Abstract
The deep sea represents the largest and least explored biome on the planet. Despite the iconic status of the Galapagos Islands and being considered one of the most pristine locations on earth, the deep-sea benthic ecosystems of the archipelago are virtually unexplored in comparison to their shallow-water counterparts. In 2015, we embarked on a multi-disciplinary scientific expedition to conduct the first systematic characterization of deep-sea benthic invertebrate communities of the Galapagos, across a range of habitats. We explored seven sites to depths of over 3,300 m using a two-part Remotely Operated Vehicle (ROV) system aboard the E/V Nautilus, and collected 90 biological specimens that were preserved and sent to experts around the world for analysis. Of those, 30 taxa were determined to be undescribed and new to science, including members of five new genera (2 sponges and 3 cnidarians). We also systematically analysed image frame grabs from over 85 h of ROV footage to investigate patterns of species diversity and document the presence of a range of underwater communities between depths of 290 and 3,373 m, including cold-water coral communities, extensive glass sponge and octocoral gardens, and soft-sediment faunal communities. This characterization of Galapagos deep-sea benthic invertebrate megafauna across a range of ecosystems represents a first step to study future changes that may result from anthropogenic impacts to the planet’s climate and oceans, and informed the creation of fully protected deep-water areas in the Galapagos Marine Reserve that may help preserve these unique communities in our changing planet.
Subject terms: Biodiversity, Community ecology
Introduction
When Charles Darwin visited the Galapagos Islands aboard the HMS Beagle in 18351, deep-sea exploration was in its infancy and the presence of life at depth was questioned2,3. The Galapagos served as inspiration for Darwin’s breakthrough theory of evolution by means of natural selection4 and ever since, the iconic islands have captivated the global scientific community. While the Galapagos terrestrial fauna and flora have been the subject of intense study5–7, and research on shallow (< 40 m) coastal marine ecosystems has been considerable over the past four decades8–12, only a limited number of expeditions have explored beyond SCUBA diving depths. Most of these deep-sea research efforts have followed on the historic discovery of hydrothermal vents on the Galapagos Spreading Centre13,14 and further investigations on the volcanic origin of the islands15,16. A limited number of expeditions have been dedicated to the study of deep biological communities, with the 1986 Johnson Sealink being the most prolific in terms of invertebrate collections. However, the 25-year sequestration period placed by the National Cancer Institute on all specimens collected has limited the number of scientific publications from this cruise until recently24. The other limited number of expeditions to the Galapagos have been focused on the chemolithoautotrophic fauna associated with hydrothermal vents17–19, specific taxa such as fish20–23 and deep-sea corals24,25, or investigating the invertebrate communities associated with soft-sediment environments by means of trawling26.
Despite our fascination for the earth’s final frontier, the proportion of the deep sea that has been explored and sampled is minimal across all ocean basins27. Recent technological advances have facilitated deep-sea exploration to unprecedented levels, resulting in the discovery of new and diverse ecosystems27, the description of thousands of species new to science28 and the documentation of unique behaviours and natural histories29–31. With an estimated one-third to two-thirds of species in the ocean still awaiting description28, the deep sea has the potential to host a large proportion of earth’s undiscovered biodiversity. As our footprint on earth keeps growing and human activities now threaten the deeper parts of our oceans, it is key that we document and inform management actions to protect the biodiversity of the deep sea before it is too late.
The volcanic and isolated nature of the Galapagos Islands, represents an oasis of life in the vastness of the Eastern Pacific abyssal plain32, with the presence of numerous seamounts around the archipelago33. Among deep-sea environments, seamounts are known to support some of the most diverse and productive habitats, as physical interactions at these topographically pronounced features and surrounding water masses create favourable conditions that enhance biodiversity, species richness and possible endemism34–37. The location of the Galapagos Islands, at the crossroads of warm and cold oceanic currents, also results in a unique oceanographic setting38, with three major biogeographical groupings reported for shallow-water reef fauna39. Whether this is reflected on deep-sea taxa is unknown. Moreover, destructive human practices such as bottom trawling, globally known to have catastrophic impacts on slow-growing sessile deep-sea communities40,41, have been historically absent across the archipelago. As a result, the Galapagos Islands are likely to harbour unique, pristine deep-sea invertebrate benthic communities. In 2015, we explored the deep-water regions of the Galapagos Islands using a two-part Remotely Operated Vehicle (ROV) system aboard the E/V Nautilus. Here we present the results of a systematic characterization of deep-sea benthic invertebrate communities of the Galapagos across a range of habitats and investigate biodiversity across the different locations and depths explored.
Results and discussion
Throughout the ROV surveys we documented the presence of a range of underwater communities between depths of 290 and 3,373 m, including cold-water coral communities, extensive glass sponge and octocoral gardens, and soft-sediment faunal communities (Fig. 1; Table 1). A total of 90 taxa from six phyla, 13 classes, 22 orders and 44 families were collected and identified to the lowest taxonomic level possible by experts. Of the specimens analysed, thirty were identified as species new to science. Eleven of these taxa were also identified as belonging to new undescribed genera (Table 2).
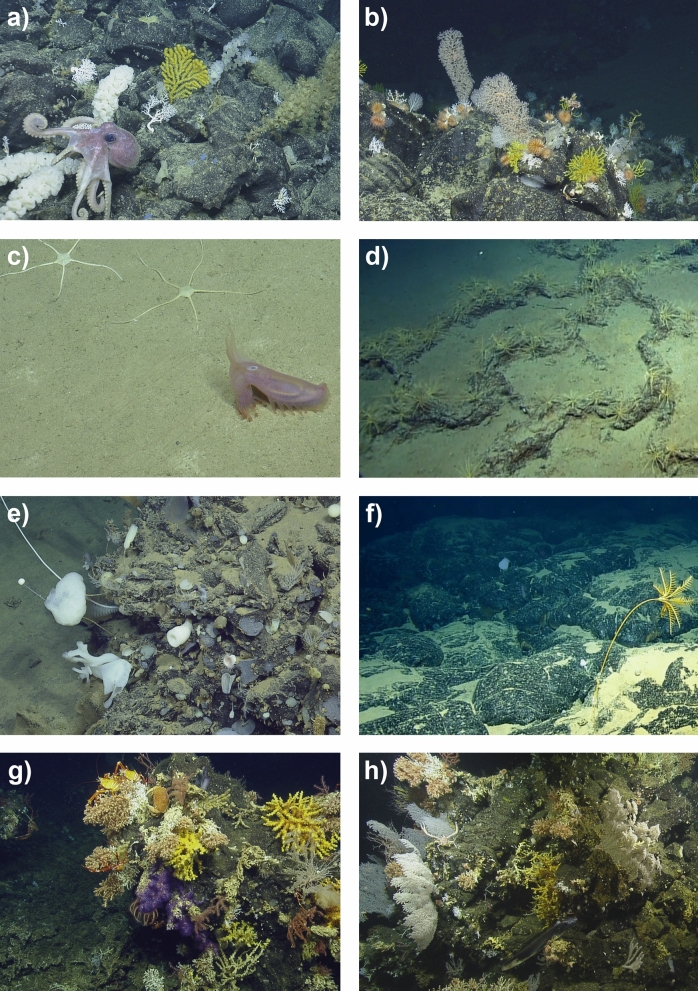
Figure 1.
Examples of the habitats and taxa present on the seamounts and lava flows of the Galapagos Marine Reserve (GMR) observed during the NA064 EV Nautilus expedition. (a) Dive H1435 seamount, depth 1,014 m. Octocorals and Hexactinellida sponges associated with volcanic substrate; (b) Dive H1435 seamount, depth 813 m. Scleractinia and Octocorallia associated with volcanic substrate; (c) Dive H1436 seamount, depth 2086 m. Ophiuroids and holothurian Peniagone indet. associated with an area of sand; (d) Dive H1440 seamount, depth 1,427 m. Aggregation of comatulid crinoids on exposed volcanic substrate; (e) Dive H1441 lava flow, depth 3,388 m. A mixed sponge and octocoral community on volcanic outcrop; (f) Dive H1442 lava flow, depth 2,922 m. A stalked sponge and Bathycrinus sp. crinoid on lava pillows; (g) Dive H1443 seamount, depth 414 m. Squat lobsters and brachyuran crabs on Scleractinia and Octocorallia colonies; (h) Dive H1443 seamount, depth 446 m. A mixed community of Scleractinia and Octocorallia colonies. Note; not all taxa presented in this figure were collected for taxonomic identification.
Table 1.
Summary of the ROV dive transects, sampling events, presence of vulnerable marine ecosystems and environmental parameters within the Galapagos Marine Reserve (GMR) during expedition NA064.
| Dive | H1435 | H1436 | H1440 | H1441 | H1442 | H1443A | H1443B |
|---|---|---|---|---|---|---|---|
| Duration | 16:48:47 | 11:14:19 | 11:23:43 | 13:34:44 | 18:22:52 | 10:02:24 | 06:39:17 |
| Feature | Seamount | Seamount | Seamount | Abyssal Plain | Abyssal Plain | Seamount | Seamount |
| Latitude | 1.2105 | 1.6584 | 1.8535 | − 0.3763 | − 0.3763 | − 0.3824 | − 0.3824 |
| Longitude | − 91.0836 | − 91.6731 | − 92.1064 | − 91.9043 | − 91.7619 | − 90.8091 | − 90.8091 |
| Geographic location | East of Wolf | East of Darwin | North of Darwin | West of Fernandina | West of Fernandina | West of Santiago | West of Santiago |
| Transect length (m) | 5,980 | 5,947 | 6,958 | 9,551 | 9,184 | 3,208 | 3,882 |
| Number of sampling events† | 19 (15) | 15 (8) | 18 (5) | 16 (3) | 25 (6) | 18 (5) | 5 (0) |
| Total number of species identified | 48 | 20 | 5 | 3 | 6 | 6 | 0 |
| Undescribed/new to science* | 16 (31%) | 5 (25%) | 3 (60%) | 2 (67%) | 1 (17%) | 3 (50%) | 0 (NA) |
| Vulnerable Marine Ecosystems (VMEs) |
Cold-water corals Coral gardens Sponge gardens |
*Indicator species present | *Indicator species present | *Indicator species present | *Indicator species present |
Cold-water corals Coral gardens Sponge gardens |
Cold-water corals Coral gardens |
| Depth range (m) | 290–1,199 | 930–2088 | 1,188–1962 | 3,307–3,373 | 2,940–3,012 | 411–640 | 248–618 |
| Temperature range (°C) | 3.88–13.45 | 2.28–4.96 | 2.54–3.84 | 1.79 | 1.76 | 7.15–9.10 | 7.15–13.61 |
| Salinity range (PSU) | 33.64–35.04 | 34.56–34.66 | 34.34–34.66 | 34.67 | 34.68 | 34.54–34.74 | 34.6–35.00 |
| O2 range (µmol/L) | 6.87–79.94 | 67.56–115.52 | 80.52–106.69 | 140.48–140.78 | 141.80–141.93 | 27.03–40.00 | 15.42–66.85 |
(†)value in brackets represents number of biological samples collected (*) value in brackets represents percentage of biological samples that are either undescribed or new to science from each dive location.
(+)Indicator species are those listed in the VME criteria under the methods section (octocoral, hexacorals, scleractinians, and erect sponges) but do not meet the required abundances.
Table 2.
List of deep-sea invertebrate megafauna sampled during the NA064 Galapagos cruise aboard the E/V Nautilus. New species are in bold.
| Sample ID | Event | Dive | Depth (m) | Phylum | Class | Order | Family | Lowest taxonomic ID | Authority | ID |
|---|---|---|---|---|---|---|---|---|---|---|
| NA064-028–01-03-A | 028 | H1436 | 1,199 | Annelida | Polychaeta | Phyllodocida | Polynoidae | Macellicephala galapagensis | (McIntosh, 1885) | GR† |
| NA064-027–01-01-A | 027 | H1436 | 1,272 | Melaenis indet. | GR† | |||||
| NA064-019–03-A | 019 | H1435 | 296 | Hyperhalosydna indet. | GR† | |||||
| NA064-034–01-02-A | 034 | H1436 | 973 | Macellicephala indet. sp. 1 | GR† | |||||
| NA064-004–08-A | 004 | H1435 | 1,153 | Macellicephala indet. sp. 2 | GR† | |||||
| NA064-029–05-A | 029 | H1436 | 1,047 | Macellicephala indet. sp. 3 | GR† | |||||
| NA064-018–01-01-A | 018 | H1435 | 299 | Polynoinae indet. sp. 1 | GR† | |||||
| NA064-014–01-09-A | 014 | H1435 | 871 | Polynoinae indet. sp. 2 | GR† | |||||
| NA064-011–01-03-A | 011 | H1435 | 1,052 | Arthropoda | Malacostraca | Decapoda | Axiidae | Eiconaxius albatrossae | Kensley, 1996 | MW |
| NA064-013–02-01-A | 013 | H1435 | 873 | Bathypalaemonellidae | Bathypalaemonella cf. serratipalma | Pequegnat, 1970 | LW | |||
| NA064-013–03-01-A | 013 | H1435 | 873 | Chirostylidae | Sternostylus defensus | (Benedict, 1902) | MW | |||
| NA064-010–01-02-A | 010 | H1435 | 1,067 | Uroptychus compressus sp. nov. | Baba & Wicksten, 2019 | MW | ||||
| NA064-007–01-03-B | 007 | H1435 | 1,041 | Uroptychus indet | MW | |||||
| NA064-013–01-01-A | 013 | H1435 | 873 | Uroptychus occidentalis | Faxon, 1893 | MW | ||||
| NA064-130–01-01-A | 130 | H1443 | 472 | Uroptychus bellus | Faxon, 1893 | MW | ||||
| NA064-031–01-01 | 031 | H1436 | 1,012 | Heteroptychus galapagos sp. nov. | Baba & Wicksten, 2019 | MW | ||||
| NA064-009–01-01-A | 009 | H1435 | 1,048 | Heteroptychus nautilus sp. nov. | Baba & Wicksten, 2019 | MW | ||||
| NA064-018–01-03-A | 018 | H1435 | 305 | Eumunididae | Eumunida subsolanus sp. nov. | Baba & Wicksten, 2019 | MW | |||
| NA064-019–02-B | 019 | H1435 | 296 | Mithracidae | Mithraculus indet. | TS† | ||||
| NA064-004–03-A | 004 | H1435 | 1,153 | Munididae | Munida perlata | Benedict, 1902 | MW | |||
| NA064-004–01-A | 004 | H1435 | 1,153 | Munidopsidae | Munidopsis indet. | MW | ||||
| NA064-028–01-04-A | 028 | H1436 | 1,199 | Munidopsis mina | Benedict, 1902 | MW | ||||
| NA064-027–01-03-A | 027 | H1436 | 1,272 | Munidopsis modesta | Benedict, 1902 | MW | ||||
| NA064-122–02-A | 122 | H1443 | 464 | Munidopsis scabra | Faxon, 1893 | MW | ||||
| NA064-022–01-01-A | 022 | H1436 | 1607 | Nematocarcinidae | Nematocarcinus indet | TS† | ||||
| NA064-130–01-02-A | 130 | H1443 | 472 | Palaemonidae | Pontonides sympathes | (De Ridder & Holthuis, 1979) | MW | |||
| NA064-019–15-A | 019 | H1435 | 296 | Pycnogonida | Pantopoda | Ammotheidae | Ammotheidae indet. | Dohrn, 1881 | MW | |
| NA064-067–01-A | 067 | H1440 | 1,222 | Cnidaria | Anthozoa | Alcyonacea | Acanthogorgiidae | Acanthogorgia indet. sp. 1 | SR | |
| NA064-005–01-A | 005 | H1435 | 1,153 | Acanthogorgia indet. sp. 2* | SR | |||||
| NA064-007–02-A | 007 | H1435 | 1,041 | Acanthogorgia indet. sp. 3 | SR | |||||
| NA064-026–01-A | 026 | H1436 | 1,286 | Acanthogorgia indet. sp. 4 | SR | |||||
| NA064-076–01-A | 076 | H1441 | 3,379 | Alcyoniidae | Bathyalcyon sp. nov.* | LW | ||||
| NA064-013–01-A | 013 | H1435 | 873 | Chrysogorgiidae | Chrysogorgia indet. | LW | ||||
| NA064-079–01-A | 079 | H1441 | 3,382 | Isididae | Bathygorgia sp. nov. | LW | ||||
| NA064-012-A | 012 | H1435 | 1,046 | Isididae gen. nov. (clade H1) | LW | |||||
| NA064-121–01-A | 121 | H1443 | 464 | Isididae gen. nov. sp. 1 | LW | |||||
| NA064-055–01-A | 055 | H1440 | 1822 | Isididae gen. nov. sp. 2 | LW | |||||
| NA064-061–01-A | 061 | H1440 | 1,407 | Isididae gen. nov. sp. 3* | LW | |||||
| NA064-099–01-A | 099 | H1442 | 2,923 | Isididae gen. nov. sp. 4 | LW | |||||
| NA064-009–01-A | 009 | H1435 | 1,048 | Isididae gen. nov. (clade 1) sp. 1 | LW | |||||
| NA064-021–01-A | 021 | H1436 | 1634 | Isididae gen. nov. (clade 1) sp. 2 | LW | |||||
| NA064-031–01-A | 031 | H1436 | 1,011 | Isididae gen. nov. (clade 1) sp. 3 | LW | |||||
| NA064-062–01-A | 062 | H1440 | 1,405 | Isididae gen. nov. (clade 1) sp. 4 | LW | |||||
| NA064-019–01-A | 019 | H1435 | 296 | Plexauridae | Swiftia indet.sp. 1 | SR | ||||
| NA064-007–01-A | 007 | H1435 | 1,041 | Swiftia indet.sp. 2 | SR | |||||
| NA064-077–01-A | 077 | H1441 | 3,381 | Primnoidae | Callozostron carlottae | Kükenthal, 1909 | SC | |||
| NA064-126–01-A | 126 | H1443 | 445 | Calyptrophora sp. nov. | SC | |||||
| NA064-125–01-A | 125 | H1443 | 446 | Parastenella sp. nov. | SC | |||||
| NA064-034–01-A | 034 | H1436 | 973 | Victorgorgiidae | Victorgorgia sp. nov.* | LW | ||||
| NA064-018–01-10-A | 018 | H1435 | 299 | Mollusca | Gastropoda | Lepetellida | Fissurellidae | Fissurellidae indet. | J. Fleming, 1822 | GR† |
| NA064-107–01-A | 107 | H1442 | 2,919 | Echinodermata | Asteroidea | Brisingida | Brisingidae | Hymenodiscus pannychia | (Fisher, 1928) | GR† |
| NA064-003–01-A | 003 | H1435 | 1,178 | Valvatida | Solasteridae | Solasteridae indet. | GR† | |||
| NA064-027–01-02-A | 027 | H1436 | 1,272 | Crinoidea | Comatulida | Antedonidae | Fariometra indet. | GR† | ||
| NA064-071–06-A | 071 | H1440 | 1,338 | Fariometra cf. parvula | (Hartlaub, 1895) | GR† | ||||
| NA064-011–01-01-A | 011 | H1435 | 1,052 | Heliometrinae indet. | GR† | |||||
| NA064-097–01-A | 097 | H1442 | 2,949 | Bathycrinidae | Bathycrinus cf. equatorialis | AH Clark, 1908 | GR† | |||
| NA064-022–01-A | 022 | H1436 | 1607 | Pentametrocrinidae | Pentametrocrinus paucispinulus | Messing, 2008 | GR† | |||
| NA064-029–02-A | 029 | H1436 | 1,047 | Thalassometridae | Thalassometridae indet. sp. 1 | GR† | ||||
| NA064-096–01-A | 096 | H1442 | 2,922 | Thalassometridae indet. sp. 2 | GR† | |||||
| NA064-028–01-A | 028 | H1436 | 1,199 | Echinoidea | Aspidodiadematoida | Aspidodiadematidae | Plesiodiadema indet. sp. 1 | GR† | ||
| NA064-103–01-A | 103 | H1442 | 2,908 | Plesiodiadema indet. sp. 2 | GR† | |||||
| NA064-017–11-A | 017 | H1435 | 352 | Cidaroida | Cidaridae | Cidaridae indet. sp. 1 | GR† | |||
| NA064-018–01-11-A | 018 | H1435 | 299 | Cidaridae indet. sp. 2 | GR† | |||||
| NA064-105–01-A | 105 | H1442 | 2,914 | Holothuroidea | Synallactida | Deimatidae | Oneirophanta setigera | (Ludwig, 1893) | GR† | |
| NA064-004–07-A | 004 | H1435 | 1,153 | Ophiuroidea | Amphilepidida | Ophiothamnidae | Histampica duplicata | (Lyman, 1875) | TO | |
| NA064-026–01-01-A | 026 | H1436 | 1,286 | Euryalida | Euryalidae | Asteroschema indet. sp. 1 | TO | |||
| NA064-005–01-01-A | 005 | H1435 | 1,153 | Ophiocreas indet. sp. 1 | TO | |||||
| NA064-017–03-A | 017 | H1435 | 352 | Ophiacanthida | Ophiacanthidae | Ophiacantha contigua | Lütken & Mortensen, 1899 | TO | ||
| NA064-015–05-A | 015 | H1435 | 376 | Ophiacantha quadrispina | H.L. Clark, 1917 | TO | ||||
| NA064-007–01-02-A | 007 | H1435 | 1,041 | Ophiacantha similis | A.H. Clark, 1916 | TO | ||||
| NA064-027–01-05-A | 027 | H1436 | 1,272 | Ophiolebes mortenseni | A.H. Clark, 1916 | TO | ||||
| NA064-015–13-A | 015 | H1435 | 376 | Ophiolimna bairdi | Lyman, 1883 | TO | ||||
| NA064-027–01-04-A | 027 | H1436 | 1,272 | Ophioplinthaca sp. nov. | TO | |||||
| NA064-015–06-A | 015 | H1435 | 376 | Ophiotreta valenciennesi | (Lyman, 1879) | TO | ||||
| NA064-004–06-A | 004 | H1435 | 1,153 | Ophiurida | Ophiuridae | Ophiocten sp. nov. | TO | |||
| NA064-004–10-A (a) | 004 | H1435 | 1,153 | Porifera | Demospongiae | Desmacellida | Desmacellidae | Desmacella sp. nov. | BO | |
| NA064-015–07-A | 015 | H1435 | 376 | Haplosclerida | Chalinidae | Haliclona sp. nov. | BO | |||
| NA064-004–10-A (b) | 004 | H1435 | 1,153 | Haliclona (Gellius) cf. perforata | Wilson, 1904 | HR | ||||
| NA064-029–04-A | 029 | H1436 | 1,047 | Niphatidae | Pachychalina sp. nov. | BO | ||||
| NA064-029–01-A | 029 | H1436 | 1,047 | Poecilosclerida | Cladorhizidae | Asbestopluma indet. | HR | |||
| NA064-010–01-A | 010 | H1435 | 1,067 | Phellodermidae | Phellodermidae gen. nov.* | HR | ||||
| NA064-018–01-08-A (c) | 018 | H1435 | 299 | Phellodermidae indet.* | BO | |||||
| NA064-019–10-A | 019 | H1435 | 296 | Polymastiida | Polymastiidae | Polymastia sp. nov.* | BO | |||
| NA064-018–01-08-A (a) | 018 | H1435 | 299 | Suberitida | Halichondriidae | Hymeniacidon sp. nov.* | BO | |||
| NA064-019–14-A | 019 | H1435 | 296 | Suberitidae | Protosuberitessp. nov.* | BO | ||||
| NA064-019–08-A | 019 | H1435 | 296 | Prosuberites sp. nov.* | BO | |||||
| NA064-018–01-08-A (b) | 018 | H1435 | 299 | Hexactinellida | Lyssacinosida | Rossellidae | Vitrollula sp. nov.* | BO | ||
| NA064-011–01-A | 011 | H1435 | 1,052 | Sceptrulophora | Euretidae | Conorete erectum | (Schulze, 1899) | HR | ||
| NA064-006-A | 006 | H1435 | 1,048 | Farreidae | Farreidae gen. nov.* | HR | ||||
| NA064-014–01-A | 014 | H1435 | 871 | Tretodictyidae | Tretodictyum sp. nov. | HR |
GR Greg Rouse, MW Mary K. Wicksten, TS Tim Shank, SR Sonia Rowley, LW Les Watling, SC Stephen Cairns, TO Tim O’Hara, BO Bruce Ott, HR Henry Reiswig.
*Indicates voucher specimen shown in Fig. 2. †Indicates specimens that were sequenced. All other identified from morphology.
Some of the new taxa discovered includes a giant solitary coral of the genus Bathyalcyon (Fig. 2c), the first known for the region; a new genus of bamboo corals of the family Isididae, with this specimen recorded at 2,923 m deep (Fig. 2d); a new genus of glass sponges of the family Farreidae, with some of the colonies encountered growing over 1 m in width (Fig. 2e); and a new demosponge genus in the family Phellodermidae, with an unusual ‘kebab-like’ growth form (Fig. 2f).
Figure 2.
Examples of voucher specimens collected and identified from the NA064 EV Nautilus expedition (a) Dive H1436, depth 974 m. NA064-034 Victorgorgia sp. nov.; (b) Dive H1435, depth 1,042 m. NA064-007 Acanthogorgia indet. sp 2; (c) Dive H1441, depth 3,382 m. NA064-076 Bathyalcyon sp. nov.; (d) Dive H1440, depth 1,404 m. NA064-061 Isididae gen. nov.; (e) Dive H1435, depth 1,048 m. NA064-006 Farreidae gen. nov.; (f) Dive H1435, depth 1,068 m. NA064-010 Phellodermidae gen. nov.; (g) Dive H1435, depth 305 m. NA064-018 Mixed sponge colony. Specimens examined within this sample have been identified to 3 different taxa. Vitrollula sp. nov; Hymeniacidon sp. nov; Phellodermidae indet.; (h) Dive H1435, depth 297 m. NA064-019 Various sponges. All specimens identified to genus: Polymastia sp. nov; Protosuberites sp. nov.; Prosuberites sp. nov.
This study represents one of the most comprehensive surveys of deep-sea invertebrate megafauna across a varied range of habitats, depth gradients and geographical locations within the Galapagos Islands. To our knowledge, this study provides the first systematic description of deep-sea specimens of several phyla, including Porifera, Echinodermata, Arthropoda and Annelida for Galapagos and also the Tropical Eastern Pacific (TEP)42. We also provide additional deep-water octocoral (Anthozoa) discoveries, a group that has previously received by far the most scientific attention from deep Galapagos waters24,25,42. Heterogeneous environments were found on many of the seamounts explored, including the presence of multiple fragile habitats, such as glass sponge gardens, coral gardens and cold-water coral colonies, that are considered Vulnerable Marine Ecosystems (VMEs) by the United Nations General Assembly43,44.
Our analysis of ROV imagery, revealed that VME indicator species were present on all the dives conducted, although their presence varied considerably among locations. According to the criteria used in our image analysis, VME communities were present on three of the sites visited, including one located on the far north bioregion of the archipelago (Dive H1435) and two sites within the central-south-eastern bioregion (Dive H1443; Table 3). The presence of cold-water corals was restricted to depths shallower than 500 m, while coral and sponge gardens, dominated by hexactinellids, were documented to depths of around 1,000 m (Table 3). The limited presence of VME communities on the remaining four sites sampled (Dives H1436; H1440; H1441; H1442) might be explained in part by the differences in the depths sampled, with ROV transects at these sites conducted at depths greater than 930 m, and the structural complexity of the seafloor. Dives H1436 and H1440, were conducted at seamounts with a much deeper, less topographically complex slope to the summit, while dives H1441 and H1442, were conducted on a predominantly sediment-covered seafloor, and only experienced a depth change of 66 m and 72 m respectively (Supplementary material I).
Table 3.
Summary of the vulnerable marine ecosystems present within the Galapagos Marine Reserve (GMR) during expedition NA064.
| Dive | Cold-water corals | Coral gardens | Sponge gardens | Total % of VMEs in images analysed |
|---|---|---|---|---|
| H1435 | ||||
| Depth range present | 359–449 m | 293–1102 m | 293–1165 m | 42% |
| Percentage presence over dive transect | 5% | 19% | 33% | |
| H1443A | ||||
| Depth range present | 414–489 m | 417–618 m | 417–618 m | 27% |
| Percentage presence over dive transect | 10% | 37% | 3% | |
| H1443B | ||||
| Depth range present | 249–335 m | 252–514 m | Not Present | 46% |
| Percentage presence over dive transect | 4% | 14% | Not Present | |
Our systematic analysis of ROV imagery documented a total of 70 benthic megafauna morphospecies (or morphospecies groups) across the locations surveyed (Supplementary Material II). The most speciose phyla observed were cnidarians (n = 31), echinoderms (n = 14), and arthropods (n = 9). Morphospecies of sponges, molluscs, annelida and tunicates were also observed (Fig. 1). In order to compare richness across dive locations and depth, morphospecies richness was derived from the number of the species present in each 100 m depth band. Analyses revealed that Dive H1435 in the Far north region of the GMR displayed the highest values in species richness within the 700–1,400 m depth strata, with a peak of 37 different morphospecies recorded at 800 m (Fig. 3). The lowest species richness was recorded at the deeper locations, with a minimum richness of four morphospecies recorded at 1,400–1,700 m on Dive H1436, also in the far north region of the GMR (Fig. 3).
Figure 3.
Depth-related trends in morphospecies richness per 100 m-depth band reported on three seamounts (H1435, H1436, H1440) and two volcanic cones (H1443A, H1443B) of the Galapagos Marine Reserve. The distribution of the Oxygen Minimum Zone (OMZ) as recorded on the ROV mounted CTD and Oxygen optode (Supplementary Material III) is highlighted in red.
The detailed species inventory from physical samples and spatial and depth related biodiversity patterns presented here provides one of the first comprehensive characterizations within the TEP, a marine ecoregion of South America where benthic marine biodiversity is presently poorly known and underestimated45. It also contributes to the global effort to publish deep-sea biodiversity inventories, a step critical to identify VME indicator species and communities in an effort to develop long-term conservation management plans for these areas on both national and regional scales. To reach these goals, there is a need to expand the taxonomic coverage of collections and compile this information from regional and global datasets46. In this study, we had the support and collaboration of taxonomic experts for each phylum from all over the world; nevertheless, taxonomy is still a major constraint on such studies as there is a general shortage of taxonomic expertise for marine biodiversity, particularly for the TEP45.
From the image analysis, we present morphospecies present on young volcanic formations (0.1- < 1 Ma) around the Darwin and Wolf lineament in the north of the GMR (H1435, H1436 and H1440), and one location relatively close (< 100 km) to the Galapagos Hotspot to the west of the archipelago47 (H1443; Fig. 4). We only recorded major differences in morphospecies richness between shallow depth strata (200–700 m) from North and Southeast regions (Fig. 3), which could suggest the presence of different biogeographical areas within the Archipelago, as it has been proposed for shallow marine communities39. However, given the limited number of locations surveyed at each region, further data is required to determine whether this biogeographic division applies to deep-sea communities. In addition, given the movement of the Nazca plate east towards the South America mainland, the islands that are formed in the Galapagos hotspot travel east, subsiding to eventually become drowned islands48. Therefore, the islands and seamounts to the east of the archipelago represent much older geological formations, with some dated to be > 8 Ma47. It is thus likely that additional explorations to the east of the archipelago might result in the discovery of distinct populations and additional new taxa. Future deep-sea research directed at investigating the effect of environmental predictors on deep-sea biodiversity is also required to better understand these relationships at the Galapagos Islands.
Figure 4.
Bathymetric map of the Galapagos Marine Reserve with ROV dive locations across the Galapagos Islands during the NA064 E/V Nautilus cruise. All dives also fall within the BY7 Cocos Plate deep-sea benthic province as proposed by Watling et al.49. Gridded bathymetric data provided by the General Bathymetric Chart of the Oceans (GEBCO) 30 arc-second grid (accessed via https://www.gebco.net/). Map created in ESRI ArcMap (version 10.3.1).
Although deep-sea communities are rarely included into spatial-planning processes, the Ecuadorian government recently reviewed the zoning process for the Galapagos Marine Reserve and prioritized the protection of seamounts and open water environments50. The information presented here contributed to this spatial planning process and promoted the protection of specific areas based on the demonstration of the presence of VME indicator species and communities and unique taxa and behaviours31,51,52. In March 2016, a new zoning plan was proposed and fully protected areas were recommended across the reserve to protect seamounts and other deep-sea habitats, including the creation of a 40,000 km2 marine sanctuary to protect the three seamounts we surveyed around Darwin and Wolf islands, a hotspot for pelagic species10,53,54. However, due to continuous political pressure from the Galapagos artisanal fishing sector, this zoning plan is still under re-evaluation55.
The discovery of these diverse deep-sea biological communities highlights yet again the ecological significance of the marine ecosystems of the Galapagos Islands. This deep-sea world that Darwin never saw represents a unique and pristine environment, particularly considering the historical absence of destructive human practices known to have catastrophic impacts upon fragile communities56,57, such as bottom trawling or deep-sea mining; and their current conservation status and level of protection granted by the Galapagos Marine Reserve. Under the present climate crisis, that will have major effects across the world’s marine ecosystems58, including the deep sea59–61, the Galapagos Islands represent an ideal location from which to monitor future changes in this poorly studied and understood biome.
Methods
NA064 Galapagos platform cruise
In June 2015, we conducted a 10-day collaborative research cruise (NA064) aboard E/V Nautilus between the Ocean Exploration Trust, the Charles Darwin Foundation and the Galapagos National Park Directorate to explore deep-sea environments of the Galapagos Marine Reserve. All methods were carried out in accordance with relevant guidelines and regulations by the Galapagos National Park Directorate under research permits PC-26–15 & PC-45–15. All experimental protocols were reviewed and approved by a Galapagos National Park Directorate’s committee that evaluates animal care in research activities.
ROV surveys and sample collection
Exploration of the seafloor was carried out using the two-body Remotely Operated Vehicle (ROV) system Argus and Hercules, each rated for 4 km water depth. Video and still images of the sites were acquired using Insite Pacific Zeus Plus HD color video cameras on both vehicles, each equipped with a 10 × mechanical zoom lens. Biological specimens were collected using the ROV manipulator arm and placed on sample boxes aboard Hercules ROV for recovery.
We conducted a total of six exploratory dives to the north, west, and central part of the Galapagos Archipelago (Fig. 4, Table 1). In general terms, sampling transects began at the base of each feature explored and we conducted a general upslope transect, following sonar and visual features along the way. Dives H1435, H1436 and H1440 explored three conical seamounts around the most northern islands of the archipelago, Darwin and Wolf. Dives H1441 and H1442 explored the abyssal plain located to the west of Fernandina and adjacent to the Galapagos. The last dive, H1443, explored a shallower volcanic cone, with two horseshoe-shaped craters, located between Santiago and Isabela islands in the central part of the Archipelago (Fig. 4, Table 1).
During each dive, biological samples were collected opportunistically using the ROV’s arms or suction sampler, with the aim of collecting a representative sample of specimens, but with a sampling bias towards large and conspicuous megafauna and their associates. Sampling involved the collection of individual organisms (or part of them) with their associated epibionts, as well as collecting rocks with organisms attached to them. When the ROV was back on deck, individual organisms were separated and photographed. Specimens were then sub-sampled and preserved following standardized protocols62 and in accordance with subsequent morphological and genetic analysis. Scleractinian corals were not included in the taxonomic analysis due to permit restrictions.
Morphological and genetic identification of collected specimens
Whole specimens or sub-samples were sent to renowned experts on specific taxa for morphological and/or genetic identification. Most specimens were identified by morphological traits to the lowest taxonomic level. Additionally, some Annelida, Arthropoda, Mollusca and Echinodermata tissue sub-samples were sequenced for genetic characterization. For the Annelida the standard Folmer (M1-M6) region (~ 650 bp) of the mitochondrial cytochrome oxidase subunit 1 gene (mtDNA COI) was sequenced63. For the Echinodermata, a hybrid enrichment phylogenetic method was used to obtain sequences from 415 housekeeping genes (285 kbp)64.
Accepted species names were verified using WoRMS (World Register of Marine Species, 2017) and WoRDSS (World Register of Deep-sea Species, 2017). Geographical distribution of identified taxa was investigated using the OBIS (Ocean Biogeographic Information System). Voucher specimens will be stored at the Charles Darwin Foundation Galapagos Biodiversity Collection. All taxonomic identifications and metadata have been formatted to DarwinCore standard65.
Presence of vulnerable marine ecosystems and imagery analysis
Due to the volume of non-quantatitive data that had been recorded from the Hercules ROV during the NA064 expedition, it was decided to analyse a series of still images rather than the full video sequence for each dive. Video files were converted to image sequences, with one frame extracted every 5 s using QuicktimePro 7. As the speed of the ROV varied over the duration of the full transect, an image was selected every 10 m for analysis rather than a set time interval. To achieve this, the ROV USBL navigation was cleaned and smoothed in ArcGIS (version 10.3) using editing and cartography toolboxes and split into 10 m segments. A shapefile for the mid-point of each 10 m segment was then generated. Using the join and relate functions in ArcGIS, the 5-s image frame (co-registered with the smoothed USBL positional data) that was in closest proximity to the 10 m mid-point, was selected for further analysis. Video data recorded during sampling events, were removed from the transect data. Dives H1441 and H1442 that were conducted on deep abyssal plains were excluded from the morphospecies analysis given the homogeneity of the terrain surveyed.
Image analysis was conducted using the BIIGLE 2.0 software66 by three analysts. To minimise bias, each analyst was randomly assigned a third of the images from each dive to analyse. To account for the variation in image quality (i.e. blue water, blurred images, variation in field of view) but to be able to obtain the maximum amount of viable data, all images were annotated using the following label trees; 1) image quality; 2) dominant and secondary substrate type; 3) presence of habitat-forming fauna (or VMEs) such as sponges, corals or bivalves; or large aggregations of benthic fauna such as brittlestars or featherstars within the field of view of the oblique high-definition camera and finally; 4) the presence of morphospecies (> 3 cm in size) identified to the lowest taxonomic level possible. Only images of sufficient quality were analysed. For the purpose of this study VME communities were defined as follows; 1) coral gardens were defined as the presence of > 5 colonies of octocorals and/or hexacorals (not including scleractinians) of the same or different morphospecies; 2) sponge gardens were defined as the presence of > 5 colonies of erect (not encrusting) of the same or different morphospecies and; 3) cold-water coral communities were defined as the presence of 3D habitat-forming scleractinian colonies. Scaling lasers set 10 cm were used to opportunistically to measure biota or geological formations. However, as these were not used consistently for the duration of the dive and owing to the oblique camera angle, densities of morphospecies have not been calculated. To ensure consistency between analysts, any conflicts in morphospecies identification or VME community identification were resolved using the “largo” re-evaluation function in BIIGLE. A total of 2077 individual images were analysed across the five ROV dives.
A species matrix was created by enumerating taxonomically distinct organisms in each image as present or absent over each 100 m depth band. Given the uncertainties of identifying morphospecies from ROV imagery we adopted best practices in analyses to provide the best possible yet conservative identifications, and where appropriate, morphospecies have been grouped at higher taxonomic levels where identifications were uncertain. Indeterminate taxa that could not be confidently distinguished or identified to phylum were excluded from the analyses.
Supplementary information
Acknowledgements
We are thankful to the Ocean Exploration Trust as well as the pilots and crew aboard the E/V Nautilus during cruise NA064 for their assistance in sample collection and exploration using the Hercules ROV. Thank you to the NOAA Office of Exploration and Research for funding the E/V Nautilus Exploration Program (NA15OAR0110220). Further acknowledgements and thanks go out to the Charles Darwin Foundation and the Galapagos National Park Directorate for their collaboration and assistance in the exploration of the Galapagos Platform conducted under research permits PC-26–15 & PC-45-15. We also gratefully recognize the Government of Ecuador via the Ecuadorian Navy for permission to operate in their territorial waters. This research was supported by a grant from the Helmsley Charitable Trust and the Gordon and Betty Moore Foundation. This publication is contribution number 2354 of the Charles Darwin Foundation for the Galapagos Islands.
Author contributions
P.S.L., E.R., C.F., N.A.R., L.M. conceived and designed the study. P.S.L., P.M.P., S.B., C.A.U., E.R., M.C., S.D.C., C.F., T.D.O., B.O., N.A.R., H.R., G.W.R., S.R., T.S., J.S., L.W., M.W., L.M. collected and analysed data and contributed to write the main manuscript text. P.S.L., C.A.U., L.M. prepared figures.
Data availability
Data cannot be shared publicly because of Galapagos National Park research permit conditions. A previous authorization from the Galapagos National Park Directorate is required for further use of this data. Access to data can be requested via this email address at the Galapagos National Park Directorate Applied Research Department: investigacion@galapagos.gob.ec.
Competing interests
The authors declare no financial and non-financial competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-70744-1.
References
- 1.Darwin, C. The Voyage of the Beagle (1839).
- 2.Forbes, E. Report on the Mollusca and Radiata of the Aegean Sea: And on Their Distribution, Considered as Bearing on Geology (1843).
- 3.De La Beche HT. Researches in Theoretical Geology. New York: FJ Huntington & co.; 1837. [Google Scholar]
- 4.Darwin C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. Hachette: John Murray; 1859. [PMC free article] [PubMed] [Google Scholar]
- 5.Caccone A, et al. Phylogeography and history of giant Galápagos tortoises. Evolution. 2002;56:2052–2066. doi: 10.1111/j.0014-3820.2002.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 6.Grant PR. Ecology and Evolution of Darwin’s Finches. Princeton: Princeton University Press; 1999. [Google Scholar]
- 7.Wikelski M, Thom C. Marine iguanas shrink to survive El Niño. Nature. 2000;403:37–38. doi: 10.1038/47396. [DOI] [PubMed] [Google Scholar]
- 8.Glynn PW, Wellington GM. Corals and Coral Reefs of the Galapagos Islands. California: University of California Press; 1983. [Google Scholar]
- 9.McCosker JE, Rosenblatt RH. The fishes of the Galápagos Archipelago: an update. Proc. Calif. Acad. Sci. 2010;61:167–195. [Google Scholar]
- 10.Salinas de León P, et al. Largest global shark biomass found in the northern Galápagos Islands of Darwin and Wolf. PeerJ. 2016;4:e1911. doi: 10.7717/peerj.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wellington, G. M. The Galápagos coastal marine environment (1975).
- 12.Witman JD, Smith F. Rapid community change at a tropical upwelling site in the Galápagos Marine Reserve. Biodivers. Conserv. 2003;12:25–45. [Google Scholar]
- 13.Corliss JB, Dymond J, Gordon LI, Edmond J. M. on the Galapagos Rift. Science. 1979;203:16. doi: 10.1126/science.203.4385.1073. [DOI] [PubMed] [Google Scholar]
- 14.Hessler, R. R. & Smithey Jr, W. M. The distribution and community structure of megafauna at the Galapagos Rift hydrothermal vents. In Hydrothermal Processes at Seafloor Spreading Centers 735–770 (Springer, 1983).
- 15.Harpp, K. S. & White, W. M. Tracing a mantle plume: isotopic and trace element variations of Galápagos seamounts. Geochem. Geophys. Geosystems2 (2001).
- 16.Lubetkin M, et al. Nontronite-bearing tubular hydrothermal deposits from a Galapagos seamount. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017 doi: 10.1016/j.dsr2.2017.09.017. [DOI] [Google Scholar]
- 17.Karl DM, Wirsen C, Jannasch H. Deep-sea primary production at the Galapagos hydrothermal vents. Sci. States. 1980;207:1345–1347. [Google Scholar]
- 18.Rhoads DC, Lutz RA, Revelas EC, Cerrato RM. Growth of bivalves at deep-sea hydrothermal vents along the Galapagos Rift. Science. 1981;214:911–913. doi: 10.1126/science.214.4523.911. [DOI] [PubMed] [Google Scholar]
- 19.Van Dover CL, Berg Jr CJ, Turner RD. Recruitment of marine invertebrates to hard substrates at deep-sea hydrothermal vents on the East Pacific Rise and Galapagos spreading center. Deep Sea Res. Part Oceanogr. 1988;35:1833–1849. [Google Scholar]
- 20.Iwamoto T, McCosker JE. Notes on Galápagos grenadiers (Pisces, Gadiformes, Macrouridae), with the description of a new species of Coryphaenoides. Rev. Biol. Trop. 2001;49:21–27. [PubMed] [Google Scholar]
- 21.Long DJ, McCosker JE, Blum S, Klapfer A. Tropical Eastern Pacific records of the prickly shark, Echinorhinus cookei (Chondrichthyes: Echinorhinidae) Pac. Sci. 2011;65:433–440. [Google Scholar]
- 22.McCosker JE, Long DJ, Baldwin CC. Description of a new species of deepwater catshark, Bythaelurus giddingsi sp. Nov., from the Galápagos Islands (Chondrichthyes: Carcharhiniformes: Scyliorhinidae) Zootaxa. 2012;59:48–59. [Google Scholar]
- 23.Cerutti-Pereyra F, Yanez A, Ebert DA, Arnés-Urgellés C, Salinas-De-León P. New record and range extension of the deepsea skate, Bathyraja Abyssicola (Chondrichthyes: Arhynchobatidae) The Galapagos Islands. 2018 doi: 10.5281/zenodo.1400829. [DOI] [Google Scholar]
- 24.Cairns SD. Deep-water octocorals (Cnidaria, Anthozoa) from the Galápagos and Cocos Islands. Part 1: Suborder Calcaxonia. ZooKeys. 2018;729:1–46. doi: 10.3897/zookeys.729.21779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cairns, S. D. New records of Stylasteridae (Hydrozoa: Hydroida) from the Galápagos and Cocos Islands (1991).
- 26.Faxon W. Reports on an exploration off the west coast of Mexico, Central and South America, and off the Galapagos Islands by the US Fish Commission steamer «Albatross» during 1891.... XV. Mem. Mus. Comp. Zool. 1895;18:1–292. [Google Scholar]
- 27.Ramirez-Llodra E, et al. Deep, diverse and definitely different: unique attributes of the world’s largest ecosystem. Biogeosciences. 2010;7:2851–2899. [Google Scholar]
- 28.Appeltans W, et al. The magnitude of global marine species diversity. Curr. Biol. 2012;22:2189–2202. doi: 10.1016/j.cub.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 29.Archer SK, et al. Pyrosome consumption by benthic organisms during blooms in the northeast Pacific and Gulf of Mexico. Ecology. 2018;99:981–984. doi: 10.1002/ecy.2097. [DOI] [PubMed] [Google Scholar]
- 30.Gates AR, Morris KJ, Jones DO, Sulak KJ. An association between a cusk eel (Bassozetus sp.) and a black coral (Schizopathes sp.) in the deep western Indian Ocean. Mar. Biodivers. 2017;47:971–977. [Google Scholar]
- 31.Salinas-de-León P, et al. Deep-sea hydrothermal vents as natural egg-case incubators at the Galapagos Rift. Sci. Rep. 2018;8:1788. doi: 10.1038/s41598-018-20046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris P, Macmillan-Lawler M, Rupp J, Baker E. Geomorphology of the oceans. Mar. Geol. 2014;352:4–24. [Google Scholar]
- 33.Geist, D. J., Snell, H., Snell, H., Goddard, C. & Kurz, M. D. A paleogeographic model of the Galápagos Islands and biogeographical and evolutionary implications. Galápagos Nat. Lab. Earth Sci. Am. Geophys. Union Wash. DC USA 145–166 (2014).
- 34.Morato T, Hoyle SD, Allain V, Nicol SJ. Seamounts are hotspots of pelagic biodiversity in the open ocean. Proc. Natl. Acad. Sci. 2010;107:9707–9711. doi: 10.1073/pnas.0910290107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitcher TJ, et al. Seamounts: Ecology, Fisheries & Conservation. Hoboken: Wiley; 2008. [Google Scholar]
- 36.Rogers A. The biology of seamounts. Adv. Mar. Biol. 1994;30:305–350. doi: 10.1016/bs.amb.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Genin A, Dayton PK, Lonsdale PF, Spiess FN. Corals on seamount peaks provide evidence of current acceleration over deep-sea topography. Nature. 1986;322:59. [Google Scholar]
- 38.Palacios DM. Seasonal patterns of sea-surface temperature and ocean color around the Galápagos: regional and local influences. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2004;51:43–57. [Google Scholar]
- 39.Edgar GJ, Banks S, Fariña JM, Calvopiña M, Martínez C. Regional biogeography of shallow reef fish and macro-invertebrate communities in the Galapagos archipelago. J. Biogeogr. 2004;31:1107–1124. [Google Scholar]
- 40.Koslow J, et al. Continental slope and deep-sea fisheries: implications for a fragile ecosystem. ICES J. Mar. Sci. 2000;57:548–557. [Google Scholar]
- 41.Watling L, Norse EA. Disturbance of the seabed by mobile fishing gear: a comparison to forest clearcutting. Conserv. Biol. 1998;12:1180–1197. [Google Scholar]
- 42.Breedy O, van Ofwegen LP, Vargas S. A new family of soft corals (Anthozoa, Octocorallia, Alcyonacea) from the aphotic tropical eastern Pacific waters revealed by integrative taxonomy. Syst. Biodivers. 2012;10:351–359. [Google Scholar]
- 43.Ardron JA, et al. A systematic approach towards the identification and protection of vulnerable marine ecosystems. Mar. Policy. 2014;49:146–154. [Google Scholar]
- 44.Halpern BS, Selkoe KA, Micheli F, Kappel CV. Evaluating and ranking the vulnerability of global marine ecosystems to anthropogenic threats. Conserv. Biol. 2007;21:1301–1315. doi: 10.1111/j.1523-1739.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 45.Miloslavich P, et al. Marine biodiversity in the atlantic and pacific coasts of south america: knowledge and gaps. PLoS ONE. 2011;6:e14631. doi: 10.1371/journal.pone.0014631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clark MR, Schlacher TA, Rowden AA, Stocks KI, Consalvey M. Science priorities for seamounts: research links to conservation and management. PLoS ONE. 2012;7:e29232. doi: 10.1371/journal.pone.0029232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinton CW, Christie DM, Duncan RA. Geochronology of Galápagos seamounts. J. Geophys. Res. Solid Earth. 1996;101:13689–13700. [Google Scholar]
- 48.Christie D, et al. Drowned islands downstream from the Galapagos hotspot imply extended speciation times. Nature. 1992;355:246. [Google Scholar]
- 49.Watling L, Guinotte J, Clark MR, Smith CR. A proposed biogeography of the deep ocean floor. Prog. Oceanogr. 2013;111:91–112. [Google Scholar]
- 50.Dirección del Parque Nacional Galápagos. Plan de Manejo de las Areas Protegidas de Galápagos par el Buen Vivir (2014).
- 51.Carey S, et al. Exploring the undersea world of the Galápagos Islands. Ocean. Mag. 2016;29:32–34. [Google Scholar]
- 52.Salinas-De-León, P., Acuña-Marrero, D., Carrión-Tacuri, J. & Sala, E. Valor ecológico de los ecosistemas marinos de Darwin y Wolf, Reserva Marina de Galápagos. 15 (2015).
- 53.Acuña-Marrero D, et al. Spatial patterns of distribution and relative abundance of coastal shark species in the Galapagos Marine Reserve. Mar. Ecol. Prog. Ser. 2018;593:73–95. [Google Scholar]
- 54.Acuña-Marrero D, et al. Whale shark (Rhincodon typus) seasonal presence, residence time and habitat use at Darwin Island Galapagos Marine Reserve. PLoS ONE. 2014;9:e115946. doi: 10.1371/journal.pone.0115946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ministerio del Ambiente del Ecuador. Acuerdo Ministerial 076/2018. (2018).
- 56.Ramirez-Llodra E, et al. Man and the last great wilderness: human impact on the deep sea. PLoS ONE. 2011;6:e22588. doi: 10.1371/journal.pone.0022588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roberts CM. Deep impact: the rising toll of fishing in the deep sea. Trends Ecol. Evol. 2002;17:242–245. [Google Scholar]
- 58.Hoegh-Guldberg O, Bruno JF. The impact of climate change on the world’s marine ecosystems. Science. 2010;328:1523–1528. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- 59.Danovaro R, Dell’Anno A, Pusceddu A. Biodiversity response to climate change in a warm deep sea: biodiversity and climate change in the deep sea. Ecol. Lett. 2004;7:821–828. [Google Scholar]
- 60.Danovaro R, Dell’Anno A, Fabiano M, Pusceddu A, Tselepides A. Deep-sea ecosystem response to climate changes: the eastern Mediterranean case study. Trends Ecol. Evol. 2001;16:505–510. [Google Scholar]
- 61.Sweetman AK, et al. Major impacts of climate change on deep-sea benthic ecosystems. Elem. Sci. Anthr. 2017;5:4. [Google Scholar]
- 62.Etnoyer, P. et al. Deep-sea coral collection protocols. NOAA Tech. Memo. NMFS-OPR28 (2006).
- 63.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 64.Lemmon AR, Emme SA, Lemmon EM. Anchored hybrid enrichment for massively high-throughput phylogenomics. Syst. Biol. 2012;61:727–744. doi: 10.1093/sysbio/sys049. [DOI] [PubMed] [Google Scholar]
- 65.Wieczorek J, et al. Darwin core: an evolving community-developed biodiversity data standard. PLoS ONE. 2012;7:e29715. doi: 10.1371/journal.pone.0029715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ontrup, J., Ehnert, N., Bergmann, M. & Nattkemper, T. W. BIIGLE-Web 2.0 Enabled Labelling and Exploring of Images from the Arctic Deep-Sea Observatory HAUSGARTEN. 1–7 (IEEE, 2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared publicly because of Galapagos National Park research permit conditions. A previous authorization from the Galapagos National Park Directorate is required for further use of this data. Access to data can be requested via this email address at the Galapagos National Park Directorate Applied Research Department: investigacion@galapagos.gob.ec.