F, Kaufmann C, Hegglin D, Mathis A. The invasive mosquito Aedes japonicus in Central Europe. Med Vet Entomol. 2009;23:448–451. doi: 10.1111/j.1365-2915.2009.00825.x. [DOI] [PubMed] [Google Scholar]
- Schneider P. Beitrag zur Kenntnis der Culiciden in der Umgebung von Bonn. Verh Naturhist Ver Preuß Rheinl Westf. 1913;70:1–54. [Google Scholar]
- Scholte E-J, Schaffner F. Waiting for the tiger: establishment and spread of the Aedes albopictus mosquito in Europe. In: Takken W, Knols BGJ, editors. Emerging pests and vector-borne diseases in Europe. Wageningen: Wageningen Academic Publishers; 2007. pp. 241–260. [Google Scholar]
- Steinbrink A, Zotzmann S, Cunze S, Klimpel S. Aedes koreicus—a new member of the genus Aedes establishing in Germany? Parasitol Res. 2019;118:1073–1076. doi: 10.1007/s00436-019-06232-x. [DOI] [PubMed] [Google Scholar]
- Tippelt L, Walther D, Kampen H. The thermophilic mosquito species Uranotaenia unguiculata (Diptera: Culicidae) moves north in Germany. Parasitol Res. 2017;116:3437–3440. doi: 10.1007/s00436-017-5652-2. [DOI] [PubMed] [Google Scholar]
- Tippelt L, Walther D, Kampen H. Further reports of Anopheles algeriensis Theobald, 1903 (Diptera: Culicidae) in Germany, with evidence of local mass development. Parasitol Res. 2018;117:2689–2696. doi: 10.1007/s00436-018-5938-z. [DOI] [PubMed] [Google Scholar]
- Vogel R. Zur Kenntnis der Stechmücken Württembergs. I Teil Jahresh Ver Vaterländ Naturk Württemb. 1929;85:258–277. [Google Scholar]
- Vogel R. Eine für Deutschland neue Stechmücke, Aedes refiki Medschid. Int Rev Ges Hydrobiol Hydrogeogr. 1931;25:257–258. [Google Scholar]
- Walther D, Kampen H. The citizen science project ‘Mueckenatlas’ helps monitor the distribution and spread of invasive mosquito species in Germany. J Med Entomol. 2017;54:1790–1794. doi: 10.1093/jme/tjx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther D, Scheuch DE, Kampen H. The invasive Asian tiger mosquito Aedes albopictus Diptera: (Culicidae) in Germany: local reproduction and overwintering. Acta Trop. 2017;166:186–192. doi: 10.1016/j.actatropica.2016.11.024. [DOI] [PubMed] [Google Scholar]
- Weitzel T, Gauch C, Becker N. Identification of Anopheles daciae in Germany through ITS2 sequencing. Parasitol Res. 2012;111:2431–2438. doi: 10.1007/s00436-012-3102-8. [DOI] [PubMed] [Google Scholar]
- Werner D, Kampen H. The further spread of Aedes japonicus japonicus (Diptera, Culicidae) towards northern Germany. Parasitol Res. 2013;112:3665–3668. doi: 10.1007/s00436-013-3564-3. [DOI] [PubMed] [Google Scholar]
- Werner D, Kampen H. Aedes albopictus breeding in southern Germany, 2014. Parasitol Res. 2015;114:831–834. doi: 10.1007/s00436-014-4244-7. [DOI] [PubMed] [Google Scholar]
- Werner D, Kronefeld M, Schaffner F, Kampen H (2012) Two invasive mosquito species, Aedes albopictus and Aedes japonicus japonicus, trapped in south-West Germany, July to August 2011. Euro Surveill 17:pii=20067 [DOI] [PubMed]
- Werner D, Zielke DE, Kampen H. First record of Aedes koreicus (Diptera: Culicidae) in Germany. Parasitol Res. 2016;115:1331–1334. doi: 10.1007/s00436-015-4848-6. [DOI] [PubMed] [Google Scholar]
- Zielke DE, Walther D, Kampen H. Newly discovered population of Aedes japonicus japonicus (Diptera: Culicidae) in upper Bavaria, Germany, and Salzburg, Austria, is closely related to the Austrian/Slovenian bush mosquito population. Parasit Vectors. 2016;9:e163. doi: 10.1186/s13071-016-1447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]